Abstract
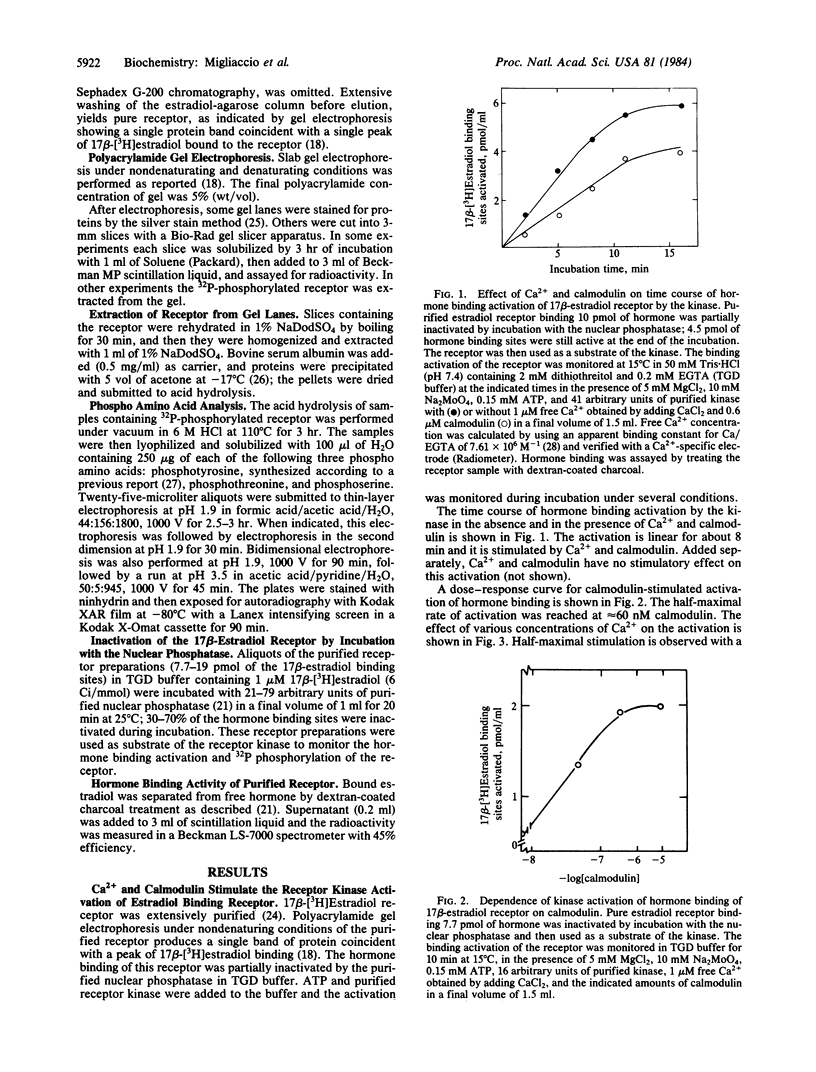
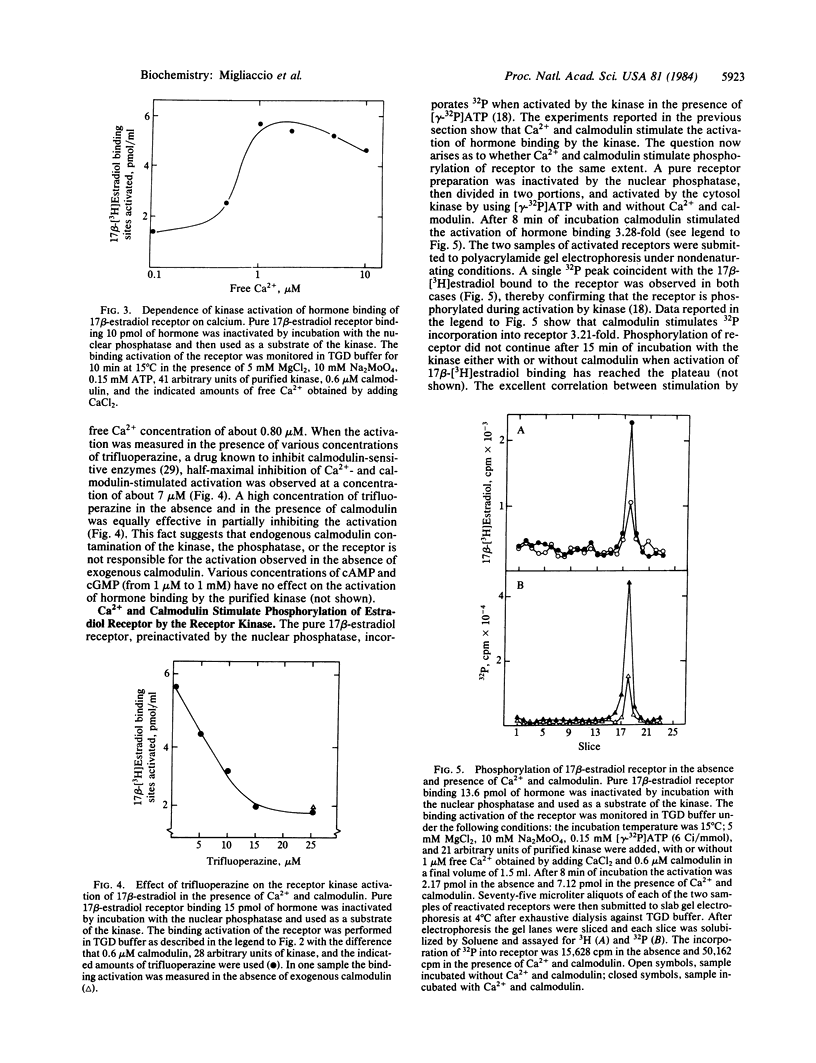
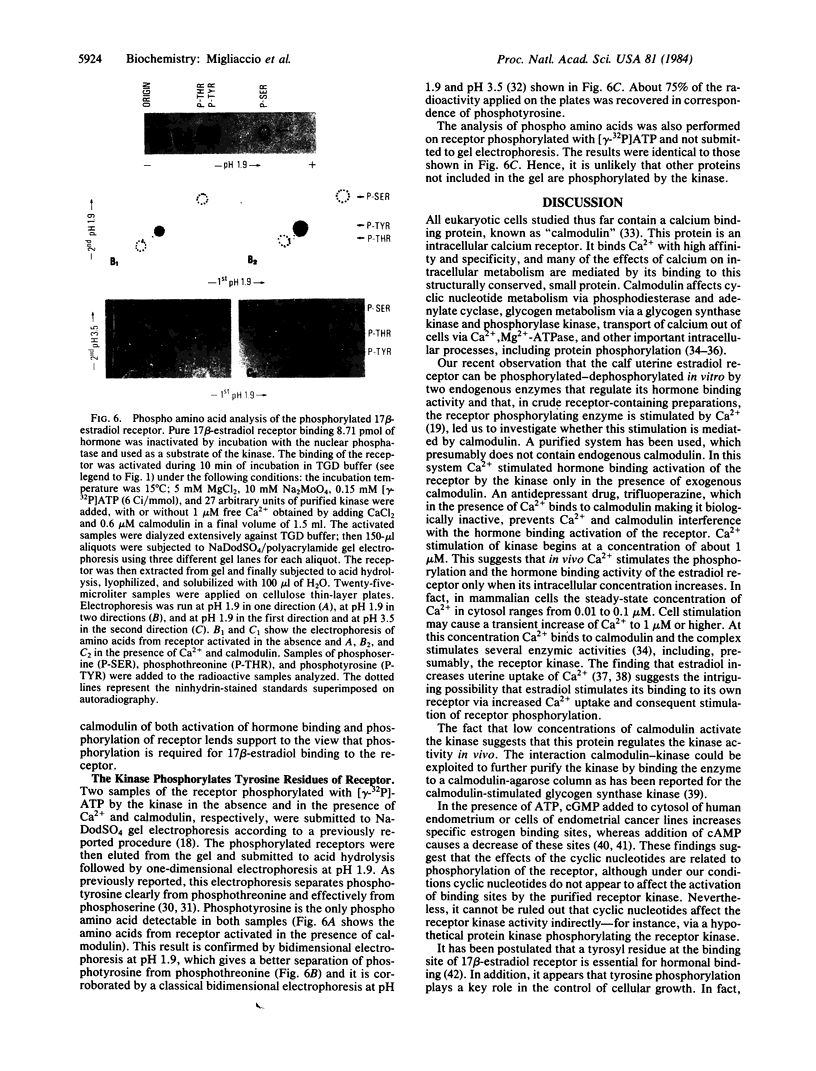
The calf uterine 17 beta-estradiol receptor is a phosphoprotein. Phosphorylation-dephosphorylation of the receptor is controlled by a cytosol receptor kinase that activates the hormone binding and by a nuclear phosphatase that inactivates this binding. This report concerns the nature of the 17 beta-estradiol receptor kinase. Highly purified calf uterus 17 beta-estradiol receptor preinactivated by the nuclear phosphatase was used as substrate of the purified receptor kinase. Ca2+ and calmodulin stimulate both the kinase-dependent activation of the hormone binding and 32P incorporation from [gamma-32P]-ATP into the receptor. Maximal stimulation of hormone binding activation requires 1 microM Ca2+ and 0.6 microM calmodulin. Fifteen micromolar trifluoperazine is the lowest concentration that will prevent completely Ca2+-calmodulin stimulation of the kinase. The receptor is phosphorylated by the receptor kinase exclusively on tyrosine. Phosphorylation of proteins on tyrosine is a rare event implicated in hormone-induced cell growth and cell transformation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad Z., DePaoli-Roach A. A., Roach P. J. Purification and characterization of a rabbit liver calmodulin-dependent protein kinase able to phosphorylate glycogen synthase. J Biol Chem. 1982 Jul 25;257(14):8348–8355. [PubMed] [Google Scholar]
- Auricchio F., Migliaccio A., Castoria G. Dephosphorylation of oestradiol nuclear receptor in vitro. A hypothesis on the mechanism of action of non-steroidal anti-oestrogens. Biochem J. 1981 Sep 15;198(3):699–702. doi: 10.1042/bj1980699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auricchio F., Migliaccio A., Castoria G., Lastoria S., Rotondi A. Evidence that in vivo estradiol receptor translocated into nuclei is dephosphorylated and released into cytoplasm. Biochem Biophys Res Commun. 1982 May 14;106(1):149–157. doi: 10.1016/0006-291x(82)92070-8. [DOI] [PubMed] [Google Scholar]
