Abstract
Bee pollen and propolis are popular, traditional health foods. The objective of the current study was to investigate the anti-mutagenic, anti-histopathologic and antioxidant effects among water extracts of Egyptian bee pollen (WEBP) and brown powder of water-soluble derivative propolis (WSDP) on cisplatin (CDDP) induced hepatic, renal, testicular and genotoxicity in male albino mice (Musmuscullus), in addition to their effects on the oxidant/antioxidant status in the tested organs. Hepatic, renal and testicular dysfunctions were evaluated histologically; while genotoxicity and cytotoxicity were evaluated by the bone marrow chromosomal aberration assay and mitotic index, respectively. Moreover, oxidative stress was explored via determination of lipid peroxidation, catalase activity and the concentration of the reduced form of glutathione. The treatment of mice with WEBP and WSDP at doses 140 and 8.4 mg/kg b. wt./day, respectively for 14 days simultaneously with CDDP (2.8 mg/kg b. wt.) resulted in significant protection. The positive control animals taken CDDP alone showed toxic histological and genetical manifestations (at P < 0.05) accompanied with an elevated content of peroxidized lipid and lowered catalase activity and glutathione concentration in the homogenate of liver, kidney and testis tissues (at P < 0.001). These toxic side effects in all tested organs were greatly ablated with a significant reduction in lipid peroxidation level and elevation in catalase activity and glutathione concentration (P < 0.001) when using both WEBP and WSDP. On the basis of the present assays, Bee pollen appears more potent in exerting an ameliorative effect and this effect was more pronounced in testis.
Keywords: Bee pollen, Propolis, Anti-mutagenic, Anti-histopathologic, Antioxidant
Introduction
The honeybee (Apis mellifera) makes various bee products from plants, flower nectar, and flower pollen, and humans make use of these products. Bee products are well known in the traditional medicine, and their use has a very long history. Todays, their uses have expanded from the health food arena into the medical one. Bee pollen is collected by honeybees as a nutrient harvest for the hive. Bee pollen is considered by many people to be a nutrient-rich perfect food, and is promoted as a commercially available food supplement. Pollen grains, which are the male reproductive cells of flowers, contain high concentrations of phytochemicals and nutrients (Markham and Campos 1996; Eraslan et al. 2009; Saric et al. 2009). Nakajima et al. (2009) reported that bee pollen is rich in carotenoids, flavonoids and phytosterols, while the exact profile of bee pollen content varies depending on the plant sources and growth conditions. The anti-oxidant activity of honeybee-collected pollen has been recognized as free radical scavenger and as lipid peroxidation inhibitor. This activity has been associated with the phenolic pollen content (Campos et al. 2003). Furthermore, Turkey bee pollen has inhibitory effects against mycelia growth and several pharmacological activities (Ozen et al. 2004).
Propolis a sticky substance that honeybees manufacture by mixing their own waxes with resinous sap obtained from the bark and leaf-buds of certain trees and flowering plants. It is used as a sealant and sterilant in honeybee nests. The composition of propolis varies from hive to hive, from district to district, and from season to season. Normally it is dark brown in color, but it can be found in green, red, black and white hues, depending on the sources of resin found in the particular hive area. Honey bees are opportunists, gathering what they need from available sources, and detailed analyses show that the chemical composition of propolis varies considerably from region to region, along with the vegetation. In northern temperate climates, for example, bees collect resins from trees, such as poplars and conifers (the biological role of resin in trees is to seal wounds and defend against bacteria, fungi and insects). “Typical” northern temperate propolis has approximately 50 constituents, primarily resins and vegetable balsams (50 %), waxes (30 %), essential oils (10 %), and pollen (5 %). In neotropical regions, in addition to a large variety of trees, bees may also gather resin from flowers of the genera Clusia and Dalechampia, which are the only known plant genera that produce floral resins to attract pollinators (Bankova 2005). Now, it is recognized that propolis has a wide range of biological activities, such as antibacterial (Souza et al. 2007), anti-inflammatory (Barros et al. 2008; Nakajima et al. 2009), antioxidative (Teixeira et al. 2008), hepatoprotective (Basnet et al. 1997) and tumoricidal (Mitamura et al. 1996) activities. Such effects have been associated with the presence of β-amylase and many phenolic compounds such as flavones, flavonones, galangin, cinnamic acid, caffeic acid and esters (Volpi 2004). Concerning Egyptian propolis, Lotfy (2006) reported thirty-nine constituents eight of them being newly described for propolis including phenolic esters (72.7 %), phenolic acids (1.1 %), aliphatic acids (2.4 %), dihydrochalcones (6.5 %), chalcones (1.7 %), flavanones (1.9 %), flavones (4.6 %), and tetrahydrofuran derivatives (0.7 %).
Cisplatin (cis-diamminedichloro platinum) is one of the most important anti-neoplastic agents used in the treatment of several types of solid tumorus (Pabla et al. 2008). Along with its high therapeutic activity, cisplatin is a potential human carcinogen with increased carcinogenic risk towards the development of secondary malignancy in patients (Greene 1992). In addition, this drug has a lot of side effects including high nephrotoxicity, reproductive toxicity, hepatotoxicity and genotoxicity which are examples of the most severe and dose limiting side effects (Nersesyan and Muradyan 2004). The cytotoxic activity of cisplatin results from its induced oxidative stress, resulting in loss of mitochondrial protein-SH, inhibition of calcium uptake and reduction in the mitochondrial membrane potential (Husain and Naseem 2008). Recently, evidences have been accumulated that lipid peroxidation and free radical formation which cause an imbalance between the generation of oxygen derived radicals and the animal antioxidant potential play a great role in this toxicity promoting cellular damage (Ozen et al. 2004; Naziroglu et al. 2004; Iraz et al. 2006). While the genotoxic effect of cisplatin was proposed to be derived from its interaction with DNA and production of DNA-platinum covalent adducts that inhibiting the fundamental cellular processes including replication, transcription, translation and DNA repair (Lee and Schmitt 2003).
The purpose of the present study was to evaluate and compare the effectiveness of total water extracts of honey bee collected pollen (WEBP) and propolis (WSDP) from Beni-Suef, Egypt, as in vivo anti-mutagenic, anti-histopathologic and antioxidant agent against cisplatin (CDDP)–induced chromosomal abnormalities in bone marrow cells, and histological alterations and oxidative stress in liver, kidney and testis tissues of mice (Mus musculus).
Materials and methods
Chemicals
Cis-platin (CDDP) (cis-diamminedicholorplatinum) was purchased from MERCK Company (Darmstadt, Germany) in a form of ampoules, each containing 25 mg of CDDP in 25 ml sterile saline solution containing hydrochloric acid and sodium hydroxide to adjust pH between 3.8 and 5.9. The solution in these ampoules appeared clear, free from turbidity or matter which deposits on standing. Other chemicals were obtained from Sigma (St. Louis, MO, USA).
Experimental animals
The experimental animals used in this work were random bred adult males of laboratory mice Musmusculus (20–30 g in weight). Animals were obtained from Ophthalmology research institute, Giza, Egypt. Experiments were performed as per internationally followed ethical standards and according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (Institute of laboratory animal resources 1996). All animals were housed in plastic cages with wired covers and kept under normal laboratory conditions for the different periods of time used. The animals were not treated with antibiotics, vitamins or insecticides and fed a standard commercials diet (ATMID Company, Giza, Egypt) and tap water ad. libitum.
Bee products
The propolis used in the present study was obtained as a brown powder of water-soluble derivative propolis (WSDP) from beekeepers in Beni-Suef, Upper Egypt. The mixture of honey bee–collected pollen was provided by local beekeepers in September 2006. The pollen source in a given area depends on the type of vegetation present and the length of their bloom period. What type of vegetation will grow in an area depends on soil texture, soil pH, soil drainage, daily maximum and minimum temperatures, precipitation, extreme minimum winter temperature, and growing degrees. These pollens collected by the bees from anthers of the flowers of the plants growing in the surrounding of the bee hives in Beni-Suef, Upper Egypt.
