Abstract
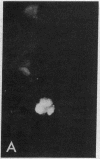
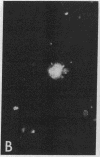
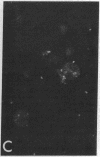
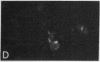
Purified EBV DNA and cloned DNA fragments were trapped in Sendai virus (SV) envelopes during envelope reconstitution. The DNA-loaded reconstituted envelopes (RSVE/DNA) served as gene-transfer vehicles using the capability of RSVE to fuse with normal and tumor cells. The efficiency of RSVE-mediated EBV DNA transfer into lymphoid tumor cells and fresh human lymphocytes was 5-10% of the enveloped 3H-labeled EcoRI fragment B of EBV DNA. Purified intracellular EBV (B95-8 strain) DNA induced EBV nuclear antigen (EBNA) in 0.2-1% of human lymphocytes, transiently stimulated cellular DNA synthesis, but did not fully transform cells. Cloned Sal I F1 fragment [approximately equal to 9 kilobase pairs (kbp)] and a smaller BamHI K (5.2 kbp) fragment from the same region of B95-8 EBV DNA induced EBNA in 2-4% of human lymphocytes but did not stimulate DNA synthesis nor transform cells. Cloned BamHI D1 fragment (approximately equal to 9 kbp) from AG-876 virus DNA, or a combination of cloned BamHI X and H fragments (approximately equal to 2 and 7 kbp, respectively) from the similar region of B95-8 virus DNA, significantly stimulated lymphocyte DNA synthesis, but EBNA could not be detected and transformation was not achieved. Early antigen and viral capsid antigen were not observed with any of the fragments tested. Our results suggest that the induction of EBNA and stimulation of lymphocyte proliferation are not controlled by the same region of EBV DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornkamm G. W., Delius H., Zimber U., Hudewentz J., Epstein M. A. Comparison of Epstein-Barr virus strains of different origin by analysis of the viral DNAs. J Virol. 1980 Sep;35(3):603–618. doi: 10.1128/jvi.35.3.603-618.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambaugh T., Beisel C., Hummel M., King W., Fennewald S., Cheung A., Heller M., Raab-Traub N., Kieff E. Epstein-Barr virus (B95-8) DNA VII: molecular cloning and detailed mapping. Proc Natl Acad Sci U S A. 1980 May;77(5):2999–3003. doi: 10.1073/pnas.77.5.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R., Boyd A., Stoerker J., Holliday J. Functional mapping of the Epstein-Barr virus genome: identification of sites coding for the restricted early antigen, the diffuse early antigen, and the nuclear antigen. Virology. 1983 Aug;129(1):188–198. doi: 10.1016/0042-6822(83)90405-1. [DOI] [PubMed] [Google Scholar]
- Graessmann A., Wolf H., Bornkamm G. W. Expression of Epstein-Barr virus genes in different cell types after microinjection of viral DNA. Proc Natl Acad Sci U S A. 1980 Jan;77(1):433–436. doi: 10.1073/pnas.77.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M., Dambaugh T., Kieff E. Epstein-Barr virus DNA. IX. Variation among viral DNAs from producer and nonproducer infected cells. J Virol. 1981 May;38(2):632–648. doi: 10.1128/jvi.38.2.632-648.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy K., Heller M., van Santen V., Kieff E. Simple repeat array in Epstein-Barr virus DNA encodes part of the Epstein-Barr nuclear antigen. Science. 1983 Jun 24;220(4604):1396–1398. doi: 10.1126/science.6304878. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M., Kieff E. Mapping of polypeptides encoded by the Epstein-Barr virus genome in productive infection. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5698–5702. doi: 10.1073/pnas.79.18.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Gergely L., Goldstein G. Two-colour immunofluorescence studies on EBV-determined antigens. Clin Exp Immunol. 1971 Apr;8(4):593–602. [PMC free article] [PubMed] [Google Scholar]
