Abstract
Olfactory disorders are not rare and affect quality of life in patients. The purpose of our study was to evaluate the outcomes of an outpatient-based diode laser inferior turbinate reduction (ITR) in otherwise therapy-refractory olfactory disorder. In a prospective clinical investigation, 20 patients (7 male, 13 female, mean age 53.2 ± 15.34) with olfactory disorder and 10 patients (8 male, 2 female, mean age 52.5 ± 17.55) without olfactory disorder underwent ITR under videoendoscopic control with a continuous diode laser in “contact” mode after topical anesthetic preparation. Treatment efficiency was assessed before and 2 months after surgery. Subjective nasal airflow (NA) and the olfactory function were rated by means of visual analogue scales (VAS). Olfactory function was assessed using the “Sniffin’ Sticks” test battery. VAS showed very low median values for intraoperative pain (0) [0–1.20] and high postoperative patient satisfaction (8) [5–9]. After 2 months there was no significant improvement of objective olfactory function as measured by the TDI score (threshold, discrimination and identification). The VAS displayed a slight significant improvement in the group of patients with olfactory disorder from 2.95 to 3.65 (P = 0.04). After 2 months, NA data revealed a statistically significant improvement of subjective VAS from 5.05 to 6.25 (P < 0.0005) and of objective NA from 353.77 to 443.50 (P = 0.007) as measured by rhinomanometry in both groups. Outpatient diode laser ITR represents an effective option providing recovery by NA improvement but not a significant improvement of the olfactory function.
Keywords: Olfactory disorder, Nasal obstruction, Rhinomanometry, Laser treatment, Diode laser, TDI
Introduction
Olfactory disorders affect quality of life [1, 2]. Sinonasal disease, upper respiratory tract infection and head trauma still seem to be the main aetiology for olfactory disorders [3, 4]. Diagnostic of olfactory disorders using standardized test battery [5, 6], olfactory evoked potentials [7, 8] and MRI [9] gain in importance but the therapeutic options available in the treatment of olfactory disorders are still disappointing [2], whereas medical or operative treatment of nasal obstruction is well demonstrated in literature [10]. The purpose of our prospective study was to evaluate the outcomes of an outpatient-based diode laser inferior turbinate reduction (ITR) in patients with olfactory dysfunction and hypertrophic inferior turbinate. We wanted to find out whether a better nasal airflow correlates with better olfactory function.
Materials and Methods
Study Design
In a clinical, controlled, prospective study, patients diagnosed with olfactory disorders underwent IT surgery under endoscopic control with a continuous diode laser in “contact” mode after topical anesthetic preparation. Clinical examination, data acquisition and olfactory-counseling took place at pre-therapeutic initial visit, during laser treatment and regular follow-up investigations. All patients showed hypertrophic inferior turbinates. Intra- and perioperative details were registered including bleeding, crusting, experienced pain and discomfort. Treatment efficiency was assessed 2 months after surgery. The subjective pre- and post-therapeutic nasal airflow (NA), subjective olfactory function, patient satisfaction and extend of experienced pain were rated by means of visual analogue scales (VAS; 0–10 points). Olfactory function was performed before and 2 months after surgery using the “Sniffin’ Sticks” test kit [5, 6].
Patients
We recruited only patients with hypertrophic inferior turbinates and olfactory dysfunction from our Consultation Service for olfactory disorders at Charité University ENT Clinic. The participants were selected following unsuccessful medical treatment with systemic or topic corticosteroids.
Selection criteria were: age older than 18 years, confirmed diagnosis with history, at least one unsuccessful conservative treatment attempt (e.g. steroid nasal spray), hypertrophic inferior turbinates and informed consent. Exclusion criteria comprised the existence of a malignant tumor, pregnancy, the coexistence of acute infection, nasal polyps, obstructive septal deviation, preceding nasal surgery, and the occurrence of atrophic Rhinitis medicamentosa.