Extracts preparation
Propolis extract was prepared by the method of (Oršolić and Bašić 2005). Under sterile conditions 16.8 mg of the brown powder of propolis (WSDP) was dissolved in 10 ml distilled water and mixed vigorously for 10 min. Finally, this suspension was centrifuged at 1,000 rpm for 10 min in room temperature. The supernatant was collected and stored under freezing condition at −20 °C until use.
Bee pollen extract was obtained according to the method of Yamaguchi et al. (2006). Briefly, the powder of bee pollen (280 mg) was suspended in distilled water (10 ml) and mixed vigorously. This suspension was stored stand overnight in dark and centrifuged at 10,000 rpm in a cooling centrifuge at 10 °C for 45 min. The supernatant fraction was carefully collected and filtrated. The filtrate was stored under freezing condition at −20 °C until used.
Experimental design
A single dose (2.8 mg/kg b. wt.) of CDDP used in present study was selected with reference to the dose range of the cytotoxicity and genotoxicity of CDDP (Pisano et al. 2003; Nersesyan and Muradyan 2004). However, propolis (WSDP) and bee pollen (WEBP) water extract concentrations used in the present study were 8.40 and 140 mg/kg b. wt. respectively. These concentrations were selected according to Mani et al. (2006) and Yamaguchi et al. (2006) respectively.
Experimental groups were organized as six groups including 11 animals each. In each group five animals were used for cytogenetic analysis while the rest of animals (six mice) were used for biochemical and histopathological analysis. The animals of the group one (G1) served as a negative control receiving 0.9 % NaCl solution by intraperitoneal injection (i.p.) twice/week for 3 weeks. The animals of the group two (G2) received i.p. injection of CDDP (2.8 mg/kg b. wt.) twice/week for 3 weeks. In group three (G3) 8.4 mg/kg b. wt. of WSDP extract was given to the animals through oral intubation once/day for 14 days consecutively. The animals of the group four (G4) received oral administration of WEBP (140 mg/kg b. wt.) once/day for 14 days. The animals of the groups five and six (G5 and G6) were injected i.p. with CDDP (2.8 mg/kg b. wt. twice/week) alone for 1 week but, for next 2 weeks these animals were given WSDP and WEBP respectively through oral intubation in parallel to i.p. injection of CDDP.
Tissues sampling
At the end of the experiment, mice in each group were sacrificed under mild anesthesia. Liver, kidneys and testes tissues were removed quickly. 0.5 g of each of them was homogenized in 5 ml of 0.9 % NaCl. The obtained homogenate was kept in deep freezer at −20 °C for measurement of oxidative stress markers including lipid peroxidation and glutathione content, and catalase activity. Moreover, pieces of liver and kidney tissues were fixed in neutral buffered formalin for histopathological studies.
Antioxidant-capacity assay
Parts of liver, kidney and testis (0.5 g) were ice-cooled and homogenized in 5 ml 0.9 % NaCl (1 % w/v) using Teflon homogenizer (Glas-Col, Terr Haute, IN, USA). The homogenate was centrifuged at 3,000 g for 15 min at 4 °C. The supernatant was collected and preserved at −20 °C till used for oxidative stress and antioxidant defense system measurement. In tested organs homogenates, lipid peroxidation (LPX) was determined by measuring the thiobarbituric acid reactive substances (TBARS) according to the method of Pressus et al. (1998). Glutathione reduced form (GSH) level was measured colorimetrically as protein-free sulfhydryl content using Ellman reagent (Beutler et al. 1963). Catalse (CAT) activity was analyzed according to the method of Cohen et al. (1970) by monitoring the enzyme-catalyzed decomposition of hydrogen peroxide using potassium permanganate.
Histopathological assay
Tissue samples of liver, kidney and testis were fixed in 10 % neutral buffered formalin (pH 6.8) for 24 h. After dehydration, tissue samples were embedded in paraffin wax, sectioned at 5 μm and stained with haematoxylin and eosin (Bancroft and Gamble 2002) for histopathological examination.
Cytogenetic assay
Bone marrow cell preparations for the analysis of chromosomal aberrations and mitotic indices were conducted by the colchicines-hypotonic technique. After completion of the treatment period, five animals from each group were sacrificed 24 h post-injection with saline, cisplatin, bee pollen or propolis, by cervical dislocation. Colchicine was given at the dose of 4 mg/Kg b. wt. intraperitoneally (i.p.) at 22 h prior to sacrificing the animals. The bone marrow smears of animals in each group were prepared according to Preston et al. (1987). For each group, slides were stained with Giemsa staining method and 50 well spread metaphase plates/animal were analyzed for chromosomal aberrations and the number of mitotic cells in 1000 cells/animal. The percentage of suppressed aberrant cells was calculated according to Shukla and Taneja (2002) as follows:
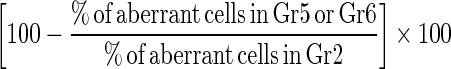 |
Statistical analysis
One way analysis of variance ANOVA (Rao et al. 1985) was used to statistically analyze the biochemical variables. (P < 0.05 was considered significant).
While statistical analysis for the difference in the mean number of chromosomal aberrations and mitotic index between groups was carried out using the student t test (P < 0.05 was considered significant).
Results
Antioxidant-capacity assay
Administration of cisplatin for 21 days caused significant increase at P < 0.001 in the lipid peroxidation level associated with a significant decrease (P < 0.001) in both glutathione content and catalase activity in all organs (liver, kidney and testis) compared to the control group. However, administration of propolis and bee pollen aqueous extracts after 1 week of cisplatin administration and in parallel with it for another 14 days significantly attenuated the changes in these parameters (P < 0.001). The lipid peroxidation was significantly reduced (P < 0.001) in groups five and six (G5 and G6) compared to the cisplatin group (G2) and this decrease was even more pronounced in G6 (CDDP + WEBP), where all values return to the normal level but still exceeding the negative control group (G1). The amelioration was more pronounced in testis (Table 1).
Table 1.
Showing the changes in lipid peroxidation content (Malondialdehyde), catalase activity and glutathione concentration in different organs of all tested experimental groups
| Groups | Lipid peroxidation (LPX) nmol/g tissue × 10 |
Catalase (CAT) k/g tissue |
Reduced glutathione (GSH) μmol/μg tissue × 103 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Kidney | Liver | Testis | Kidney | Liver | Testis | Kidney | Liver | Testis | |
| G1 (saline) | 4.466 ± 0.09d | 3.2 ± 0.10e | 3.2 ± 0.09e | 0.766 ± 0.01a | 0.81 ± 0.01b | 0.69 ± 0.01b | 3.3 ± 0.10b, c | 4.53 ± 0.09b | 3.866 ± 0.12c |
| G2 (CDDP) | 7.466 ± 0.15a | 5.1 ± 0.10a | 6.1 ± 0.11a | 0.466 ± 0.02e | 0.58 ± 0.02e | 0.44 ± 0.02e | 2.1 ± 0.13e | 2.67 ± 0.24d | 2.900 ± 0.10d |
| G3 (WSDP) | 5.466 ± 0.12c | 4.8 ± 0.04b | 4.5 ± 0.10c | 0.606 ± 0.01c | 0.73 ± 0.01c | 0.64 ± 0.01c | 3.0 ± 0.07c, d | 3.67 ± 0.21c | 3.633 ± 0.14c |
| G4 (WEBP) | 3.666 ± 0.21e | 3.1 ± 0.06e | 2.4 ± 0.07f | 0.723 ± 0.01a | 0.93 ± 0.01a | 0.80 ± 0.01a | 4.2 ± 0.08a | 5.33 ± 0.09a | 5.366 ± 0.15a |
| G5 (CDDP + WSDP) | 5.966 ± 0.15b | 4.5 ± 0.08c | 5.2 ± 0.08b | 0.550 ± 0.03d | 0.63 ± 0.01d | 0.59 ± 0.01d | 2.9 ± 0.04d | 2.97 ± 0.02d | 2.766 ± 0.09d |
| G6 (CDDP + WEBP) | 5.366 ± 0.13c | 4.1 ± 0.08d | 3.6 ± 0.15d | 0.663 ± 0.01b | 0.71 ± 0.03c | 0.63 ± 0.01c | 3.3 ± 0.18b | 3.43 ± 0.11c | 4.300 ± 0.04b |
Data are expressed as mean ± standard error (SE)
Numbers of animals tested are six per group
Values sharing the same superscript symbol are considered non-significant at P < 0.001
Contrary, the level of glutathione and activity of catalase were significantly elevated with both treatments (P < 0.001) (G5 and G6). Bee pollen extract in G6 had also a better effect than propolis extract in G5 leading to significantly elevation of most of the recorded values above the CDDP group (G2) (Table 1).
