Abstract
We present a study of up-regulation of genes responsible for pancreatic development in glucose-sensitive insulin-secreting mesenchymal stem cells (IS-MSC) generated and differentiated from human adipose tissue (h-AD), with use of our specific differentiation media and without use of any xenogenic material. Anterior wall abdominal fat was collected from 56 volunteers and cultured in self-designed proliferation medium for 10 days. Cells were harvested by trypsinization and differentiated into insulin-expressing cells using self-designed differentiation medium for 3 days followed by evaluation for transcriptional factors Pax-6, Ipf-1, Isl-1, C-peptide and insulin secretion. Generated IS-MSC showed expression of Pax-6, Pdx-6 and Isl-1. Non-differentiated MSC as well as their further culture in absence of differentiation medium were used as negative controls. Generated 56 IS-MSC cell-lines were glucose responsive i.e. mean C-Peptide and insulin secretion levels were measured 0.41 ng/ml and 13.13 μU/ml, respectively, in absence of glucose which rose to 1.18 ng/ml and 83.42 μU/ml, respectively, following glucose challenge (p < 0.001). The mean rise in C-peptide and insulin secretion levels was 2.88 and 6.35 fold, respectively. To conclude insulin-secreting h-AD-MSC can be generated safely and effectively with application of specific differentiation media without xenogeneic material/any genetic modification, showing expression of transcriptional factors Pax-6, Ipf-1 and Isl-1.
Keywords: Transcriptional factors, Insulin secreting cells, Human adipose tissue, Differentiation media
Introduction
Transcription factors (TFs) are molecules involved in regulating gene expression. They are usually proteins in groups or complexes, involved in multiple interactions necessary to achieve varying degrees of control over transcription rates. Considerable progress has been made in the understanding of sequential activation of signal transduction pathways and the expression of transcription factors during pancreas development. Much of this knowledge has been obtained from analysis of phenotypes of knock-out mice models. A straightforward therapeutic strategy in the field of stem cell-based regenerative medicine is the transplantation of functional differentiated cells as cell replacement for the lost or defective cells affected by disease. However, this strategy requires the capacity to regulate stem cell differentiation toward the desired cell fate. This therapeutic approach assumes the capability to direct mesenchymal stem cells (MSC) differentiation toward diverse cell fates, by a process termed transdifferentiation. The capacity of MSCs to undergo functional transdifferentiation has been questioned over the years. Specifically, forced expression of certain transcription factors can lead to reprogramming and alter cell fate. Using such a method, fully differentiated lymphocytes have been reprogrammed to become macrophages and, remarkably, somatic cells have been reprogrammed to become embryonic stem-like cells. The past and current research aimed at transdifferentiating MSCs, a process with applications could revolutionize regenerative medicine. Understanding of the importance of transcription factor genes during pancreas development provides insight into the pathogenesis of diabetes mellitus (DM) in which the mass of insulin-producing beta cells is reduced or has become ineffective (Habener et al. 2005).
The expression of transcription factors Pax-6, Ipf-1 and Isl-1 are required for pancreas development, including the differentiation and function of beta-cells. Homozygosis or mutations in these genes in mice results in a complete pancreatic agenesis (Jonsson et al. 1994). There is an increasing body of evidence suggesting that MSC are able to differentiate into insulin producing cells and to correct hyperglycaemia, at least in rodents (Kodama et al. 2003; Tang et al. 2004).
We present our experience with up-regulation of transcriptional factors Pax-6, Ipf-1 and Isl-1 without any genetic manipulation, associated with insulin secretion from human adipose tissue-derived mesenchymal stem cells (h-AD-MSCs).
Materials and methods
Isolation of MSC from h-AD
After Institutional Review Board approval of the methodology and informed consent forms from voluntary donors, undergoing any other surgeries. 10 gram adipose tissue was resected from anterior abdominal wall under local anesthesia after making a small incision on left lateral side below umbilicus. Sutures were taken after hemostasis was secured. This adipose tissue was collected in self-designed proliferation medium comprising of Minimum Essential Medium with alpha modification (α-MEM) (Sigma, St. Louis, MO, USA), 20 % human albumin (Reliance Life Sciences, Navi Mumbai, India), 5 ng/ml Basic-Human Fibroblast Growth Factor (B-hFGF) (Sigma, USA), 1 % Sodium pyruvate (Hi-media, India) and standard antibiotics which included 1 % penicillin-streptomycin-cefotaxime solution (Hi-media, Mumbai, India) and anti-fungal fluconazol (1 mg/10 ml) (Life sciences, Haryana, India).