A consecutive sample of 20 patients (7 men, 13 women, mean age 53.2 ± 15.34 years with anosmia or hyposmia of different aetiology (n = 4 after upper respiratory tract infection, n = 9 sinonasal, n = 3 post-traumatic, n = 4 idiopathic) with therapy-refractory olfactory disorder was recruited for laser surgery including follow-up examinations.
The duration of the olfactory dysfunction was median 19 months (14–57 months).
At the first consultation, a detailed case history was taken and the patients underwent an extensive ENT examination including endoscopy of the nose and the olfactory cleft. Our laser device consisted of a continuous-wave semiconductor diode laser, which emits wavelengths in the near infrared area of the light spectrum (λ = 830 nm; Lumenis, Dreieich, Germany). The laser light application was performed in “contact” mode by using a flexible, plastic-clad silica fiber (400-μm core diameter) coupled to an aiming beam.
A control group of 10 patients (8 male, 2 female, mean age 52.5 ± 17.55) without subjective olfactory disorder but with hypertrophic inferior turbinates who underwent the same operation due to nasal obstruction was evaluated using the same procedure.
Surgical Procedure
Patients were asked to lie down on the examination couch. Before laser treatment, topical local anesthetics were applied for about 10 min using cotton pads imbibed with a mixture of 0.5% tetracaine and 0.1% naphazoline solution (50:50). Subsequently, 5% lidocaine hydrochloride gel was repeatedly applied onto the entire IT and bottom of the nose with a cotton applicator for about 3 min. When the nose was completely analgized, the laser output power was set to 4.8 W and hyperplastic IT were treated in “contact” mode. Two parallel posteroanterior mucosal cuts were made at the mediobasal part and optional some additional laser spots onto the head of hypertrophic IT. Postoperatively, the patients were observed for about 10 min in the department before being discharged home. They were put on nasal irrigation with saline, nursing dexpanthenol ointment (Bepanthen®) and if necessary naphazoline hydrochloride ointment (Siozwo®) for maximum 2 weeks.
Instruments for Examination
Visual analogue scales (VAS) served to acquire and compare the subjective pre-, intra- and posttherapeutic data. VAS were presented as lines anchored by bipolar descriptions on a numeric scale from 0 to 10 (0 = not existing, 10 = maximal prevalence). The participants were asked to rate their momentary state of olfactory function by marking the corresponding location. Objective NA was quantified by active anterior rhinomanometry (Hortmann, Neckartenzlingen, Germany) under standardized conditions at a pressure of 150 Pa [11, 12]. Preoperatively, the measurements were done prior to and 10 min after nasal decongestion with 1% phenylephrine solution. The same procedure was done 2 months after surgery.
The olfactory function of all our patients (olfactory disorder and control group) was determined with the ‘TDI-score’ at the beginning and 2 months after finishing therapy. The TDI-score is a tripartite instrument measuring threshold, discrimination and identification with each 16 odoriferous markers (“Sniffin’ Sticks” test battery) [5]. Patients are partly blinded and have to make a choice (’forced choice’) in this procedure to identify their olfactory function in preferably an objective manner. The TDI-score is a reliable and validated tool [6] reflecting the olfactory function. In general a score of 15 and less indicates anosmia, a score up to 30 hyposmia and above 30.5 normosmia. The change in the TDI-score was calculated, too. At each visit, the TDI score was measured by the same person.
The change in TDI score was therefore linked to descriptive statistics (aetiology, age, duration of olfactory disorder, gender).
Data Analysis
Statistical analysis was performed using SPSS (16.0, Chicago, USA). Descriptive statistics are used to characterize the basic quantitative features of the data. Means ± standard deviation (SD), medians, first and third quartiles and ranges were calculated for all subjective and objective parameters depending on the distribution of measured values.
For numeric data, Wilcoxon matched pairs test was used to compare the parameters before and after treatment. Mann–Whitney-U test and Kruskal–Wallis test were used for independent samples. Correlations as assessed by means of bivariate regression analysis were used to evaluate the influence of co-variables (age, duration of olfactory disorder, initial NA value and initial TDI score at preoperative baseline level, and postoperative change in NA value) on the outcome after ITR as measured by change in inspiratory NA at 150 Pa and in TDI score. A difference was considered significant at a P value of <0.05.