However, propolis extract administration to mice in G3 led to a significant increase in the level of lipid peroxidation significantly compared to the control (G1) and reduced catalase activity, while the glutathione level was nearly unchanged in all organs. In addition significantly lowered values of glutathione content in G3 were recorded in comparison to the control (G1) and bee pollen (G4) groups.
Histopathological assay
Liver
The histological examination of the liver sections of control animals showed normal liver architecture. The central area is formed of a central vein form which hepatic cords arise. These cords are separated by a number of sinusoids lined with Kupffer cells (Fig. 1a).
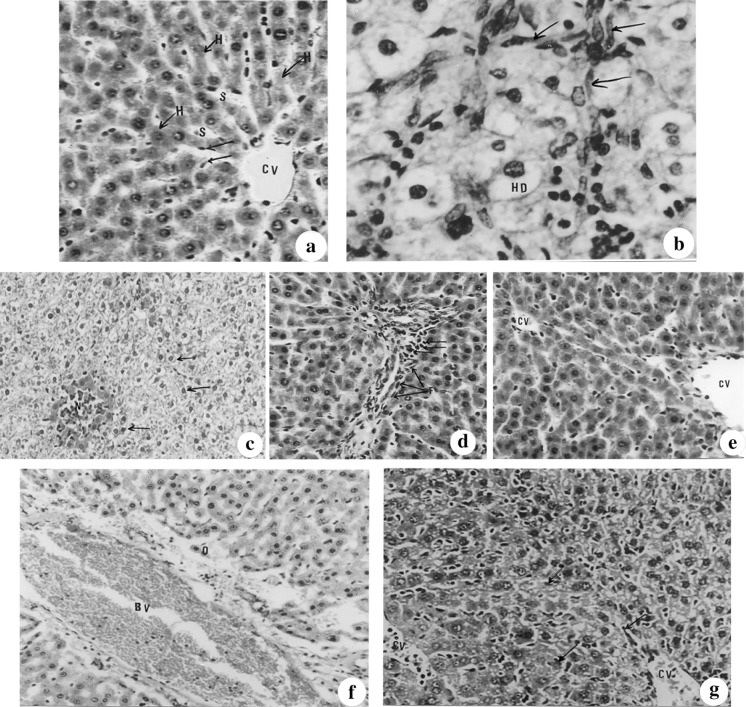
Fig. 1.
(a–g): Photomicrographs of liver sections of mice stained with haematoxylin and eosin showing a control liver section showing the central vein (CV), the hepatic sinusoids (S), the hepatic strands (H) and the Kupffer cells (X 128), b hydrobic degenerative changes (HD), fibroblastic proliferation (downward arrow) seen in the hepatic parenchyma of cisplatin injected mice for twenty-one days (×512), c necrotic foci (N) seen in the parenchyma with hydrobic degenerative changes (downward arrow) of liver section of cisplatin-treated mice surrounded by highly eosinophilic hepatocytes and occupied with mononuclear leucocytes (×100), d minimal fibrosis (downward arrow) seen in the portal area of propolis-treated animals (X 128), e normal hepatic tissue organization registered in bee pollen-treated mice (×128), f periportal odema (O) and portal vein congestion with normal hepatocytes organization and appearance, recorded in animals treated with propolis after cisplatin administration (×100) and g diffuse proliferation of Kupffer cells (downward arrow) noticed in the hepatic parenchyma in the central area of animals treated with bee pollen after cisplatin injection (×128)
Administration of cisplatin for 21 days caused severe disruption to the normal structure. The hepatocytes showed several degenerative changes (Fig. 1b), the parenchyma in the central area was infiltrated with a number of fibroblasts (Fig. 1b) and revealed numerous necrotic foci (Fig. 1c), while the portal area showed odema, fibrosis and congested portal vein. However, mice given propolis or bee pollen had nearly normal liver architecture and organization (Fig. 1d, e) except for the infiltration with few inflammatory cells noticed in the portal area of the liver sections of propolis-treated animals (Fig. 1d).
Administration of propolis and bee pollen after and concomitant with cisplatin, greatly ameliorated the hepatic histopathological lesions and the hepatic parenchyma attained nearly normal structure and organization. Since few portal areas showed portal odema accompanied with portal vein congestion (Fig. 1f) in the propolis treated group while proliferation of Kupffer cell’s was recorded in bee pollen group (Fig. 1g).
Kidney
The microscopical examination of the kidney of control mice revealed the normal cortical and medullary histological structure. The cortex is formed of a number of Malpigian corpuscles with its glomeruli and urinary space, proximal tubules and distal tubules (Fig. 2a).
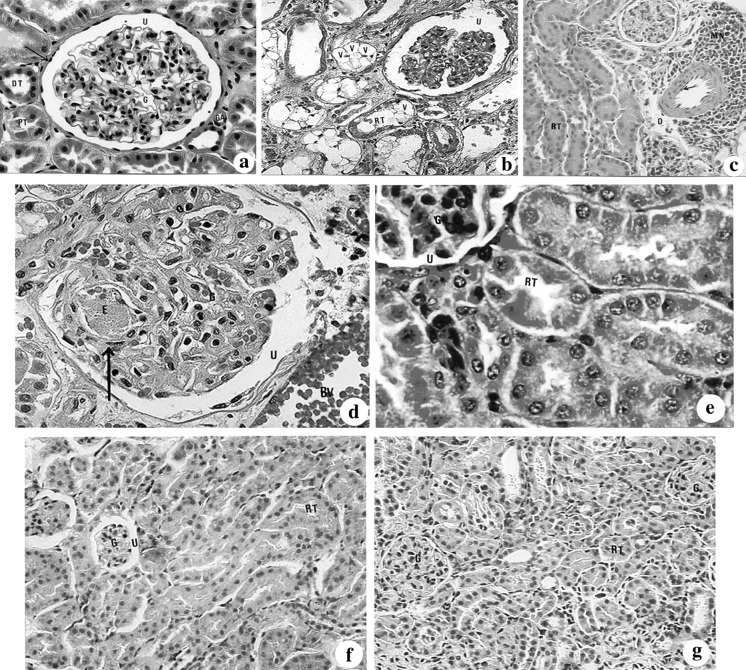
Fig. 2.