The adipose tissue was minced with knife into fine pieces with addition of Collagenase type I (10 mg/10 ml) in 75 cm2 tissue culture dishes. After mincing the tissue, the culture dish was placed in an incubator at 37 °C with a shaker arranged at 35 RPM (self-designed) for 1 h for removal of extra-cellular matrix from the adipose tissue. Subsequently the contents were transferred to 15 ml centrifuge tubes and centrifuged at 780 RPM for 8 min. Subsequently the supernatant and pellets were separately cultured in proliferation medium on 100 and 25 cm2 cell+ plates (Sarstedt, Newton, NC, USA), respectively, at 37 °C with 5 % CO2 for 10 days. Medium was changed every other day without doing any passaging.
On 10th day of the culture in proliferation medium, the tissue culture dishes containing cells were washed with Phosphate Buffered Saline (1 N, PBS) and MSCs were harvested by means of trypsinization (0.25 % Trypsin EDTA solution (Hi Media, India). Harvested MSCs were checked for viability using trypan blue, sterility (Bactec, Franklin Lakes, NJ, USA) and cell-counts in a modified Neubauer chamber. MSC characteristics were confirmed by flow cytometric analysis with CD 45 (PerCP) negativity and CD90 (FITC)/CD73 (PE) (Beckton Dickinson, Franklin Lakes, NJ, USA) positivity. Negative isotype controls were carried out by flow cytometric analysis to confirm the analysis. A small aliquot was also stained by hematoxylin and eosin stain for morphologic analysis.
Differentiation of h-AD-MSC into insulin secreting cells
Harvested MSCs were further subjected to differentiation to insulin-secreting cells using differentiation medium comprising of Dulbecco’s Modified Eagle’s Medium (DMEM) (4,500 mg glucose/l) (Sigma, USA), DMEM: F-12, (1:1) (Sigma, USA), growth factors and serum supplements like nicotinamide (10 mM) (Hi-media, India), activin A (2 nM) (Sigma, USA), exendin (10 nM) (Sigma, USA), pentagastrin (10 nM) (Sigma, USA), Hepatocyte Growth Factor (HGF) (100 pM) (Sigma, USA), B-27 (2 %) (Sigma, USA), N-2 (2 %) (Sigma, USA) and antibiotics. No xenogenic material was used.
The cells were kept in differentiation medium for 3 days in and then were subjected to isolation on Ficoll Hypaque (density: 1.077 ± 0.007 g/ml; Osmolality: 290 ± 15 ml Osmol) (Invitrogen, Karlsruhe, Germany) by density gradient centrifugation for possible selective isolation of IS-MSC at the 1,000 RPM for 8 min. After centrifugation prepared cell pellet was diluted with Phosphate Buffered Saline (PBS) (1 N, Hi-media).
Cells were tested for sterility, viability and cell counts and subsequently subjected to immunofluorescence (IF) study for paired box genes-6 (Pax-6): key regulator for normal islet cell development, insulin promoter factor-1 (Ipf-1) (in humans) or also known as pancreatic and duodenal homobox 1(Pdx-1): regulator of β-cell specific gene expression, function and for self-renewal of β progenitor cells (in animal), islet 1 transcriptional factor (Isl-1): the gene up-regulating expression of insulin.
C-peptide and insulin secretion from cells were measured by a chemiluminescence assay (Lumax, Lake Forest, CA, USA). To check glucose responsiveness of the cells, they were first incubated in absence of glucose and then after addition of glucose. Insulin and C-peptide levels secreted by the cells were measured on both occasions by chemiluminescence assay.