- Auricchio F., Migliaccio A., Castoria G., Lastoria S., Schiavone E. ATP-dependent enzyme activating hormone binding of estradiol receptor. Biochem Biophys Res Commun. 1981 Aug 31;101(4):1171–1178. doi: 10.1016/0006-291x(81)91571-0. [DOI] [PubMed] [Google Scholar]
- Auricchio F., Migliaccio A. In vitro inactivation of oestrogen receptor by nuclei: prevention by phosphatase inhibitors. FEBS Lett. 1980 Aug 11;117(1):224–226. doi: 10.1016/0014-5793(80)80950-1. [DOI] [PubMed] [Google Scholar]
- Auricchio F., Migliaccio A., Rotondi A. Inactivation of oestrogen receptor in vitro by nuclear dephosphorylation. Biochem J. 1981 Feb 15;194(2):569–574. doi: 10.1042/bj1940569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avruch J., Nemenoff R. A., Blackshear P. J., Pierce M. W., Osathanondh R. Insulin-stimulated tyrosine phosphorylation of the insulin receptor in detergent extracts of human placental membranes. Comparison to epidermal growth factor-stimulated phosphorylation. J Biol Chem. 1982 Dec 25;257(24):15162–15166. [PubMed] [Google Scholar]
- Batra S., Sjögren C. Effect of estrogen treatment on calcium uptake by the rat uterine smooth muscle. Life Sci. 1983 Jan 24;32(4):315–319. doi: 10.1016/0024-3205(83)90076-0. [DOI] [PubMed] [Google Scholar]
- Campbell K. P., MacLennan D. H. A calmodulin-dependent protein kinase system from skeletal muscle sarcoplasmic reticulum. Phosphorylation of a 60,000-dalton protein. J Biol Chem. 1982 Feb 10;257(3):1238–1246. [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Cohen S., Carpenter G., King L., Jr Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980 May 25;255(10):4834–4842. [PubMed] [Google Scholar]