(a–g): Showed sections of kidney in different examined groups stained with haematoxylin and eosin. a A photomicrograph of the cortical region of a kidney of control mice showing the golmerulus (G) with its Bowman’s capsule (downward arrow), urinary space (U), glomerular apparatus (GA), the proximal tubule (PT) and distal tubule (DT) (×400). b A photomicrograph of the cortical region of kidney section of mice administered cisplatin illustrating widening of the urinary space (U), atrophy of the glomerulus (G), and vacuolar degenerative changes in the renal tubules (V) (×1,000). c A light micrograph showing perivascular focal mononuclear leucocytic aggregation (MN) and odema (O). Renal tubular (RT) epithelial cells showed hyperplasia with obliterated lumen (×400). d A light micrograph of the glomerulus of propolis-treated group showing eosinophilic bodies (E) (downward arrow) and congested blood vessels (BV) (×400). e A photomicrograph of the kidney section of administered bee pollen showing nearly normal cortical structure. (G: glomerulus, U: urinary space, RT: renal tubule) (×400). f Lightmicrogarph of the kidney section of mice administered cisplatin and treated with propolis having renal tubular (RT) normal structures with hyperemic glomeruli (G) (×150). g A photomicrograph of the kidney of mice given cisplatin and treated with bee pollen illustrating normal renal tubules (RT) and glomeruli (G) (×150)
In cisplatin-injected group, numerous severe histological alterations were noticed. These alterations include tubular and glomerular degenerative changes (Fig. 2b) associated with tubular and periglomerular accumulation of the albuminous material and medullary fibrosis in most animals (Fig. 2d). In few cases, the renal tubules showed hypertrophied epithelial cells with obliterated lumens (Fig. 2c) and the glomeruli showed hypercellularity, accompanied with periglomerular leucocytic aggregation (Fig. 2c). However, kidneys from animals administered with propolis and bee pollen extracts (groups G3 and G4 respectively), showed normal histological structure compared to the cisplatin (G2) and control (G1) groups (Fig. 2d, e). Though hyperemic glomeruli and congested blood vessels were rarely seen in propolis group (Fig. 2d).
Administration of propolis or bee pollen together with cisplatin minimized the degenerative changes, the accumulation of albuminous material, the medullary fibrosis, the glomerular hypercellularity and hypertrophy of epithelial cells of renal tubules (Fig. 2f, g). This amelioration effect was better in bee pollen group (G4) where only hypertrophied epithelial cells were still noticed.
Testis
Studying the testis sections of control animals showed that it is formed of a number of seminiferous tubules. Each of them was separated by a number of Leydig cells. These tubules are lined with germ cells in various stages and Sertoli cells in between. The spermatogenic lineage is represented by a number of spermatogonia, primary and secondary spermatocytes, spermatids and spermatozoa (Fig. 3a).
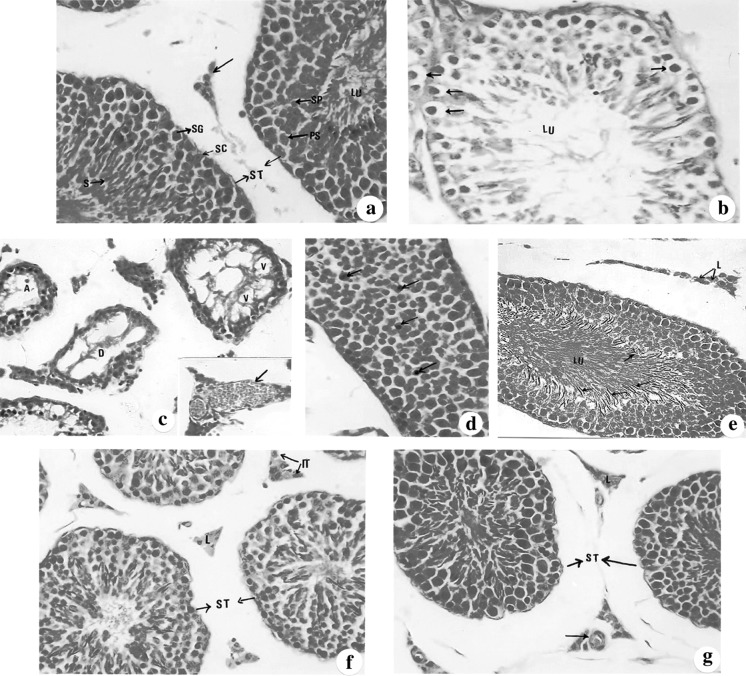
Fig. 3.
(a–g): Sections of testis in different examined groups stained with haematoxylin and eosin. a A control seminiferous tubules (ST) and interstitial cells (downward arrow) showing the normal spermatogenic lineage; spermatogonia (SG). Note the sertoli cells (SC) are seen between the spermatogonia. LU: lumen (×400). b A photomicrograph of the testis section of cisplatin injected mice showing the seminiferous tubule with few number of spermatogenic lineage and numerous vacuolated spermatogonia (downward arrow) LU: lumen (×1,000). c A lightmicrograph of the testis section of a cisplatin-injected mice showing atrophied (A) seminiferous tubules or degenerated ones (D) with severe vacuolation (V).The insertion: congested blood vessel in the interstitial tissue (×400). d A photomicrograph of the testis section of mice administered propolis showing a normal seminiferous tubule invaded with macrophages (downward arrow) (×400). e A light micrograph of a testis section of mice given bee pollen illustrating normal interstitial tissue with Leydig cells (L), seminiferous tubules with respect number of sperms (downward arrow).Lu: lumen (×400). f A light micrograph of the testis section of mice given cisplatin and treated with bee pollen illustrating control seminiferous tubules (ST) and interstitial tissues (IT) with Leydig cells (L) (×400). g A photomicrograph of the testis section of mice injected with cisplatin and treated with propolis showing normal seminiferous tubules (ST) with normal lineage and interstitial tissue with Leydig cells (L) and blood vessels (downward arrow) (×400)
Injection of cisplatin for 21 days induced numerous destructive changes in the seminiferous tubules including vacuolation of the spermatogonia, loss of spermatids and sperms (Figs. 3b, c) and disorganization of the tubular cells. Besides, there was congestion in the interstitial tissue (Fig. 3c insert) compared to the control animals. Propolis or bee pollen administration caused no histopathological alteration in the testis (Fig. 3d, e) respect to the control group, despite few macrophage were noticed in the propolis group (Fig. 3d).
On the other hand, administration of propolis and bee pollen extracts after and concomitant with cisplatin led to a decrease in the destructive histopathological alterations induced by cisplatin in testis tissues including decrease in vacuolation of the spermatogonia and increase in the organization of tubular cells (Fig. 3f, g). The bee pollen effect was most potent than propolis (Fig. 3f).
Cytogenetic assay
According to the cytogenetic results illustrated in Tables 2 and 3, seven structural and numerical chromosomal aberrations were detected in the control and the experimental groups (Fig. 4). The results obtained in the first phase of cell cycle (24 h sampling time), revealed that cisplatin (CDDP) when given at a dose of 2.8 mg/kg b.wt, twice/week for 3 weeks (G2) induced a high frequency of chromosomal aberrations in bone marrow cells of mice when compared with the control (G1) group (Tables 2, 3). The chromatid breakages were the most frequent chromosomal aberration. Other structural and numerical aberrations increased significantly at P < 0.05 over the control group (G1), while the mitotic index was significantly decreased to 37.75, compared to control value of 83.29 (P < 0.05), indicating bone marrow cytotoxicity (Table 3).
Table 2.