IF study for Pax-6, Isl-1 and Pdx-1
IF study for Pax-6, Ipf-1 (Pdx-1) and Isl-1 (genes responsible for insulin secretion) was carried out after cells were isolated. The isolated IS-MSC were transferred on poly-l-lysine coated slides and incubated overnight in DMEM/F-12 (1:1) (Sigma) with 20 % Human Albumin (Reliance Biopharmaceuticals, Navi Mumbai, India). All cells were then fixed in 4 % paraformaldehyde (prepared in 1× PBS; Hi-media, India) for 30 min at 4 °C in dark. After several rinses in PBS the cells were permeabilized with chilled methanol for 10 min. Unspecific binding was prevented by incubation with 10 % heat inactivated human serum prepared in PBS (1×, Hi-media, India) for 45 min. Fixed cells were then incubated with primary anti-sera overnight at 4 °C in dark, rinsed off with PBS and incubated with secondary anti-sera for 1 h in dark. After several rinses with PBS the cells were cover-slipped with non-fluorescing mounting medium (DAPI) for 10 min at 37 °C and were examined under fluorescence microscope (Nikon Eclipse 90i, Tokyo, Japan). Primary antibodies used were Isl-1 (10 μg/ml) (Sigma, USA), Pdx-1/Ipf-1 (10 μg/ml) (Sigma, USA) and Pax-6 (10 μg/ml) (Sigma, USA). Secondary antibodies used were goat anti-mouse IgG (Sigma, USA) coupled with Alexa flour 488 (Sigma, USA), diluted in PBS (1:1,000) (Hi-media, India).
Chemiluminescence study
C-peptide and insulin concentration were determined from cultured cells using a commercially available chemiluminescence system and kit (LUMAX-Semi Automated Chemiluminescence System, ACCULITE & INLITE Vast Enabled Reagents System; CLIA, USA). The procedure was carried out as per the kit insert. At first 25 μl of samples were taken in test-well and 50 μl tracer reagent was added into it. They were mixed well and incubated at 25 °C for 120 and 90 min for insulin and C-peptide, respectively. The cells were washed and 50 μl signal reagent was added. The cells were again incubated for 5 min at 25 °C and values were measured as relative light units (RLU) emitted.
Controls
For controls, a small aliquot of MSCs from each cell line was isolated from proliferation media on the 10th day of culture, followed by tests for sterility, quantification, morphological and phenotypical characterization by hematoxylin and eosin stain and flowcytometry, respectively. These MSCs were subjected to IF study for transcriptional factors Pax 6, Pdx-1/Ipf-1 and Isl-1 and subsequently cultured further in the same proliferation medium for the next 3 days without addition of specific differentiation medium. Again at the end of the culture, cells were isolated and subjected to IF for analysis of transcriptional factors. Flowcytometric isotype controls were also carried out for CD45−/90+/73+.
Statistical analysis
Statistical analysis was performed using SPSS version 12. Data are expressed as mean ± SD (min–max) for continuous variables. Continuous variables were compared using Wilcoxon signed rank test. p < 0.05 was considered to be statistically significant.
Results
Totally 56 cell lines of IS-MSC were generated from h-AD-MSC from 56 volunteers. h-AD-MSC when stained with hematoxylin and eosin stain, showed large basophilic nucleus with distinct margin surrounded by eosinophilic cytoplasm (Fig. 1). The mean IS-MSC quantum was 3.17 ± 0.5 ml (range 2–4 ml), their mean cell count was, 5.48 ± 2.34 × 103/μl (1.5–9 × 103/μl).
Fig. 1.
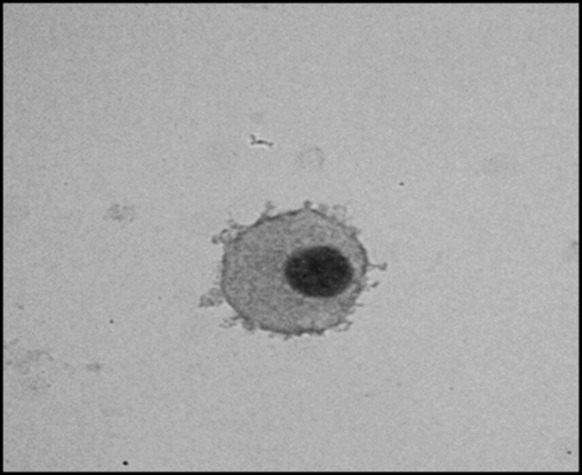
MSC showing large basophilic nucleus with distinct margin surrounded by eosinophilic cytoplasm, hematoxylin and eosin stain, ×200
Flow-cytometric analysis
Proliferating MSC and differentiated IS-MSC both were negative for CD45, a tyrosine phosphatase A (a well known haematopoietic stem cell marker of human myeloid progenitor cells) according to their non-haematopoietic origin and showed a strong expression of CD90 and CD73 (markers which are typically expressed in MSC).