Results
Sample Description and Preoperative Assessment
Altogether, 20 patients with confirmed diagnosis of olfactory disorder as well as nasal obstruction due to hypertrophic IT and 10 patients without subjective olfactory disorder but with nasal obstruction due to hypertrophic IT and completely documented data records entered therapy and further evaluation. Videoendoscopy displayed hypertrophic IT in all patients.
Patients with olfactory disorder and controls were comparable with regard to age, initial NA, visibility of the olfactory cleft and smoking habits, but they were not comparable concerning distribution of gender (P = 0.05). Patient characteristics are depicted in Tables 1 and 2.
Table 1.
Patient characteristics. Gender, olfactory cleft and olfactory disorder
| Patients with olfactory disorder | Controls | P | |||
|---|---|---|---|---|---|
| Number of patients | % of all patients (n = 20) | Number of patients | % of all patients (n = 10) | ||
| Gender | |||||
| Male | 7 | 35 | 8 | 80 | 0.05 |
| Female | 13 | 65 | 2 | 20 | |
| Olfactory cleft visibility | |||||
| Bothsided | 18 | 90 | 9 | 90 | 1.00 |
| Onesided | 2 | 10 | 1 | 10 | |
| Smoking | |||||
| Yes | 4 | 20 | 1 | 10 | 1.00 |
| No | 14 | 70 | 9 | 90 | |
| Unknown | 2 | 10 | – | – | |
| Etiology of olfactory disorder | |||||
| Sinunasal | 9 | 45 | |||
| Post-URTI | 4 | 20 | |||
| Post trauma | 3 | 15 | |||
| Idiopathic | 4 | 20 | |||
URTI upper respiratory tract infection
Table 2.
Patient characteristics. Results
| Patients with olfactory disorder (n = 20) | Controls (n = 10) | P | |
|---|---|---|---|
| Age (in years) | 53.50 (±14.59) | 52.50 (±17.55) | 0.87 |
| Duration of olfactory disorder (in months) | 19.00 (14.00–57.00) | – | – |
| VAS | |||
| Pain | 0.00 (0.00–1.00) | 0.00 (0.00–3.50) | 0.44 |
| Satisfaction | 8.00 (5.75–8.25) | 7.00 (5.00–9.50) | 0.89 |
| NA before ITR | 5.08 (±2.61) | 5.00 (±1.58) | 0.97 |
| NA after ITR | 6.25 (±2.78) | 6.25 (±1.99) | 0.70 |
| OF before ITR | 2.95 (±2.33) | 9.20 (±1.03) | <0.0005 |
| OF after ITR | 3.65 (±2.44) | 8.80 (±1.03) | <0.0005 |
VAS visual analogue scale, NA nasal airflow, OF olfactory function, ITR inferior turbinate reduction, Pa pascal, TDI score of olfactory threshold, discrimination, identification
VAS showed mean olfactory function values of 2.95 points (±2.33, range 0–6.5) in patients with olfactory disorder and 9.2 points (±1.03, range 8–10) in the control group. TDI score resulted in 18.48 points (±9.68, range 1–29.5) for patients with olfactory disorder and in 28.55 points (±2.79, range 24–34) in the controls.
Subjective VAS showed mean NA values of 5.05 points (±2.99, range 0–9) in the entire sample of patients: 5.08 points (±2.61, range 0–9) in the study group and 5 points (±1.58, range 2–7) in the control group. Objective rhinomanometry before nasal decongestion resulted in 353.77 cm3/s (±162.25, range 65–656 cm3/s) inspiratory NA at 150 Pa in the means in the entire sample of patients: 341.05 cm3/s (±166.43, range 65–656 cm3/s) in the study group and 379.20 cm3/s (±158.95, range 182–640 cm3/s) in the control group.