- Klein G., Giovanella B., Westman A., Stehlin J. S., Mumford D. An EBV-genome-negative cell line established from an American Burkitt lymphoma; receptor characteristics. EBV infectibility and permanent conversion into EBV-positive sublines by in vitro infection. Intervirology. 1975;5(6):319–334. doi: 10.1159/000149930. [DOI] [PubMed] [Google Scholar]
- Klein G., Luka J., Zeuthen J. Transformation induced by Epstein-Barr virus and the role of the nuclear antigen. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):253–261. doi: 10.1101/sqb.1980.044.01.029. [DOI] [PubMed] [Google Scholar]
- Laudano A. P., Doolittle R. F. Influence of calcium ion on the binding of fibrin amino terminal peptides to fibrinogen. Science. 1981 Apr 24;212(4493):457–459. doi: 10.1126/science.7209542. [DOI] [PubMed] [Google Scholar]
- Loyter A., Vainstein A., Graessmann M., Graessmann A. Fusion-mediated injection of SV40-DNA. Introduction of SV40-DNA into tissue culture cells by the use of DNA-loaded reconstituted Sendai virus envelopes. Exp Cell Res. 1983 Feb;143(2):415–425. doi: 10.1016/0014-4827(83)90068-x. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Comparison of the yield of infectious virus from clones of human and simian lymphoblastoid lines transformed by Epstein-Barr virus. J Exp Med. 1973 Dec 1;138(6):1398–1412. doi: 10.1084/jem.138.6.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab-Traub N., Dambaugh T., Kieff E. DNA of Epstein-Barr virus VIII: B95-8, the previous prototype, is an unusual deletion derivative. Cell. 1980 Nov;22(1 Pt 1):257–267. doi: 10.1016/0092-8674(80)90173-7. [DOI] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Rymo L. Identification of transcribed regions of Epstein-Barr virus DNA in Burkitt lymphoma-derived cells. J Virol. 1979 Oct;32(1):8–18. doi: 10.1128/jvi.32.1.8-18.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro I. M., Klein G., Volsky D. J. Epstein-Barr virus co-reconstituted with Sendai virus envelopes infects Epstein-Barr virus-receptor negative cells. Biochim Biophys Acta. 1981 Aug 5;676(1):19–24. doi: 10.1016/0304-4165(81)90004-0. [DOI] [PubMed] [Google Scholar]
- Stoerker J., Glaser R. Rescue of transforming Epstein-Barr virus (EBV) from EBV-genome-positive epithelial hybrid cells transfected with subgenomic fragments of EBV DNA. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1726–1729. doi: 10.1073/pnas.80.6.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoerker J., Holliday J. E., Glaser R. Identification of a region of the Epstein-Barr virus (B95-8) genome required for transformation. Virology. 1983 Aug;129(1):199–206. doi: 10.1016/0042-6822(83)90406-3. [DOI] [PubMed] [Google Scholar]
- Stoerker J., Parris D., Yajima Y., Glaser R. Pleiotropic expression of Epstein--Barr virus DNA in human epithelial cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5852–5855. doi: 10.1073/pnas.78.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. P., Grogan E. A., Shedd D., Robert M., Liu C. R., Miller G. Stable expression in mouse cells of nuclear neoantigen after transfer of a 3.4-megadalton cloned fragment of Epstein-Barr virus DNA. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5688–5692. doi: 10.1073/pnas.79.18.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volsky D. J., Loyter A. An efficient method for reassembly of fusogenic Sendai virus envelopes after solubilization of intact virions with Triton X-100. FEBS Lett. 1978 Aug 15;92(2):190–194. doi: 10.1016/0014-5793(78)80751-0. [DOI] [PubMed] [Google Scholar]
- Volsky D. J., Shapiro I. M., Klein G. Transfer of Epstein-Barr virus receptors to receptor-negative cells permits virus penetration and antigen expression. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5453–5457. doi: 10.1073/pnas.77.9.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]