Abstract
First branchial cleft anomalies (FBCA) represent a small subset of congenital malformations in neck. Prime objective of this study is to share our experience with FBCA, emphasize its relevance in otolaryngology and deal with its pediatric perspective. Embryology, pathologic anatomy and varied spectra of clinical presentations of FBCA are discussed. Along with this we have illustrated three different cases; all of them were of pediatric age group and were misdiagnosed by their treating specialists elsewhere. In this article we have also laid special emphasis on its pediatric considerations. FBCA are mostly misdiagnosed due to their unfamiliar clinical signs and symptoms. Swellings may masquerade as other neck masses. Majority of patients give a history of previous incision and drainage. While dealing with pediatric patients the important factors to be kept in mind are the age of child, superficial course of facial nerve, any associated agenesis of parotid gland. Alteration in surgical technique may be required in children. A thorough medical examination with high index of clinical suspicion should be kept in mind while dealing with such anomalies. Owing to their complex presentation and close relation with facial nerve they are challenging lesions for surgeons.
Keywords: Branchial cleft, Anomaly, Multifaceted presentations, Pediatric considerations
Introduction
First branchial cleft anomalies (FBCA) originate from branchial apparatus and are very unusual cause of congenital neck swellings. Embryologically branchial apparatus is a transient structure that is formed between 4th and 7th weeks of fetal development. Its incomplete obliteration results in development of these anomalies [1]. Branchial cleft anomalies can manifest in different morphologic patterns such as a cyst (a closed pouch with no opening), a sinus (a tract with one opening), or a fistula (a tract with two openings) [2]. Of all the branchial cleft anomalies majority are second branchial cleft defects. FBCA form just 1–8% of these defects [3, 4]. The annual incidence of FBCA has been cited as 1 per 1,000,000 [3, 5] and they are commoner in females (69%) as compared with males (31%) [3]. FBCA have been categorized into two types. Type I anomalies are cysts or sinus opening medial, inferior, or possibly posterior to conchal cartilage and pinna. Tracts, when present, run parallel to the external auditory meatus. Type II anomalies are regarded as duplication of membranous and cartilaginous parts of external auditory canal. They consist of a fistula running from floor of ear canal to neck with opening of the sinus being localized above the hyoid bone anterior to the sternocleidomastoid muscle. Type II FBCA are more common than type I [6]. In either case, the course of the tract is quite variable.
The clinical features vary from recurrent periauricular swelling, a sinus in neck, sinus in external auditory meatus presenting as ear discharge or fistula below angle of mandible. Their rarity and diverse presentation often leads to misdiagnosis resulting into inappropriate treatment. Eventually, diagnosis of FBCA is often made only after multiple incision and drainage procedures have been performed on what is thought to be a simple cyst or periauricular node. Successful treatment necessitates a thorough understanding of the complex embryology involved in the pathogenesis of these anomalies, close relationship of the branchial anomaly to the facial nerve and wide range of clinical presentation of such branchial anomalies.
Embryology and Pathologic Anatomy
The complex embryology of neck almost guarantees a plethora of congenital anomalies. Proper knowledge of regional embryology and pathologic anatomy play key role in establishing an early diagnosis at initial presentation.
Branchial apparatus was first described by VonBaer in 1827 but developmental anomalies were credited by Von Ascheron [7]. This apparatus resembles primitive gill slits [8] having six transverse mesodermal arches divided by five clefts (ectoderm) externally and five pouches (endoderm) internally. A closing membrane is located at the interface between the pouches and the clefts. By the 7th week, these arches fuse and the corresponding clefts are obliterated.
FBCA originate from subtotal closure of ectodermal portion of the first branchial cleft. Whether the defect is a fistula, sinus, or cyst depends on the degree of closure. The anomaly begins on the floor of the external auditory canal either at the level of the bony-cartilaginous junction or in the cartilaginous portion, follows the seam between the mandibular and hyoid arches, and ends in the submandibular region more or less distally, depending on the extent of the disturbance of the fusion. Since the obliteration of cleft proceeds from ventral to dorsal side thus the chances of occurrence of malformations near the ear and parotid region are greater than that occurring at the hyoid area [9].
FBCA have intimate and variable association with parotid gland and facial nerve. This can be attributed to somewhat later (6th–8th week) embryologic development of the gland and upward migration of facial nerve as compared to branchial cleft obliteration. The time of onset of the anomaly in relation to the change in character of the lateral surface of the fetal pharynx may well be an important factor in determining the final course.
Several classifications of these anomalies have been proposed to assist clinical diagnosis. Arnot [10] described the first anatomic classification for FBCA in 1971. He described type I defect as cyst or sinus in parotid gland, lined by squamous epithelium and manifesting in early or middle adult life. He proposed that these anomalies developed due to embryonic cell rests buried during obliteration of cleft. Type II anomalies (cyst or sinus) present during childhood in anterior triangle of neck and often have communication with external auditory canal. These anomalies emerge due to incomplete obliteration of cleft.