Intra- and Perioperative Results
The total energy applied for each IT ranged from 1,152 to 2,304 Joule (mean, 1,440 J). Subjectively, the surgical procedure was well tolerated. VAS showed very low values for intraoperative pain and discomfort. The pain median score was 0 [0–1.19] for the entire sample of patients: 0 [0–1] in the study group and 0 [0–3.5] in the control group. In the completely topically analgized nose, no relevant local or systemic side effects occurred. Most patients declared a local feeling of slight pressure produced by the endonasal laser application system, and cauterization induced intraoperative olfactory annoyance (“barbecue smell”). Neither major bleeding requiring nasal packing nor other acute intra- and perioperative complications were observed. Of all patients, 21 declared no major postoperative complications, 6 declared the occurrence of slight bleeding, 2 reported incrustation and one patient reported on a minor infection.
Postoperative Results
Treatment success was assessed on the basis of follow-up investigations 2 months after ITR. The short-term results showed in all subjects that the postoperative edema disappeared within 8 weeks after surgery.
Objective olfactory function did not change with statistical significance neither in the study group nor in the control group. The mean initial TDI score of 18.48 (±9.68) raised to 20.03 points (±8.94, P = 0.19) in patients with olfactory disorder postoperatively, indicating a mean difference of 1.55 points (±5.06), which is mainly due to an increase of the olfactory threshold section of the TDI score. Patients only with the symptoms of nasal obstruction reached a mean TDI score of 29.70 (±3.34, P = 0.35) 2 months after surgery from initially 28.55 points (±2.79), which results in mean TDI score increase of 1.15 points (±3.7), again caused by an elevation of the olfactory threshold. There was no statistically difference under the different aetiologies (P = 0.83).
The VAS assessing subjective olfactory function raised from mean 2.95 (±2.33) to 3.65 points (±2.44, P = 0.04) in the study group after ITR, indicating a mean improvement of 0.70 points (±1.38). In the control group, the mean preoperative value was 9.2 (±1.03) and it decreased to 8.8 (±1.03, P = 0.17), meaning a decline of −0.40 points (±0.84 in the means, which did not reach statistical significance.
A relevant increase in the TDI-score (3 points) was seen in 6 of 20 patients (30%) with olfactory dysfunction and in 2 of 10 (20%) patients without olfactory disorder. Sixty-five percentage of the patients with olfactory disorder experienced no change, while 7 of 10 patients without olfactory disorder displayed no clinically relevant change in the TDI score. A change of 6 points or more was seen in 4 patients in the study group and in 2 patients in the controls. A deteriorated olfactory function was observed in one patient from the study group and in one patient of the control group.
All these differences between the study and control group did not prove to be statistically significant (P = 0.96).
The VAS results revealed a statistically significant improvement of subjective NA. The mean scores changed from preoperatively 5.08 (±2.61) to postoperatively 6.25 points (±2.78, P = 0.002) in the study group and from 5 (±1.58) to 6.25 points (±1.99, P = 0.04) in the control group. This means a subjective increase of NA of 1.17 points (±1.47) in patients with olfactory disorder and of 1.25 points (±1.69) in the controls.
These subjective data were confirmed by objective rhinomanometry, which showed a significant increase of mean NA from 341.05 (±166.43) to 462.40 (±203.20) cm3/s total nose inspiration at 150 Pa postoperatively in the olfactory disorder group (P = 0.01) and a not significant increase of mean NA from 379.20 (±158.95) to 405.70 (±175.55) cm3/s after ITR (P = 0.32) in the control group. There was no statistically difference under the different aetiologies (P = 0.98).