- Dougherty J. J., Puri R. K., Toft D. O. Phosphorylation in vivo of chicken oviduct progesterone receptor. J Biol Chem. 1982 Dec 10;257(23):14226–14230. [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Fewtrell C., Davis C. L., Metzger H. Phosphorylation of the receptor of immunoglobulin E. Biochemistry. 1982 Apr 27;21(9):2004–2010. doi: 10.1021/bi00538a005. [DOI] [PubMed] [Google Scholar]
- Fleming H., Blumenthal R., Gurpide E. Effects of cyclic nucleotides on estradiol binding in human endometrium. Endocrinology. 1982 Nov;111(5):1671–1677. doi: 10.1210/endo-111-5-1671. [DOI] [PubMed] [Google Scholar]
- Fleming H., Blumenthal R., Gurpide E. Rapid changes in specific estrogen binding elicited by cGMP or cAMP in cytosol from human endometrial cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2486–2490. doi: 10.1073/pnas.80.9.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenring J. R., Gonzalez B., McGuire J. S., Jr, DeLorenzo R. J. Purification and characterization of a calmodulin-dependent kinase from rat brain cytosol able to phosphorylate tubulin and microtubule-associated proteins. J Biol Chem. 1983 Oct 25;258(20):12632–12640. [PubMed] [Google Scholar]
- Gordon A. S., Davis C. G., Milfay D., Diamond I. Phosphorylation of acetylcholine receptor by endogenous membrane protein kinase in receptor-enriched membranes of Torpedo californica. Nature. 1977 Jun 9;267(5611):539–540. doi: 10.1038/267539a0. [DOI] [PubMed] [Google Scholar]
- Housley P. R., Pratt W. B. Direct demonstration of glucocorticoid receptor phosphorylation by intact L-cells. J Biol Chem. 1983 Apr 10;258(7):4630–4635. [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalimi M., Colman P., Feigelson P. The "activated" hepatic glucocorticoid-receptor complex. Its generation and properties. J Biol Chem. 1975 Feb 10;250(3):1080–1086. [PubMed] [Google Scholar]