Later on basis of their histology, Work [11] in 1972, classified FBCA into two types; type I and type II. Type I FBCA are usually seen in younger adults and are ectodermal in origin. They are considered to be duplication of the membranous external auditory canal and travel parallel to the external auditory canal ending in a cul-de-sac on or near a bony plate at the level of mesotympanum. If infected, they can open inferior, medial, or posterior to the pinna. In most of the cases they present as a peri-auricular cyst without external communication. Histologically these are lined by simple squamous epithelium with no mesenchymal elements. They usually lie lateral to the facial nerve. Type II FBCA usually presents in early childhood and have both ectodermal and mesodermal origin. They are regarded as duplication of both membranous and cartilaginous parts of external canal and contain skin with adnexal structures. Here the fistula runs from floor of the external auditory canal to the upper neck below the mandible passing through the parotid gland. It may lie either medial, lateral or in between branches of the facial nerve. The cysts when present are closely associated with the parotid gland and if infected, they usually drain near the angle of mandible. Type I anomalies are very rare, type II being more frequent. The middle ear is normal in both types of FBCA with rare exceptions [12, 13].
Karmody [14], further extended the concept of FBCA including congenital anomalies of the external ear. He proposed four types of anomalies including aplasia, atresia, stenosis, and duplication. In 1980, Olsen et al. [15] proposed a simplified classification subdividing the FBCA into cysts, sinuses, and fistulas. Classification proposed by Karmody and Olsen et al. has been found to be clinically more relevant [5].
Clinical Presentation
Patients with branchial cleft cysts are usually older children or young adults in contrast to patients with fistula, who are usually infants or young children [16]. Triglia et al. [9] noted a delay of 3.5 years between the initial presentation and adequate treatment. This can be attributed to its multifaceted presentation because of which the patient lands up with physician, pediatrician, or general surgeon before reaching an otolaryngologist. The varied spectra of manifestations include:
Otologic Manifestations
FBCA may manifest as cysts or sinuses in relation to the external auditory canal and auricle [17]. The most frequent otological manifestation is otorrhoea or recurrent otitis externa with infective exacerbation. Along with this there may be complaints of episodic swelling draining into external auditory canal. Patients may complain of hearing loss and sensation of stuffiness in the ear either due to obstruction of ear canal or due to oedema associated with otitis externa. Usual sites of external opening of fistulas and sinuses are external auditory canal (40%), upper neck (32.5%), concha (20%), and post-auricular area (7.5%) [3].Other sites include middle ear, parapharyngeal space [18, 19] and Eustachian tube [20]. A sinus or fistulous opening may manifest as a pit at the site of its entrance commonly into the external auditory canal. The ear canal may be partially or completely obstructed by peri-auricular cystic swelling pushing the canal walls. There may however be complete absence of signs in the external auditory canal. Sichel et al. [21] stated that type II FBCA were associated with a myringeal web i.e. an epidermal web extending from floor of external auditory canal to umbo or pars tensa of the tympanic membrane [9, 21, 22]. Usually tympanic membrane and middle ear are normal. Yalcin et al. [22] reported a case where a first branchial cleft lesion was associated with cholesteatoma of mastoid and middle ear, microtia and aural atresia. Bilateral ear involvement in FBCA has also been reported [19].
Thus a thorough otological examination must be exercised in all patients having complains of recurrent or chronic otorrhoea (mucopurulent/purulent) in absence of middle/external ear infection.
Head and Neck Manifestations
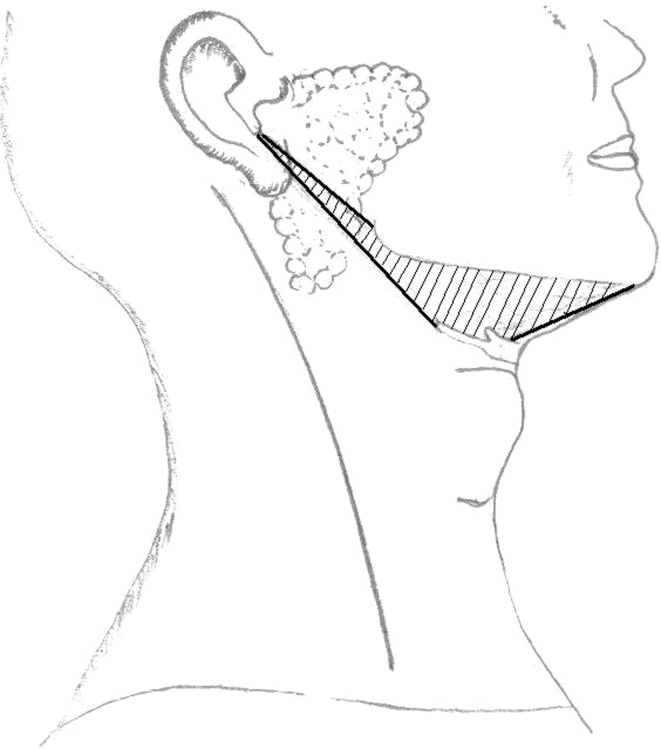
The commonest head and neck manifestation is swelling in the periauricular (24%), parotid (35%) or cervical region (35%) [9, 23]. Anatomically first branchial cleft cysts or their sinus orifice are located in Pochet’s triangle [21]. This triangle (Fig. 1) is bounded by external auditory canal superiorly, the mental region anteriorly and the hyoid bone inferiorly. They are often accompanied with repeated episodes of infection. Pathognomonic of Type II anomaly is a history of multiple incision and drainage for an abscess [24]. Recurrent surgical incision and drainage before definitive surgical procedure has been reported in 35–48% of cases [9, 25]. These anomalies are usually found close to the superficial lobe of parotid gland, majority of cysts occur in region of lower pole. They are frequently misdiagnosed as benign parotid tumors or innocent superficial sinus or cysts thus contributing to long clinical course and delays in diagnosis and treatment [15].