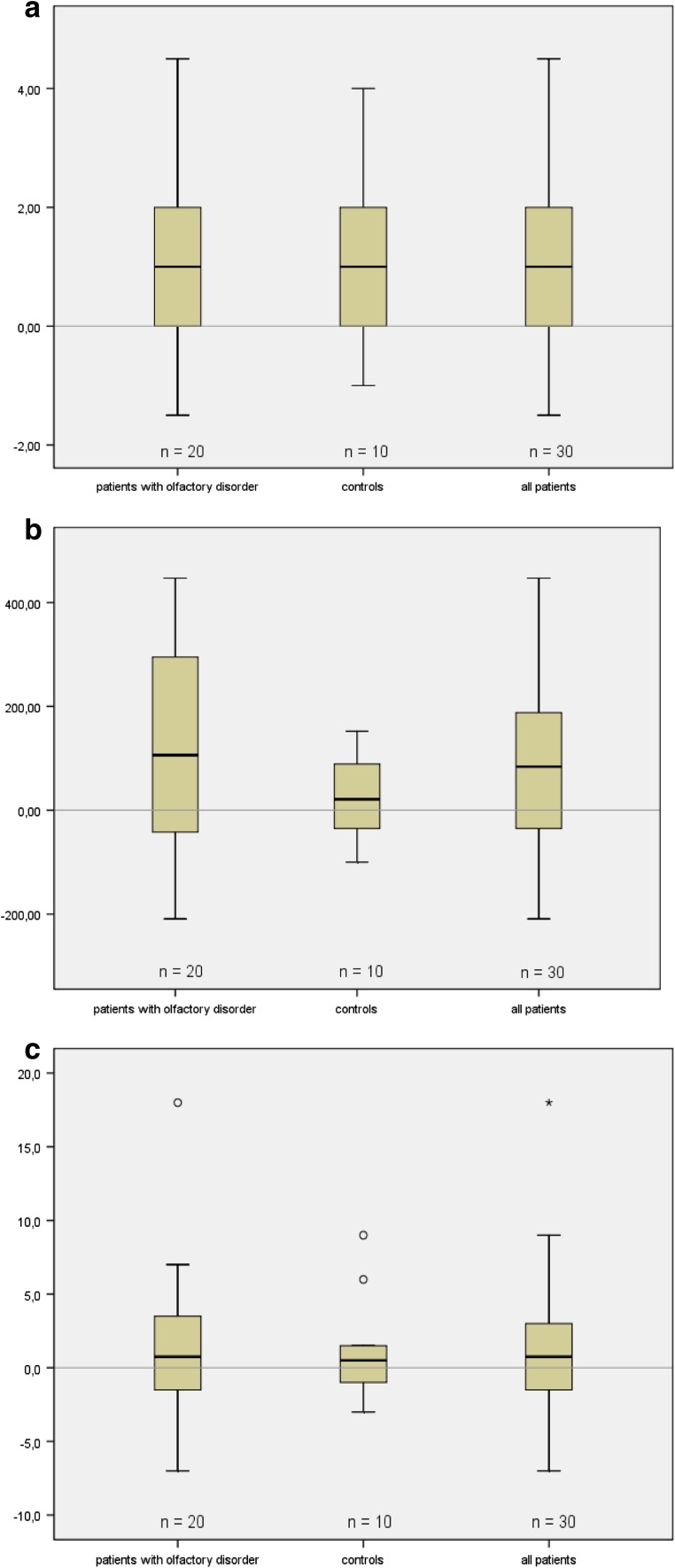
Distribution of differences between pre- and postoperative NA data and TDI scores for all patients are depicted in Fig. 1a–c.
Fig. 1.
Results of subjective improvement (a Differences between pre- and postoperative values of VAS measuring NA) and objective improvement (b Rhinomanometric differences between pre- and postoperative values of total nose inspiration at 150 pa) of nasal airflow (NA) and TDI score (c Differences between pre- and postoperative values in TDI). Data expressed as differences between pre- and postoperative values 2 months after ITR for all patients (n = 30). Box plots display the median, the quartiles, the range of values covered by the data and any outliers (single spots)
The differences between pre- and postoperative data indicated that ITR results in a significant objective NA improvement but not in a significant objective improvement of the olfactory function in these patients.
This treatment success was accompanied by high overall patient satisfaction with a median score of 8 [5–9] in the entire sample of patients: 8 [5.75–8.25] in the study group and 7 [5–9.5] in the control group.