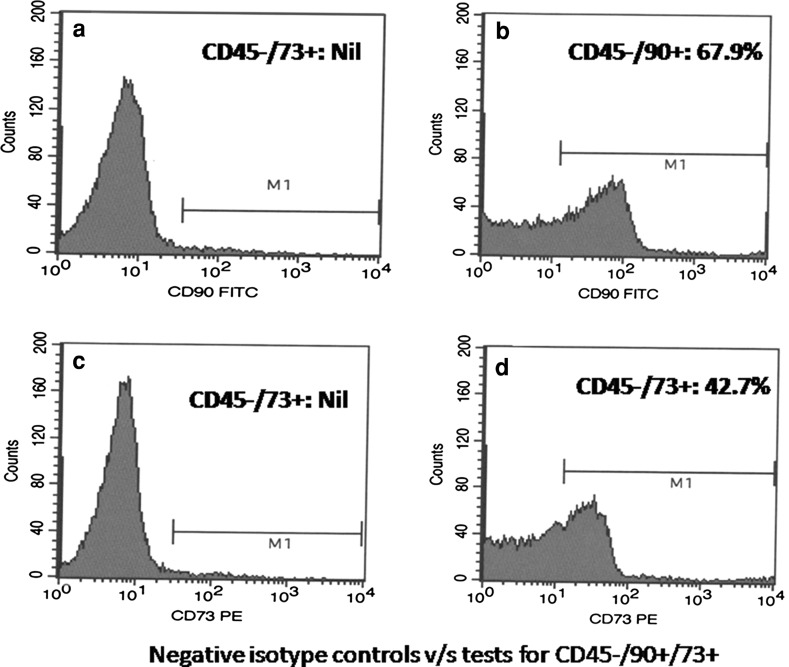
Flow-cytometric analysis revealed negligible amount of CD45−/90+/73+ cells at very first day of culture from h-AD. Increase in the CD45−/90+/73+ population was found after culture of the MSC. After differentiation of MSC into IS-MSC, they still continued to express CD45−/90+/73+. All flowcytometer analysis of CD45−/90+/73+ for IS-MSC were confirmed with negative isotype control, where CD45−/90+/73+ were found present in high amount in IS-MSC when compared to isotype control showing no expression of CD45−/90+/73+ (Fig. 2).
Fig. 2.
Flow-cytometric analysis for CD45−/90+/73+ for: a isotype control for CD45−/90+ showing no expression, b IS-MSC showing increased percentage of CD45−/90+ cells, c isotype control for CD45−/73+ showing no expression, d IS-MSC showing increased percentage of CD45−/73+ cells
CD45−/90+ expression from h-AD on day 1 of culture was 3.25 ± 1.25 % (range 1.24–4.29 %) and mean CD45-/73+ was 1.5 ± 0.75 % (range 0.59–2.1 %). After 10 days of culture from harvested MSC, mean CD45−/90+ cells were 43.51 ± 15.32 % (range 12.6–81.0 %) and mean CD45−/73+ cells were 24.6 ± 12.88 % (range 2.68–65.7 %). Mean CD45−/90+ cells from isolated IS-MSC cells after further 3 more days culture were 46.78 ± 30.69 % (range 26.28–51.8 %) and mean CD45−/73+ cells were 21.6 ± 17.6 % (range 11.8–25.4 %).
Chemiluminescence assay
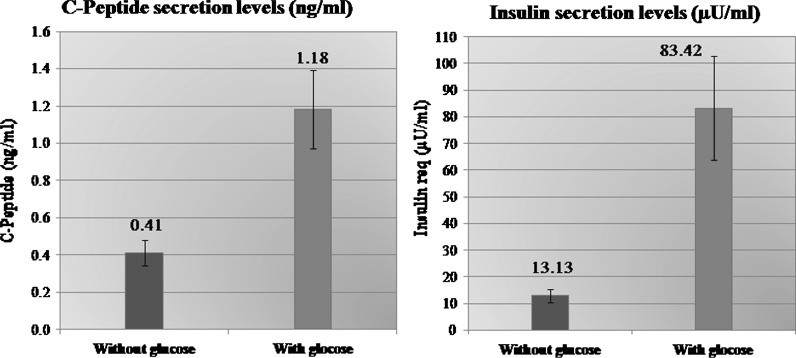
Mean insulin secretion by the isolated cells IS-MSC was 13.13 ± 18.52 μU/ml (range 0.5–95.5 μU/ml) in absence of glucose which rose to mean 83.42 ± 146.18 μU/ml (range 15–732 μU/ml) 2 h after addition of glucose (at 37 °C). Mean C-peptide level secreted by the cells IS-MSC was 0.41 ± 0.5 ng/ml (range 0–2.26 ng/ml) in absence of glucose, and 1.18 ± 1.58 ng/ml (0.1–9.35 ng/ml) 2 h after glucose addition. There was significant rise in insulin (p = 0.007) and C-peptide levels (p = 0.002) 2 h after addition of glucose to IS-MSC (Fig. 4).