Protective effects of propolis and bee pollen aqueous extracts against cisplatin induced structural and numerical chromosomal aberrations in mouse bone marrow cells
| Groups | Number of structural chromosomal aberrationsa | Number of numerical chromosomala aberrations | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Chromatid breakage | Chromatid gap | Centromeric attenuation | Centric fusion | End to end association | TSA | Polyploidy | Endomitosis | TNA | |
| G1 (saline) |
18 (3.6 ± 0.60) |
– | 2 (0.40 ± 0.24) |
1 (0.20 ± 0.20) |
2 (0.40 ± 0.24) |
23 (4.60 ± 0.68) |
– | 8 (1.60 ± 0.24) |
8 (1.60 ± 0.24) |
| G2 (CDDP) |
101b
(20.20 ± 1.46) |
14b
(2.80 ± 1.16) |
– | 11b
(2.20 ± 1.07) |
19b
(3.80 ± 0.86) |
145b
(29.00 ± 3.42) |
– | 23b
(4.60 ± 0.40) |
23b
(4.60 ± 0.40) |
| G3 (WSDP) |
45b
(9.00 ± 0.45) |
– | – | 2 (0.40 ± 0.24) |
2 (0.40 ± 0.24) |
49b
(9.80 ± 0.37) |
– | – | – |
| G4 (WEBP) |
32b
(6.40 ± 0.51) |
– | – | – | 2 (0.40 ± 0.24) |
34b
(6.80 ± 0.37) |
– | – | – |
| G5 (CDDP + WSDP) |
82c
(16.40 ± 2.04) |
7c
(1.40 ± 0.40) |
1 (0.20 ± 0.20) |
3c
(0.60 ± 0.24) |
9c
(1.80 ± 0.37) |
102c
(20.40 ± 2.04) |
1 (0.20 ± 0.20) |
1c
(0.20 ± 0.20) |
2c
(0.40 ± 0.24) |
| G6 (CDDP + WEBP) |
55c
(11.00 ± 0.71) |
4c
(0.80 ± 0.20) |
3c
(0.60 ± 0.40) |
6c
(1.20 ± 0.49) |
11c
(2.20 ± 0.20) |
79c
(15.80 ± 0.49) |
3c
(0.60 ± 0.40) |
5c
(1.00 ± 0.45) |
8c
(1.60 ± 0.68) |
Number of examined metaphase cells in each group 250 cells
Chromatid Breakage: total number of chromatid breaks + chromatid deletions
TSA total structural aberration, TNA total numerical aberration
aValues between practices represent mean ± SE of five animals
bSignificantly different from untreated control (G1) P < 0.05
cSignificantly different from positive control (G2) P < 0.05
Table 3.
Protective effects of propolis and bee pollen aqueous extracts against cisplatin induced cytotoxicity and genotoxicity in mouse bone marrow cells
| Groups | Mitotic indexa | Number of cellsa with one aberration | Number of cellsa with more than one aberration | Incidence ofa aberrant cells (%) | Number ofa aberrations/cell | Suppression (%) |
|---|---|---|---|---|---|---|
| G1 (saline) | 83.29 ± 1.05 | 6.20 ± 0.80 | – | 12.40 ± 1.60 | 0.124 ± 0.02 | |
| G2 (CDDP) | 37.75 ± 8.60b | 17.80 ± 0.97b | 6.40 ± 1.36b | 50.00 ± 4.15b | 0.672 ± 0.02b | |
| G3 (WSDP) | 76.58 ± 1.39b | 7.60 ± 0.40b | 1.20 ± 0.20b | 17.50 ± 1.17b | 0.180 ± 0.01b | |
| G4 (WEBP) | 85.65 ± 3.74 | 5.20 ± 0.73b | 0.60 ± 0.24b | 11.60 ± 0.98 | 0.136 ± 0.01 | |
| G5(CDDP + WSDP) | 73.28 ± 1.12c | 13.60 ± 1.36c | 3.80 ± 0.37c | 34.80 ± 3.38c | 0.416 ± 0.04c | 30.40 |
| G6(CDDP + WEBP) | 77.74 ± 1.66c | 14.00 ± 0.95c | 1.40 ± 0.40c | 30.80 ± 1.74c | 0.348 ± 0.01c | 38.40 |
aValues represent mean ± SE of five animals
bSignificantly different from untreated control (G1) P < 0.05
cSignificantly different from positive control (G2) P < 0.05
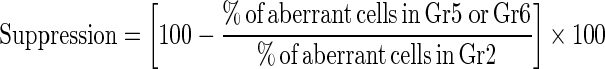
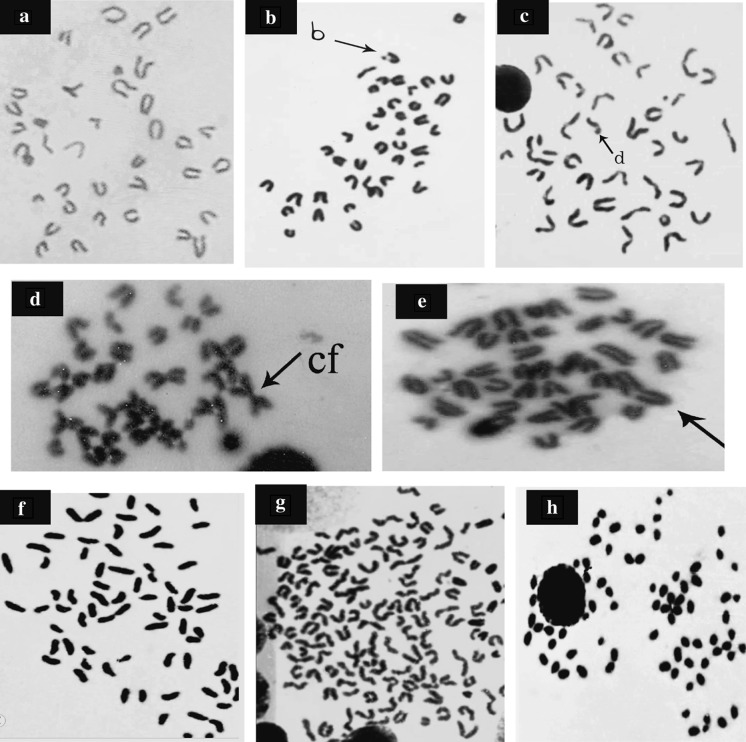
Fig. 4.
(A–H): Metaphase spreads from mouse bone marrow cells showing A normal cell, B chromatid break (b), C chromatid deletion (d), D centric fusion (cf), E end to end association (arrow), F centric attenuation, G polyploidy and H endomitosis (1,000)
Data in Tables 2 and 3 showed that, when propolis extract (WSDP) was given alone at dose 8.4 mg/kg once daily for 14 days did not induce or increase in number of some chromosomal aberrations over control group (G1) like gaps, centric attenuation, end to end association, centric fusion, polyploidy and endomitosis and the value of total numerical aberrations. While the number of chromatid breakage, value of total structural aberrations, number of cells with one aberration, and with more than one aberration, percentage of incidence of aberrant cells and number of aberration/cell increased significantly over the control group (G1) (at P < 0.05). The WSDP was also not found to be cytotoxic at the given dose (8.4 mg/kg b.wt), as there was no significant changes in mitotic index over G1 (Table 3). The group treated with the aqueous bee pollen extract (WEBP) (G4) was compared with the control group (G1) in terms of gaps, centromeric attenuations, centric fusions, end to end associations, polyploidy, endomitosis, mean total number of numerical aberrations, percentage of incidence of aberrant cells and number of aberration/cell and did not show significant differences (P < 0.05) confirming its non-mutagenicity (Tables 2, 3). The WEBP was also not found to be cytotoxic at the given dose (140 mg/kg b.wt), as there was no significant changes in mitotic index over G1 (Table 3).
On the other hand, administration of propolis and bee pollen extracts after and concomitant with cisplatin (treatment groups G5 and G6 respectively) led to a significant decrease in rates of clastogenetic changes compared with the CDDP treatment group (Tables 2, 3). All types of chromosomal aberrations induced by CDDP including breaks, gaps, end to end association, centric fusion, centromeric attenuation, and other multiple damages were found to be reduced by WSDP and WEBP but they were still significantly higher than negative control group (G1). The mitotic index was also found to be significantly increased (P < 0.05) over G2, indicating its anti-cytotoxicity towards CDDP (Table 3). The percentages of aberrant cells which were found to be 50.00 ± 4.147 in the CDDP treated animals, were reduced to 34.80 ± 3.382 and 30.80 ± 1.743 (P < 0.05) by WSDP and WEBP, respectively (Table 3). In addition a significant decrease in the number of aberrations per cell was observed in G5 and G6 over the CDDP treatment group (G2). The calculated suppressive effect was 30.40 and 38.40 %, by WSDP and WEBP, respectively (Table 3).
Discussion
The practice of disease prevention and/or inhibition, delay, or reversal of the process of carcinogenesis is the most cost effective mean for improving human health. The target of much research has been the discovery of natural and synthetic compounds that are used in the prevention and/or treatment of cancer (Heo et al. 2001).