Concerning the impact of co-variables on the outcome after ITR, regression analysis revealed the initial TDI score to have a statistically significant influence (rc = −0.36, P = 0.05), indicating that lower initial scores resulted in higher postoperative increases in TDI. In addition, the duration of olfactory disorder prior to ITR showed a statistically significant influence on the increase of the TDI score (rc = 0.62, P = 0.008), suggesting that longer preexistence of olfactory disorder resulted in higher increases in TDI. In the case of the change in the TDI score, all other factors investigated (age, gender, initial NA, change in NA after ITR) had no significant influence.
When looking at co-variables with an impact on the change in NA, regression analysis showed an influence of the initial NA value (regression coefficient rc = –0.33 P = 0.08), which approaches statistical significance. Age as well displayed an influence on the inspiratory NA 2 months after ITR (rc = −0.35, P = 0.06), again approaching statistical significance. Older age caused a higher increase in the inspiratory NA at 150 Pa. All other factors investigated (gender, duration of olfactory disorder, TDi score before ITR) had no significant influence on of the effectiveness of ITR as measured by the difference in NA.
Discussion
Very few prospective studies of operative treatment of olfactory disorders have been performed [13, 14]. There exists no prior research treating patients with olfactory disorder and hypertrophic inferior turbinates and compare these results with a control group showing nasal obstruction due to hypertrophic inferior turbinates.
The topic corticosteroid therapy is a well-established part in the treatment of allergic rhinitis [15] and chronic sinusitis [16, 17]. Mometasone nasal spray showed the greatest effectiveness in reducing fibroblast cultures in nasal polyps followed by beclomethasone [18]. Recent results tend to prove that patients with an olfactory dysfunction after upper respiratory tract infection, idiopathic and sinonasal olfactory dysfunction benefit from a topic application of a corticosteroid, too [19, 20].
This study is the first of its kind demonstrating that in otherwise therapy-refractory olfactory disorder the diode laser reduction of hyperplastic IT is effective improving nasal obstruction but not significantly in the treatment of olfactory dysfunction. The long-term VAS revealed considerable changes of NA median scores from 5.05 points to 6.25 points for the entire sample of patients, but not in the TDI score. However, the correlation between subjective NA sensation and the objective measurement of nasal resistance in rhinomanometry is usually regarded as poor [21–23]. In contrast our study shows both: a subjective and objective improvement of NA. But our results indicate that all patients not benefited significantly from ITR as a possible treatment for olfactory disorder, even due to sinonasal, upper respiratory tract infection, idiopathic or post-traumatic. All other investigated factors such as age, gender, duration of olfactory disorder had no influence on of the effectiveness of ITR.
In general, the surgical procedure was well tolerated. Due to the almost pain- and bloodless laser therapy the patients acceptance was very high. VAS showed very low median values for intraoperative pain and high postoperative patient satisfaction. Important contributing factors were: topical lidocaine gel application instead of local infiltration anesthesia, no requirement of nasal package, and discharge immediately after treatment. No bleedings and almost no adverse events or complications were observed intra- and postoperatively. The healing process in all patients showed that the minimally invasive diode laser treatment is not associated with an increased risk of local infections. Normally, the superficial coagulation zone protects against germs, changes into necrotic tissue and detaches from the healthy underground within 8 weeks after surgery. No excessive increase of secretions and crusts occurred due to the controllable coagulation and ablation of soft IT tissue without exposed bony edges.
Other surgical techniques for removal of hyperplastic IT such as conchotomy, inferior turbinoplasty, turbinectomy, lateral outfracture or cryotherapy had a wider range of side effects and higher risk of complications [24, 25]. In contrast, the correct laser in the correct clinical situation offers significant benefits over standard non-laser methods: e.g., rapid and bloodless operations, less postoperative edema, less pain, and good spontaneous epithelialization of defects. The diode laser has various advantages like a laser emission penetration of 1–3 mm into soft IT tissue. This kind of laser absorbed by water and blood. Therefore, this laser type provides good hemostatic, vaporization and excellent coagulation capabilities. With regard to socioeconomic aspects, diode laser IT surgery can be performed as an outpatient procedure with a small-sized portable device at low costs (acquisition, operating, consumables).