Fig. 4.
Graphical presentation (bar chart) showing insulin secretion and C-peptide secretion by insulin-secreting mesenchymal stem cells before and after glucose addition of glucose (with standard deviation)
IF study for Pax-6, Isl-1 and Pdx-1
IF study revealed green fluorescing cells expressing presence of all three transcriptional factors Pax-6, Isl-1 and Pdx-1 (Ipf 1) in IS-MSC (Fig. 3).
Fig. 3.
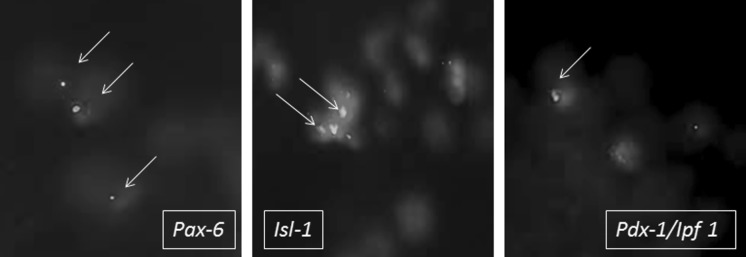
Immunofluorescence study revealing green fluorescing cells expressing transcriptional factors Pax-6, Isl-1 and Ipf-1 in IS-MSC (from left to right), ×100. (Color figure online)
Controls
All cells from the control group; undifferentiated cells and cells further proliferated in absence of differentiation medium failed to express the three transcriptional factors: Isl-1, Pax-6 and Ipf-1. They also failed to show secretion of both insulin and C-peptide in absence of glucose as well as after addition of glucose.
Discussion
Islet cell replacement is considered as the optimal treatment for type I diabetes. However, the availability of human pancreatic islets for transplantation is limited. The differentiation of murine and human embryonic stem cells (ESCs) into pancreatic cell types has been shown by several methods including spontaneous differentiation, formation of multi-lineage progenitors and lineage selection or transgene expression. However, these strategies lead to a mixture of cells of all three primary germ layers and only a low percentage of definitive endoderm cells giving rise to pancreas. To reproducibly generate functional insulin-producing cells, ES cells have to be differentiated via definitive endoderm and pancreatic endocrine progenitors recapitulating the in vivo development. A number of experiments have been reported that certain adult stem cell types can differentiate into cell types seen in organs or tissues other than those expected from the predicted cell lineage.
Few ESC differentiation protocols have reported the generation of insulin secreting cells by mimicking the in vivo developmental stages of pancreatic organogenesis, until mature beta-cells are obtained. These studies provide proof of concept that recapitulating pancreatic development in vitro offers a useful strategy for generating beta-cells. Our current differentiation protocols employ a bewildering variety of growth factors, hormones and nutrient agents i.e. we used serum free medium supplemented with factors known for their beneficial effects on differentiation of precursor cells into insulin producing cells (i.e., high glucose, exendin-4, pentagastrin, activin-A, nicotinamide and hepatocyte growth factor). Thus we induced an activation of pancreatic transcription factors Pax 6, Ipf 1 and Isl 1, as well as the islet protein insulin. Signals in the differentiation cocktail that triggered expression of these transcriptional factors in a population of AD-MSC could be the high glucose concentration along with all factors together. The exact mechanisms however, remain to be elucidated.
Multipotent stem cells have been described within pancreatic islets and in non-endocrine compartments of the pancreas and these cells have the capacity of differentiating into pancreatic islet-like structures (Hardikar et al. 2003; Gao et al. 2007).
Furthermore, cells that do not reside within the pancreas, such as ESC, hepatic oval cells, cells within spleen, have been differentiated into pancreatic endocrine hormone-producing cells in vitro and in vivo (Hori et al. 2002; Kim et al. 2003).