- Kasuga M., Karlsson F. A., Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science. 1982 Jan 8;215(4529):185–187. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- MITCHELL H. K., LUNAN K. D. TYROSINE-O-PHOSPHATE IN DROSOPHILA. Arch Biochem Biophys. 1964 Jul 20;106:219–222. doi: 10.1016/0003-9861(64)90179-1. [DOI] [PubMed] [Google Scholar]
- Means A. R., Chafouleas J. G. Calmodulin in endocrine cells. Annu Rev Physiol. 1982;44:667–682. doi: 10.1146/annurev.ph.44.030182.003315. [DOI] [PubMed] [Google Scholar]
- Means A. R., Dedman J. R. Calmodulin--an intracellular calcium receptor. Nature. 1980 May 8;285(5760):73–77. doi: 10.1038/285073a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Migliaccio A., Lastoria S., Moncharmont B., Rotondi A., Auricchio F. Phosphorylation of calf uterus 17 beta-estradiol receptor by endogenous Ca2+-stimulated kinase activating the hormone binding of the receptor. Biochem Biophys Res Commun. 1982 Dec 15;109(3):1002–1010. doi: 10.1016/0006-291x(82)92039-3. [DOI] [PubMed] [Google Scholar]
- Miller J. B., Toft D. O. Requirement for activation in the binding of progesterone receptor to ATP-Sepharose. Biochemistry. 1978 Jan 10;17(1):173–177. doi: 10.1021/bi00594a025. [DOI] [PubMed] [Google Scholar]
- Moudgil V. K., Eessalu T. E. Activation of estradiol--receptor complex by ATP in vitro. FEBS Lett. 1980 Dec 29;122(2):189–192. doi: 10.1016/0014-5793(80)80434-0. [DOI] [PubMed] [Google Scholar]
- Munck A., Brinck-Johnsen T. Specific and nonspecific physicochemical interactions of glucocorticoids and related steroids with rat thymus cells in vitro. J Biol Chem. 1968 Nov 10;243(21):5556–5565. [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Pietras R. J., Szego C. M. Endometrial cell calcium and oestrogen action. Nature. 1975 Jan 31;253(5490):357–359. doi: 10.1038/253357a0. [DOI] [PubMed] [Google Scholar]