Propolis and bee-collected pollen are apicultural products which are composed of nutritionally valuable substances and contain considerable amounts of polyphenol substances which may act as potent antioxidants. Development and utilization of more effective antioxidants of natural origin are desired. Naturally occurring polyphenols are expected to help reducing the risk of various life-threatening diseases, including cancer, due to their antioxidant activity (Teixeira et al. 2008). In addition, phenolic compounds are known to counteract oxidative stress in the human body by helping maintaining a balance between oxidant and antioxidant substance (Siddhuraju 2006).
Flavonoids and phenolic acids are major classes of polyphenolic compounds, whose structure–antioxidant activity relationships in aqueous or lipophilic systems have been extensively reported (Gardjeva et al. 2007). In addition to anti-oxidant activity, many phenolic compounds have been shown to exert anti-carcinogenic or anti-mutagenic activity to a greater or lesser extent (Awale et al. 2005). Mechanisms of antioxidant action of these compounds may include suppression of oxygen reactive species (ROS) formation, removal or inactivation of oxygen reactive species and up-regulation or protection of antioxidant defenses (Gardjeva et al. 2007). In this context, one of the most important intracellular antioxidant systems is the glutathione redox cycle. Glutathione is one of the essential compounds for maintaining cell integrity because of its reducing properties and participation in the cell metabolism (Abdella and Ahmed 2008). On another side, catalase, an enzyme involved in the antioxidant defense of cells and tissues has the ability to convert hydrogen peroxide to water (Ahmed and Abdella 2009).
Earlier studies conducted with bee pollen and propolis indicated that, the antioxidant activity of honey bee-collected pollen has been recognized as free radical scavenger and as an inhibitor of lipid peroxidation. This activity has been associated with the phenolic content of pollen (Campos et al. 2003). Bee pollen is rich in carotenoids, flavonoids and phytosterols. The exact profile varies depending on the plant sources and growth conditions (Maruyama et al. 2010). In addition, propolis possesses antioxidant activity, its constituents includes caffeic acid, galangin, quercetin and chrysin being able to scavenge free radicals (Pietta 2000; Kumazawa et al. 2003; Oršolić and Bašić 2005; Gardjeva et al. 2007; Capucho et al. 2012).
The present study revealed the chemoprotective potential of bee pollen or water extracts of propolis with respect to chromosomal and histological damages induced by cisplatin (CDDP) in bone marrow, liver, kidney and testis tissues of mice associated with antioxidant effect related to attenuated lipid peroxidation and elevated antioxidant activity of catalase and glutathione concentration.
CDDP is an inorganic platinum compound with a broad spectrum anti-neoplastic activity against different types of human tumors (Siddik 2003). CDDP has been demonstrated to have the potential for initiating genetic modifications in non-tumor cells in humans and animals, Aly et al. (2003) reported that, the genotoxicity of CDDP is due to its ability to bind with DNA, block and prolong the cell division in the G2 phase of the cell cycle. The blockage of cells in the G2 phase is related to the inhibition of chromatin condensation. Nevertheless, both clinical and experimental studies report a dose-limiting nephrotoxicity which restricts the usefulness of cisplatin in cancer chemotherapy (Nersesyan et al. 2003).
The results of the present investigation revealed that, administration of CDDP at a single dose of 2.8 mg/kg b.wt, twice/week for 3 weeks induced cytogenotoxic effects, histopathologic alterations and oxidative stress. These results are consistent with those previously reported (Nakajima et al. 2009; Maruyama et al. 2010).
The current results indicated a significant decrease in glutathione level and catalase activity in all tested tissues of cisplatin-induced toxicity in mice. Sueishi et al. (2002) registered lowered glutathione levels in the kidney of rats injected with cisplatin. In addition, glutathione peroxidase (GSHPx) and reduced glutathione (GSH) levels were significantly reduced in the liver and kidney of cisplatininjected animals (Naziroglu et al. 2004). Whereas, renal catalase showed a significant decrease after cisplatin administration to rats (Yildirim et al. 2003). Moreover, Amin and Hamza (2006) mentioned that the testicular toxicity of cisplatin is associated with a reduced glutathione content, catalase and superoxide dismutase activity.
A possible explanation for the enhancement of oxidative stress in cisplatin-injected mice may be the decreased formation of antioxidants in the tissues of cisplatin-injected animals which have the ability to scavenge hydroperoxides and lipid peroxides. In addition, the elevation of reactive oxygen species (ROS) in the tissues and depletion of antioxidants have been recognized as primary promoters of cellular damage where inhibition of membrane transport protein and increased lipid peroxidation are considered merely as a marker of cell damage (Naziroglu et al. 2004).
In the present study numerous histopathological alterations were noticed in cisplatin-injected mice. These changes include necrosis, fibrosis and hydrobic degenerative changes in liver, tubular and glomerular degeneration with albuminous cast deposition in the kidney in addition to a disorganization of seminiferous tubular cells with germ cell loss especially spermatids and sperms and congestion of blood vessels in the interstitial tissue of testes.
Numerous reports have previously reported the adverse effects of cisplatin on kidneys, liver and testis histological structures. Shirwaikar et al. (2004) reported glomerular congestion, tubular casts, epithelial degeneration, interstitial oedema, blood vessel congestion and infiltration by inflammatory cells, in the kidneys of Wistar rats administered cisplatin at a dose of 5 mg/kg b. wt. In addition, Yildirim et al. (2003) and Ozen et al. (2004) observed a remarkable proximal tubular necrosis with extensive epithelial vacuolization, swelling and tubular dilatation in the kidney of Wistar rats injected with 5 or 7 mg/kg b. wt of cisplatin, respectively. On the other side, liver sections from mice treated with cisplatin at a dose of 45 mg/kg b.wt, showed degeneration and vacuolization but without necrosis (Lu and Cederbaum 2006). Concerning testes, cross sections of mice exposed to 2.5 mg/kg b. wt. of cisplatin (G2), revealed extremely severe damage to the seminiferous epithelium with drastic reduction in tubular diameter, decreased cellularity, absence of specific cell population and absence of spermatids (Sawhney et al. 2005).
The results of the present investigation evidenced that, administration of CDDP at a single dose of 2.8 mg/kg b.wt, twice/week for 3 weeks induced cytogenotoxic effects revealed from the increase in incidence of chromosomal aberrations and decrease in mitotic indices. These results are consistent with those reported by Nersesyan et al. (2003), Tohamy et al. (2003), Nersesyan and Muradyan (2004), who demonstrated that cisplatin caused the development of chromosomal aberrations in bone marrow cells of mice.
The antioxidant, the anti-histopathologic and the anti-mutagenic actions of aqueous bee propolis extract involve enhancement of the level of glutathione S-transferase (GST), inhibition of cytochrome P-450 activity and interaction with microsome-generated proximate mutagens to generate an inactive complex. These effects were associated with inhibition of cell cycle progression, accelerating the detoxification of mutagens and carcinogens and induction of apoptosis (El-khawaga et al. 2003). Lotfy (2006) indicated also that, Egyptian propolis is characterized by the presence of unusual esters of caffeic acid with mainly saturated C12–C16 fatty alcohols. Flavonoid glycones and especially flavanones are typical components of propolis. These constituents of crude Egyptian propolis have led to an increase in its pharmaceutical demand and have rendered it an interesting subject of study.
In addition, Mello et al. (2010) reported that, propolis has a variable and complex chemical composition with high concentration of flavonoids and phenolic compounds present in the extract. The extract varies with the solvent used in extraction. Ethanol extracts contain higher levels of phenolic acid and polar compounds than water extracts. The most common propolis extraction process uses ethanol as the solvent. However, this has some disadvantages such as the strong residual flavor, adverse reactions and intolerance to alcohol of some people (Konishi et al. 2004). Researchers are interested in producing a new type of extract with the same compounds extracted by the ethanolic method, but without the related disadvantages. Water has been tested as the solvent, but resulted in a product containing reduced level of extracted compounds (Park et al. 1998).