Different types of stem cells require different culture and induction media for differentiation of insulin-secreting cells to take place. However, some common themes seem to appear in various induction methods. Firstly, induction of stem or progenitor cells into insulin-secreting cells usually requires a multistage protocol. Most IS-MSC generation protocol requires two to six stage protocols (Chen et al. 2004). It is observed that induction of ESC generally requires more stages compared to stem cells of other origins (Soria et al. 2000). Secondly, the induction period varies greatly with the type of cells used. It may last from several days to several months. For example, in a study carried out by Chen et al., the protocol involved a two-stage protocol during which rat bone marrow mesenchymal stem cells were preinduced for 24 h and reinduced for an additional 10 h. The entire induction process took less than 48 h (Chen et al. 2004). On the other hand, in another study done by Tang et al. (2004) using mouse bone marrow-derived stem cells, clusters of insulin-secreting cells started to appear in 2 months with better-defined clusters appearing in 4 months. Such wide variations in the induction period are not uncommon in published literature.
There is an increasing body of evidence suggesting that MSC are able to differentiate into insulin producing cells and to correct hyperglycaemia at least in rodents (Timper et al. 2006; Kodama et al. 2003; Tang et al. 2004). Transdifferentiation is a reported phenomenon whereby one cell type committed to and progressing along a specific developmental lineage switches into another cell type of a different lineage (Song and Tuan 2004).
In our study the transdifferentiation of h-AD-MSCs into IS-MSC with the ability to secrete insulin and C-peptide in response to glucose, were confirmed by examining the role of transcription factor expression, particularly Pax-6, Isl-1 and Pdx-1/Ipf-1; which were shown to be essential in pancreatic development, growth and activation of the insulin gene, leading to insulin expression (Timper et al. 2006).
Pax 6 is expressed during the early stages of pancreatic development and in mature endocrine cells (St-Onge et al. 1997). Genetic and biochemical evidence has been well established stating that PAX 6 is the key regulator of pancreatic islet hormone gene transcription and is required for normal islet development. In embryos homozygous for a mutant allele of the Pax 6 gene, the numbers of all four types of endocrine cells in the pancreas decreases significantly, and islet morphology becomes abnormal. Biochemical studies also identifies wild-type PAX 6 protein as the transcription factor that binds to a common element in glucagon, insulin, and somatostatin promoters, and transactivates glucagon and insulin promoters (Sander et al. 1997).
Pancreatic and duodenal homeobox (Pdx1) also known as insulin promoter factor 1 (Ipf 1) in humans, is a transcription factor necessary for pancreatic development and β-cell maturation (Stoffel et al. 1995). Pdx-1 gene is expressed in duodenum and pancreas bud and in their prospective endodermal regions and Pdx-1 plays an important role in pancreatic bud formation by allowing cells to invade the mesenchyme (Jonsson et al. 1994; Offield et al. 1996). Pdx-1 is restricted to β cells that produce insulin. When Pdx-1 is inactivated, the pancreas bud is initiated and a few glucagon cells differentiate, but the expansion of the bud is limited and it does not branch properly. In addition, the ability of Pdx-1 to bind the insulin and Glut2 promoters and transactivate these genes suggests that it is required for the function of β cells. A conditional knockout that allows pancreas formation, but affects late Pdx-1 expression in β cells results in defective insulin secretion and a diabetic condition. Altogether, Pdx-1 is known as a selector gene for generation and renewal of β cells of the pancreatic domain (Ahlgren et al. 1997; Kushner et al. 2002).
Insulin gene enhancer Islet-1 (ISL-1) is a protein, in humans it is encoded by the ISL 1 gene (Tanizawa et al. 1994). The encoded protein binds to the enhancer region of the insulin gene, and plays an important role in regulating insulin gene expression. The encoded protein is central to the development of embryogenesis of pancreatic islets of Langerhans and pancreatic cell lineages. Mutations in this gene have been associated with maturity-onset diabetes of the young. Isl-1-deficient endocrine precursors failed to mature into functional islet cells. Impairment occurs in postnatal expansion of endocrine cell mass and consequently Isl-1 deficient mice become diabetic. In addition, MafA (musculoaponeurotic fibrosarcoma oncogene homolog A), a potent regulator of the Insulin gene and beta-cell function, has been identified as the direct transcriptional target of Isl-1. The requirement for Isl-1 by virtue of its requirement for the formation of dorsal mesenchyme and also its function in pancreatic endodermal cells is required for maturation, proliferation and survival of the hormone-producing islet cells (Ahlgren et al. 1997; Du et al. 2009).