We suggested that a better nasal airflow might influence the olfactory function, at least it can facilitate the approach to areas in middle and superior turbinate with olfactory epithelium [26, 27], and so the patients had a possibility to gain more olfactory sensations.
These results show that a better nasal airflow did not correlate with better olfactory function.
Conclusions
Outpatient diode laser reduction of hypertrophic IT under topical anesthesia represents an effective and well tolerated treatment option for nasal obstruction but did not improve the olfactory function.
Acknowledgments
Conflict of interest
None to declare
References
- 1.Nordin S, Brämerson A. Complaints of olfactory disorders: epidemiology, assessment and clinical implications. Curr Opin Allergy Clin Immunol. 2008;8:10–15. doi: 10.1097/ACI.0b013e3282f3f473. [DOI] [PubMed] [Google Scholar]
- 2.Hummel T, Nordin S. Olfactory disorders and their consequences for quality of life–a review. Acta Oto-Laryngol. 2005;125:116–121. doi: 10.1080/00016480410022787. [DOI] [PubMed] [Google Scholar]
- 3.Damm M, Temmel A, Welge-Lüssen A, et al. Epidemiologie und Therapie von Riechstörungen in Deutschland, Österreich und der Schweiz. HNO. 2004;52:112–120. doi: 10.1007/s00106-003-0877-z. [DOI] [PubMed] [Google Scholar]
- 4.Reden J, Mueller A, Mueller C, et al. Recovery of olfactory function following closed head injuries or infections of the upper respiratory tract. Arch Otolaryngol Head Neck Surg. 2006;132:265–269. doi: 10.1001/archotol.132.3.265. [DOI] [PubMed] [Google Scholar]
- 5.Hummel T, Sekinger B, Wolf SR, et al. Sniffin’ sticks: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22:39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- 6.Kobal G, Klimek L, Wolfensberger M, et al. Multicenter investigation of 1036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur Arch Ororhinolaryngol. 2000;257:205–211. doi: 10.1007/s004050050223. [DOI] [PubMed] [Google Scholar]
- 7.Welge-Lüssen A, Wolfensberger M, Kobal G, et al. Basics, methods and indications for objective olfactometry. Laryngorhinootologie. 2002;81:661–667. doi: 10.1055/s-2002-34449. [DOI] [PubMed] [Google Scholar]
- 8.Hummel T, Klimek L, Welge-Lüssen A, et al. Chemosensory evoked potentials for clinical diagnosis of olfactory disorders. HNO. 2000;48:481–485. doi: 10.1007/s001060050602. [DOI] [PubMed] [Google Scholar]
- 9.Goektas O, Fleiner F, Sedlmaier B, et al. Correlation of olfactory dysfunction of different etiologies in MRI and comparison with subjective and objective olfactometry. Eur J Radiol. 2009;71(3):469–473. doi: 10.1016/j.ejrad.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 10.Caffier PP, Frieler K, Scherer H, et al. Rhinitis medicamentosa: therapeutic effect of diode laser inferior turbinate reduction on nasal obstruction and decongestant abuse. Am J Rhinol. 2008;22:433–439. doi: 10.2500/ajr.2008.22.3199. [DOI] [PubMed] [Google Scholar]
- 11.Clement P. Committee report on standardisation of rhinomanometry. Rhinology. 1984;22:151–155. [PubMed] [Google Scholar]
- 12.Gordon AS, McCaffrey TV, Kern EB, et al. Rhinomanometry for preoperative and postoperative assessment of nasal obstruction. Otolaryngol Head Neck Surg. 1989;101:20–26. doi: 10.1177/019459988910100105. [DOI] [PubMed] [Google Scholar]