In conclusion both bee pollen and propolis aqueous extracts appear to provide strong protective activities against histological and genotoxic effects of cisplatin. This activity seems to be dependent on the antioxidant activities exerted by both extracts. However, the aqueous extract of pollen gave more pronounced results than propolis since its effects on the decrease of lipid peroxidation marker was significantly higher than those of propolis and its ability to increase the antioxidant activity involved in the elimination of free radicals was better than that of propolis resulting in higher potential activity in ameliorating both genotoxic and histopathologic side effects of cisplatin administration.
References
- Abdella E, Ahmed R. Suppression of doxorubicin apoptotic, histopathologic, mutagenic and oxidative stress effect in mice bone marrow and tested tissue by aqueous Rosemary leave extract. Egypt J Zool. 2008;51:305–330. [Google Scholar]
- Ahmed R, Abdella E. Modulatory effect of Rosemary leave aqueous extract on doxorubicin-induced histological lesions, apoptosis and oxidative stress in mice. J Egypt Ger Soc Zool. 2009;57C:105–137. [Google Scholar]
- Aly M, Ashour M, El Nahas S, Abo Zeid M. Genotoxicity and cytotoxicity of the anticancer drugs gemcitabine and cisplatin, separately and in comination: in vivo studies. J Biol Sci. 2003;3(11):961–972. doi: 10.3923/jbs.2003.961.972. [DOI] [Google Scholar]
- Amin A, Hamza A. Effects of Roselle and Ginger on cisplatin-induced reproductive toxicity in rats. Asian J Androl. 2006;8(5):607–612. doi: 10.1111/j.1745-7262.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- Awale S, Shrestha SP, Tezuka Y, Ueda J, Matsushige K, Kadota S. Neoflavonoids and related constituents from Nepalese propolis and their nitric oxide production inhibitory activity. J Nat Prod. 2005;68:858–864. doi: 10.1021/np050009k. [DOI] [PubMed] [Google Scholar]
- Bancroft J, Gamble (2002) Theory and practice of histological techniques, 5th edn. Edinburgh, Churchill Livingstone Pub., pp 172–175
- Bankova V. Recent trends and important developments in propolis research. Evid Based Complement Alternat Med. 2005;2(1):29–32. doi: 10.1093/ecam/neh059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros L, Falcão S, Baptista P, Freire C, Vilas-Boas M, Ferreira IC. Antioxidant activity of Agaricus sp. mushrooms by chemical, biochemical and electrochemical assays. Food Chem. 2008;111:61–66. doi: 10.1016/j.foodchem.2008.03.033. [DOI] [Google Scholar]
- Basnet P, Matsuno T, Neidlein R. Potent free radical scavenging activity of propolis isolated from Brazilian propolis. Z Naturforsch C. 1997;52:828–833. doi: 10.1515/znc-1997-11-1217. [DOI] [PubMed] [Google Scholar]
- Beutler E, Duron O, Kelly B. Improved method for determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Campos M, Webby R, Markham K, Mitchall K, Cunha A. Age-induced diminution of free radical scavenging capacity in bee pollens and the contribution of constituent flavonoids. J Agric Food Chem. 2003;51(3):742–745. doi: 10.1021/jf0206466. [DOI] [PubMed] [Google Scholar]
- Capucho C, Sette R, Fabrícia de Souza P, Juliana de Castro M, Pigoso A, Barbieri R, Dolder MA, Grasiela DC. Green Brazilian propolis effects on sperm count and epididymis morphology and oxidative stress. Food Chem Toxicol. 2012;50(11):3956–3962. doi: 10.1016/j.fct.2012.08.027. [DOI] [PubMed] [Google Scholar]
- Cohen G, Demblec D, Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem. 1970;34:30–38. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- El-khawaga OM, Salem T, Elshal M. Protective role of Egyptian propolis against tumor in mice. Clin Chim Acta. 2003;338:11–16. doi: 10.1016/S0009-8981(03)00323-1. [DOI] [PubMed] [Google Scholar]
- Eraslan G, Kanbur M, Silici S, Liman B, Altinordulu S, Sarica Z. Evaluation of protective effect of bee pollen against propoxur toxicity in rat. Ecotoxicol Environ Saf. 2009;72:931–937. doi: 10.1016/j.ecoenv.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Gardjeva PA, Dimitrova SZ, Kostadinov ID, Murdjeva MA, Peyche LP, Lukanov LK, Stanimirova IV, Alexandrov AS. A study of chemical composition and antimicrobial activity of Bulgarian propolis. Folia Med (Plovdiv) 2007;49:63–69. [PubMed] [Google Scholar]
- Greene MH. Is cisplatin a human carcinogen? J Natl Cancer Inst. 1992;84:306–312. doi: 10.1093/jnci/84.5.306. [DOI] [PubMed] [Google Scholar]
- Heo M, Sohn S, Au W. Antigenotoxicity of galangin as cancer chemopreventive agent candidate. Mutat Res. 2001;488:135–150. doi: 10.1016/S1383-5742(01)00054-0. [DOI] [PubMed] [Google Scholar]
- Husain E, Naseem I. Riboflavin-mediated cellular photoinhibition of cisplatin-induced oxidative DNA breakage in mice epidermal keratinocytes. Photodermatol Photoimmunol Photomed. 2008;24(6):301–307. doi: 10.1111/j.1600-0781.2008.00380.x. [DOI] [PubMed] [Google Scholar]
- Institute of laboratory animal resources (1996) Guide for the care and use of laboratory animals. Commission on life sciences. National research council National academy press, Washington, DC
- Iraz M, Ozerol E, Gulec M, Tasdemir S, Idiz N, Fadillioglu E, Naziroglu M, kyol O. Protective effect of caffeic acid phenethyl ester (CAPE) administration on cisplatin-induced oxidative damage to liver in rat. Cell Biochem Funct. 2006;24:357–361. doi: 10.1002/cbf.1232. [DOI] [PubMed] [Google Scholar]
- Konishi S, Sawaya A, Custódio AR, Cunha I, Shimizu M. Influence of solubilising agents on antimicrobial activity of propolis extracts and of hydroalcoholic spray formula. Mensagem Doce. 2004;75:22–25. [Google Scholar]
- Kumazawa S, Yoneda M, Shibata I, Kanaeda J, Hamasaka T, Nakayama T. Direct evidence for the plant origin of Brazilian propolis by the observation of honeybee behavior and phytochemical analysis. Chem Pharm Bull. 2003;51(6):740–742. doi: 10.1248/cpb.51.740. [DOI] [PubMed] [Google Scholar]
- Lee S, Schmitt C. Chemotherapy response and resistance. Curr OpinGenet Dev. 2003;13:90–96. doi: 10.1016/S0959-437X(02)00014-X. [DOI] [PubMed] [Google Scholar]
- Lotfy M. Biological activity of bee propolis in health and disease. Asian Pac J Cancer Prev. 2006;7:22–31. [PubMed] [Google Scholar]
- Lu Y, Cederbaum A. Cisplatin-induced hepatotoxicity is enhanced by elevated expression of cytochrome P4502E1. Toxicol Sci. 2006;89:515–523. doi: 10.1093/toxsci/kfj031. [DOI] [PubMed] [Google Scholar]
- Mani F, Damasceno H, Novelli E, Martins E, Sforcin J (2006) Propolis: effect of different concentrations, extracts and intake period on seric biochemical variables. J Ethnopharmacol 105:95–98 [DOI] [PubMed]
- Markham K, Campos M. 7-a-8-O-Methylherbacetin-3-O-sophorosides from bee pollens and some structure/activity observations. Phytochemistry. 1996;43(4):763–767. doi: 10.1016/0031-9422(96)00286-5. [DOI] [Google Scholar]