In our study we used serum free medium supplemented with factors known for their beneficial effects on differentiation of precursor cells into insulin producing cells (i.e. high glucose, exendin-4, pentagastrin, activin-A, nicotinamide and hepatocyte growth factor). We have induced activation of pancreatic transcription factors including Pax 6, Ipf 1 and Isl 1, as well as the islet protein insulin. Signals in the differentiation cocktail that triggered expression of these transcriptional factors in a subpopulation of MSC could be high glucose concentration or all factors together. The exact mechanisms however, remain to be elucidated.
Developmental biologist and biochemist (Tosh and Slack 2002) has restricted the definition of transdifferentiation to irreversible switches of one differentiated cell type to another. The cells did switch on some of the genes that would be used in their ‘new’ type but not in their ‘old’, but they did not switch off all of their old genes. It is still an open question whether transdifferentiation could cause a complete change of cell type, and whether such a change would remain active after the cell has been re-implanted in the body (DiBerardino et al. 1984).
Very interestingly, with our experience of IS-MSC, we observed very good expression of CD45−/90+/73+ from h-AD derived MSC which remained persistent (positive) even after their further transdifferentiation into IS-MSC; giving us an idea that still IS-MSC possesses characteristics of MSC even after transdifferentiation.
In addition, these insulin producing cells possessed glucose sensitivity, which means that in response to addition of glucose, secretion of insulin was up-regulated and in absence of glucose insulin production was switched off. A cohort of diabetic patients in whom insulin secreting MSC were transplanted had no side/adverse effects or malignancy noted so far over a follow-up of 2.5 years (Trivedi et al. 2008).
There are very sporadic reports of successful use of adipose tissue for IS-MSC generation and their clinical application to treat type 1 diabetes. From our experience we conclude that (1) the present study is only a first step towards the use of ADSC as a cell-based treatment of type 1 DM, and (2) h-ADSC is an ideal population for cell replacement therapy and also h-ADSCs could be induced to differentiate into physiologically competent functional insulin secreting cells, which may provide as a source of alternative islets for cell replacement therapy in type 1 diabetes. Thus the transplantation of insulin-producing cells is a promising approach for the treatment of type 1 diabetes.
Finally we conclude that insulin-secreting h-AD-MSC can be generated safely and effectively with application of specific differentiation media without use of any xenogeneic material and without any genetic modification, showing expression of transcriptional factors Pax-6, Ipf-1 and Isl-1.
Acknowledgments
The authors are thankful to Ms. Sengunthar Shobhanarani for statistical analysis, C. N. Patel, J. V. Patel, A. G. Bhargava, and P. N. Bhavsar for carrying out all the laboratory tests including flow-cytometry analysis and chemiluminescence assays and librarian Ms. Jyotsna Suthar for literature survey.
Abbreviations
- α-MEM
Alpha modified Minimum Essential Media
- B-hFGF
Basic-Human Fibroblast Growth Factor
- DMEM
Dulbecco’s Modified Eagle’s Medium
- ESC
Embryonic stem cells
- h-AD
Human adipose tissue
- h-AD-MSC
Human adipose tissue-derived mesenchymal stem cells
- IF
Immunofluorescence
- Ipf1
Insulin promoter factor 1
- ISL-1
Islet-1
- IS-MSC
Insulin secreting mesenchymal stem cells
- MafA
Musculoaponeurotic fibrosarcoma oncogene homolog A
- Pax 6
Paired box gene 6
- PBS
Phosphate buffer saline
- Pdx1
Pancreatic and duodenal homeobox 1
- RPM
Revolution per minute
- TFs
Transcription factors
References
- Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- Chen LB, Jiang XB, Yang L. Diderentiation of rat marrow mesenchymal stem cells into pancreatic islet betacells. World J Gastroenterol. 2004;10:3016–3020. doi: 10.3748/wjg.v10.i20.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBerardino MA, Hoffner JN, Etkin LD (1984) Activation of dormant genes in specialized cells. Science 224:946–952 [DOI] [PubMed]