- 13.Seiden AM. Olfactory loss secondary to nasal and sinus pathology. In: Seiden AM, editor. Taste and smell disorders. New York: Thieme; 1997. pp. 52–71. [Google Scholar]
- 14.Fukazawa K. A local steroid injection method for olfactory loss due to upper respiratory infection. Chem Senses. 2005;30:212–213. doi: 10.1093/chemse/bjh189. [DOI] [PubMed] [Google Scholar]
- 15.Holm AF, Fokkens PJ. Topical corticosteroids in allergic rhinitis; effects on inflammatory cells and mucosa. Clin Exp Allergy. 2001;31:529–535. doi: 10.1046/j.1365-2222.2001.01091.x. [DOI] [PubMed] [Google Scholar]
- 16.Mygind N, Lildholdt T. Nasal polyps treatment: medical management. Allergy Asthma Proc. 1996;17:275–282. doi: 10.2500/108854196778662228. [DOI] [PubMed] [Google Scholar]
- 17.Lildholdt T, Rundcrantz H, Lindqvist N. Efficacy of topical corticosteroid powder for nasal polyps: a double-blind, placebo-controlled study of budesonide. Clin Otolaryngol. 1995;20:26–30. doi: 10.1111/j.1365-2273.1995.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 18.Ostwald J, Graumüller S, Dommerich S, et al. Influence of rhinologic usual and unusual drugs on fibroblasts from nasal polyps in cell culture. Laryngorhinootologie. 2003;82:408–415. doi: 10.1055/s-2003-40541. [DOI] [PubMed] [Google Scholar]
- 19.Heilmann S, Just T, Goektas O, et al. Effects of systemic or topical administration of corticosteroids and vitamin B in patients with olfactory loss. Laryngorhinootologie. 2004;83:729–734. doi: 10.1055/s-2004-825676. [DOI] [PubMed] [Google Scholar]
- 20.Stuck BA, Blum A, Hagner AE, et al. Mometasone furoate nasal spray improves olfactory performance in seasonal allergic rhinitis. Allergy. 2003;58:1195. doi: 10.1034/j.1398-9995.2003.00162.x. [DOI] [PubMed] [Google Scholar]
- 21.Panagou P, Loukides S, Tsipra S, et al. Evaluation of nasal patency: comparison of patient and clinician assessments with rhinomanometry. Acta Otolaryngol. 1998;118:847–851. doi: 10.1080/00016489850182567. [DOI] [PubMed] [Google Scholar]
- 22.Yaniv E, Hadar T, Shvero J, et al. Objective and subjective nasal airflow. Am J Otolaryngol. 1997;18:29–32. doi: 10.1016/S0196-0709(97)90045-4. [DOI] [PubMed] [Google Scholar]
- 23.Sroka R, Janda P, Killian T, et al. Comparison of long term results after Ho:YAG and diode laser treatment of hyperplastic inferior nasal turbinates. Lasers Surg Med. 2007;39:324–331. doi: 10.1002/lsm.20479. [DOI] [PubMed] [Google Scholar]
- 24.Jackson LE, Koch RJ. Controversies in the management of inferior turbinate hypertrophy: a comprehensive review. Plast Reconstr Surg. 1999;103:300–312. doi: 10.1097/00006534-199901000-00049. [DOI] [PubMed] [Google Scholar]
- 25.Chang CW, Ries WR. Surgical treatment of the inferior turbinate: new techniques. Curr Opin Otolaryngol Head Neck Surg. 2004;12:53–57. doi: 10.1097/00020840-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Feron F, Perry C, McGrath JJ, et al. New techniques for biopsy and culture of human olfactory epithelial neurons. Arch Otolaryngol Head Neck Surg. 1998;124:861–866. doi: 10.1001/archotol.124.8.861. [DOI] [PubMed] [Google Scholar]
- 27.Leopold DA, Hummel T, Schwob JE, et al. Anterior distribution of human olfactory epithelium. Laryngoscope. 2000;110:417–421. doi: 10.1097/00005537-200003000-00016. [DOI] [PubMed] [Google Scholar]