- Maruyama H, Sakamoto T, Araki Y, Hara H. Anti-inflammatory effect of bee pollen ethanol extract from Cistus sp. of Spanish on carrageenan-induced rat hind paw edema. BMC Complement Altern Med. 2010;10:30–40. doi: 10.1186/1472-6882-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello B, Petrus J, Hubinger M. Concentration of flavonoids and phenolic compounds in aqueous and ethanolic propolis extracts through nanofiltration. J Food Eng. 2010;96:533–539. doi: 10.1016/j.jfoodeng.2009.08.040. [DOI] [Google Scholar]
- Mitamura T, Matsuno T, Sakamoto S, Maemura M, Kudo H, Suzuki S, Kuwa K, Yoshimura S, Sassa S, Nakayama T, Nagasawa H. Effects of a new clerodane diterpenoid isolated from propolis on chemically induced skin tumors in mice. Anticancer Res. 1996;16:2669–2672. [PubMed] [Google Scholar]
- Nakajima Y, Tsuruma K, Shimazawa M, Mishima S, Hara H. Comparison of bee products based on assays of antioxidant capacities. BMC Complement Altern Med. 2009;9:4–12. doi: 10.1186/1472-6882-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naziroglu M, Karaoglu A, Aksoy A. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicol. 2004;195:221–230. doi: 10.1016/j.tox.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Nersesyan A, Muradyan R. Sea-buckthorn juice protects mice against genotoxicty action of cisplatin. Exp Oncol. 2004;26(2):153–155. [PubMed] [Google Scholar]
- Nersesyan A, Perrone E, Roggieri P, Bolognesi C. Genotoxic action of cycloplatam, a new platinum antitumor drug, on mammalian cells in vivo and in vitro. Chemotherapy. 2003;49:132–137. doi: 10.1159/000070619. [DOI] [PubMed] [Google Scholar]
- Oršolić N, Bašić I. Antitumor, hematostimulative and radioprotective action of water-soluble derivative of propolis (WSDP) Biomed Pharmacother. 2005;59:561–570. doi: 10.1016/j.biopha.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Ozen S, Akyol O, Iraz M, Sogut S, Ozugurlu F, Ozyurt H, Odaci E, Yildirim Z. Role of caffeic acid phenethyl ester, an active component of propolis, against cisplatin-induced nephrotoxicity in rats. J Appl Toxicol. 2004;24:27–35. doi: 10.1002/jat.941. [DOI] [PubMed] [Google Scholar]
- Pabla N, Huang S, Mi Q, Daniel R, Dong Z. ATR-Chk2 signaling in P53 activation and DNA damage response during cisplatin-induced apoptosis. J Biol Chem. 2008;283(10):6572–6583. doi: 10.1074/jbc.M707568200. [DOI] [PubMed] [Google Scholar]
- Park YK, Ikegaki M, Abreu JAS, Alcici NMF. Study of the preparation of propolis extracts and its applications. Ciênc Tecnologia Aliment. 1998;18:313–318. doi: 10.1590/S0101-20611998000300011. [DOI] [Google Scholar]
- Pietta P. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- Pisano C, Pratesi G, Laccabue D, Zunino F, Lo Giudice P, Bellucci A, Pacifici L, Camerini B, Vesci L, Castorina M, Cicuzza S, Tredici G, Marmiroli P, Nicolini G, Galbiati S, Calvani M, Carminati P, Cavaletti G. Paclitaxel and cisplatin-induced neurotoxicity: a protective role of acetyl-l-carnitine. Clin Cancer Res. 2003;9:5756–5767. [PubMed] [Google Scholar]
- Pressus H, Jarrel S, Scheckenbach R, Lieberman S, Anderson R. Comparative effects of chromium, vanadium and Gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J Am Coll Nutr. 1998;17(2):116–123. doi: 10.1080/07315724.1998.10718736. [DOI] [PubMed] [Google Scholar]
- Preston R, Dean B, Galloway S, Holden H, Mc-fee A, Shelby M. Mammalian in vivo cytogenetic assays-analysis of chromosomal aberrations in bone marrow cells. Mutat Res. 1987;189:157–165. doi: 10.1016/0165-1218(87)90021-8. [DOI] [PubMed] [Google Scholar]
- Rao M, Blane K, Zonneberg M (1985) PC-STAT, one and two way analysis of variance. The University of Georgia Programs Version 1A (C) copyright
- Saric A, Balog T, Sobocanec S, Kusic B, Sverko V, Rusak G, Likic S, Bubalo D, Pintp B, Reali D, Marotti T. Antioxidant effects of flavonoid from Croatian Cystus incanus L. rich bee pollen. Food Chem Toxicol. 2009;47:547–554. doi: 10.1016/j.fct.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Sawhney P, Giammona J, Meistrich M, Richburg J. Cisplatin-induced long-term failure of spermatogenesis in adult C57/Bl/6 J mice. J Androl. 2005;26(1):136–145. [PubMed] [Google Scholar]
- Shirwaikar A, Deepti Issac D, Malini S. Effect of Aerva lanata on cisplatin and gentamicin models of acute renal failure. J Ethnopharmacol. 2004;90:81–86. doi: 10.1016/j.jep.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Shukla Y, Taneja P. Antimutagenic effects of garlic extract on chromosomal aberrations. Cancer Lett. 2002;176:31–36. doi: 10.1016/S0304-3835(01)00774-1. [DOI] [PubMed] [Google Scholar]
- Siddhuraju P. The antioxidant activity and free radical-scavenging capacity of phenolics of raw and dry heated moth bean (Vigna aconitifolia) (Jacq.) Marcchal seed extracts. Food Chem. 2006;99:149–157. doi: 10.1016/j.foodchem.2005.07.029. [DOI] [Google Scholar]
- Siddik Z. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- Souza RM, de Souza MC, Patitucci ML, Silva JF. Evaluation of antioxidant and antimicrobial activities and characterization of bioactive components of two Brazilian propolis samples using a pKa-guided fractionation. Z Naturforsch. 2007;62C:801–807. doi: 10.1515/znc-2007-11-1205. [DOI] [PubMed] [Google Scholar]
- Sueishi K, Mishima K, Makino K, Itoh Y, Tsuruya K, Hirakata H, Oishi R. Protection by a radical scavenger edaravone against cisplatin-induced nephrotoxicity in rats. Eur J Pharmacol. 2002;451:203–208. doi: 10.1016/S0014-2999(02)02251-3. [DOI] [PubMed] [Google Scholar]
- Teixeira E, Message D, Negri G, Salatino A, Stringheta P. Seasonal variation, chemical composition and antioxidant activity of Brazilian propolis samples. Evid Based Complement Alternat Med. 2008;7:307–315. doi: 10.1093/ecam/nem177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohamy A, El Ghor A, El nahas S, Noshy M. Beta-glucan inhibits the genotoxicity of cyclophosphamide, adriamycin and cisplatin. Mutat Res. 2003;541:45–53. doi: 10.1016/S1383-5718(03)00184-0. [DOI] [PubMed] [Google Scholar]
- Volpi N. Separation of flavonoids and phenolic acids from propolis by capillary zone electrophoresis. Electrophoresis. 2004;25:1872–1878. doi: 10.1002/elps.200405949. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Hamamoto R, Uchiyama S, Ishiyama K, Hashimoto K. Anabolic effects of bee pollen Cistus ladaniferus extract on bone components in the femoral diaphyseal and metaphyseal tissues of rats in vitro and in vivo. J Health Sci. 2006;52(1):43–49. doi: 10.1248/jhs.52.43. [DOI] [Google Scholar]
- Yildirim Z, Sogut S, Odaci E, Iraz M, Ozyurt H, Kotuk M, Akyol O. Oral erdosteine administration attenuates cisplatin-induced renal tubular damage in rats. Pharmacol Res. 2003;47:149–156. doi: 10.1016/S1043-6618(02)00282-7. [DOI] [PubMed] [Google Scholar]