- Du A, Hunter CS, Murray J, Noble D, Cai CL, Evans SM, Stein R, May CL. Islet-1 is required for the maturation, proliferation, and survival of the endocrine pancreas. Diabetes. 2009;58:2059–2069. doi: 10.2337/db08-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Ustinov J, Pulkkinen MA, Lundin K, Korsgren O, Otonkoski T. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes. 2007;52:2007–2015. doi: 10.2337/diabetes.52.8.2007. [DOI] [PubMed] [Google Scholar]
- Habener JF, Kemp DM, Thomas MK. Transcriptional regulation in pancreatic development. Endocrinology. 2005;146:1025–1034. doi: 10.1210/en.2004-1576. [DOI] [PubMed] [Google Scholar]
- Hardikar AA, Marcus-Samuels B, Geras-Raaka E, Raaka BM, Gershengorn MC. Human pancreatic precursor cells secrete FGF2 to stimulate clustering into hormone-expressing islet-like cell aggregates. Proc Natl Acad Sci USA. 2003;100:7117–7122. doi: 10.1073/pnas.1232230100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori Y, Rulifson IC, Tsai BC, Heit JJ, Cahoy JD, Kim SK. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:16105–16110. doi: 10.1073/pnas.252618999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- Kim D, Gu Y, Ishii M, Fujimiya M, Qi M, Nakamura N, Yoshikawa T, Sumi S, Inoue K. In vivo functioning and transplant table mature pancreatic islet-like cell clusters differentiated from embryonic stem cell. Pancreas. 2003;27:E34–E41. doi: 10.1097/00006676-200308000-00021. [DOI] [PubMed] [Google Scholar]
- Kodama S, Kühtreiber W, Fujimura S, Dale EA, Faustman DL. Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science. 2003;302:1223–1227. doi: 10.1126/science.1088949. [DOI] [PubMed] [Google Scholar]
- Kushner JA, Ye J, Schubert M, Burks DJ, Dow MA, Flint CL, Dutta S, Wright CV, Montminy MR, White MF. Pdx1 restores β-cell function in Irs2 knockout mice. J Clin Invest. 2002;109:1193–1201. doi: 10.1172/JCI0214439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Sander M, Neubüser A, Kalamaras J, Ee HC, Martin GR, German MS. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 1997;11:1662–1673. doi: 10.1101/gad.11.13.1662. [DOI] [PubMed] [Google Scholar]
- Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18:980–982. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- Soria B, Roche E, Berná G, León-Quinto T, Reig JA, Martín F (2000) Insulin-secreting cells derived from embryonic stem cells normalise glycaemia in streptozotocin-induced diabetic mice. Diabetes 49:1–6 [DOI] [PubMed]
- Stoffel M, Stein R, Wright CV, Espinosa R, 3rd, Le Beau MM, Bell GI. Localization of human homeodomain transcription factor insulin promoter factor 1 (IPF1) to chromosome band 13q12.1. Genomics. 1995;28:125–156. doi: 10.1006/geno.1995.1120. [DOI] [PubMed] [Google Scholar]
- St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature. 1997;387:406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- Tang DQ, Cao LZ, Burkhardt BR, Xia CQ, Litherland SA, Atkinson MA, Yang LJ. In vivo and in vitro characterization of insulin producing cells obtained from murine bone marrow. Diabetes. 2004;53:1721–1732. doi: 10.2337/diabetes.53.7.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanizawa Y, Riggs AC, Dagogo-Jack S, Vaxillaire M, Froguel P, Liu L, Donis-Keller H, Permutt MA. Isolation of the human LIM/homeodomain gene islet-1 and identification of a simple sequence repeat polymorphism. Diabetes. 1994;43:935–941. doi: 10.2337/diab.43.7.935. [DOI] [PubMed] [Google Scholar]
- Timper K, Seboek D, Eberhardt M, Linscheid P, Christ-Crain M, Keller U, Müller B, Zulewski H. Human adipose-tissue derived mesenchymal stem cells differentiate into insulin, somatostatin and glucagon expressing cells. Biochem Biophys Res Commun. 2006;341:1135–1140. doi: 10.1016/j.bbrc.2006.01.072. [DOI] [PubMed] [Google Scholar]
- Tosh D, Slack JM (2002) How cells change their phenotype. Nat Rev Mol Cell Biol 3:187–194 [DOI] [PubMed]
- Trivedi HL, Vanikar AV, Thakker U, Firoze A, Dave SD, Patel CN, Patel JV, Bhargava AB, Shankar V (2008) Human adipose tissue-derived mesenchymal stem cells combined with hematopoietic stem cell transplantation synthesize insulin. Transplant Proc 40:1135–1139 [DOI] [PubMed]