- Puca G. A., Bresciani F. Binding activity of oestrogen receptors destroyed by iodination. Nature. 1970 Mar 28;225(5239):1251–1252. doi: 10.1038/2251251a0. [DOI] [PubMed] [Google Scholar]
- Puca G. A., Medici N., Molinari A. M., Moncharmont B., Nola E., Sica V. Estrogen receptor of calf uterus: an easy and fast purification procedure. J Steroid Biochem. 1980 Jan;12:105–113. doi: 10.1016/0022-4731(80)90259-9. [DOI] [PubMed] [Google Scholar]
- Ramakrishna S., Pucci D. L., Benjamin W. B. ATP-citrate lyase kinase and cyclic AMP-dependent protein kinase phosphorylate different sites on ATP-citrate lyase. J Biol Chem. 1981 Oct 25;256(20):10213–10216. [PubMed] [Google Scholar]
- Sando J. J., Hammond N. D., Stratford C. A., Pratt W. B. Activation of thymocyte glucocorticoid receptors to the steroid binding form. The roles of reduction agents, ATP, and heat-stable factors. J Biol Chem. 1979 Jun 10;254(11):4779–4789. [PubMed] [Google Scholar]
- Sando J. J., La Forest A. C., Pratt W. B. ATP-dependent activation of L cell glucocorticoid receptors to the steroid binding form. J Biol Chem. 1979 Jun 10;254(11):4772–4778. [PubMed] [Google Scholar]
- Teichberg V. I., Sobel A., Changeux J. P. In vitro phosphorylation of the acetylcholine receptor. Nature. 1977 Jun 9;267(5611):540–542. doi: 10.1038/267540a0. [DOI] [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- Weigel N. L., Tash J. S., Means A. R., Schrader W. T., O'Malley B. W. Phosphorylation of hen progesterone receptor by cAMP dependent protein kinase. Biochem Biophys Res Commun. 1981 Sep 16;102(1):513–519. doi: 10.1016/0006-291x(81)91549-7. [DOI] [PubMed] [Google Scholar]
- Weiss B., Levin R. M. Mechanism for selectively inhibiting the activation of cyclic nucleotide phosphodiesterase and adenylate cyclase by antipsychotic agents. Adv Cyclic Nucleotide Res. 1978;9:285–303. [PubMed] [Google Scholar]