Fig. 1.
Pochet’s triangle bounded superiorly by external auditory canal, anteriorly by mental region and inferiorly hyoid bone, lying anterior to sternocleidomastoid muscle. Anatomically first branchial cleft cysts or their sinus orifice are located in this triangle
The relation between the facial nerve and the sinus or fistulous tract seems to be variable. The course of fistulous tracts in relation to facial nerve can be lateral (41%), medial (37%), or between the branches (22%) whereas sinus tracts usually have a superficial course (76%) [3].
Further adding to the difficulty in diagnosis is lack of association between these anomalies and other facial malformations.
Pediatric Considerations
The best possible outcome necessitates early diagnosis at primary presentation and necessary surgical expertise. The mainstay of diagnosis is thorough clinical examination with high suspicion on its occurrence. While managing these lesions, it is prudent to remember that any excisional procedure in acute inflammatory stage may complicate the future definitive treatment. The recurrence rate of these lesions is doubled by infection, incomplete resection and non-curative interventions. A superficial parotidectomy with a wide exposure under general anaesthesia is crucial for the complete removal of these lesions. Surgical approach must meet the criteria to provide wide exposure of the entire area, allow excision of the fistulous or sinus tract with careful identification and preservation of facial nerve and minimize the resulting surgical scar by giving incision along the lines of contour of involved cervico-facial area [26]. It is advised that if branchial cleft anomalies are noted in newborns or just after then, operation plans should be deferred up to 6 months of age due to their undeveloped auricular cartilage and mastoid. Their variable relationship with the facial nerve and scarring due to previous infections can make the excision difficult especially in pediatric age group. Dissection and complete removal are easy in absence of any previous infection and surgery can be safely performed at any time after the 6 months of age [27]. In young children the facial nerve is more prone to injuries as it lies more superficially, and surgical landmarks to correctly locate it may be somewhat difficult to find. Thus for wide and safe excision a modification in usual surgical methods may be required [28]. Murthy et al. [28] proposed a retroauricular incision in children to avoid injury of the facial nerve. A sinus or fistulous opening in the external auditory canal requires excision along with skin and cartilage. If the tympanic membrane or middle ear structures are encroached, reconstructive ear surgery may be needed [12].
Another difficult condition while surgery, especially in children is the agenesis of parotid gland. This can be suspected if there is presence of a dimple anterior to tragus [28] and it can be confirmed on CT scan.
Illustrative Cases
We have illustrated three cases of FBCA, all of pediatric age group. Common factor among all three cases is the misdiagnosis and definitive treatment delay in these patients.
Case 1
A 13 year old boy presented in our out patient department with complaints of recurrent episodes of painful swelling in right parotid region since 2 years for which he underwent incision and drainage twice in past. On examination the swelling was 2 × 2 cm, firm in consistency, slightly tender, mobile and the overlying skin had scar marks of previous incision and drainage. His previous records revealed three FNAC reports, two suggestive of chronic nonspecific sialoadenitis and third had features suggestive of an abscess. Thus the patient was being treated by a pediatrician for the same since past two years. The FNAC in our institute was suggestive of an infected retention cyst. CT scan revealed cystic lesion in superficial lobe of parotid with wall enhancement suggesting the possibilities of abscess or infected retention cyst with inflamed parotid tissue. Patient was subjected to superficial parotidectomy. Intraoperatively (Fig. 2) a cystic mass was found in superficial lobe of parotid lying partly superficial and partly deep to facial nerve. Along with this there was an aberrant facial nerve. Superficial parotidectomy was done preserving the facial nerve and specimen was sent for HPE. His biopsy report revealed Work’s type I FBCA with chronic sialoadenitis.
Fig. 2.
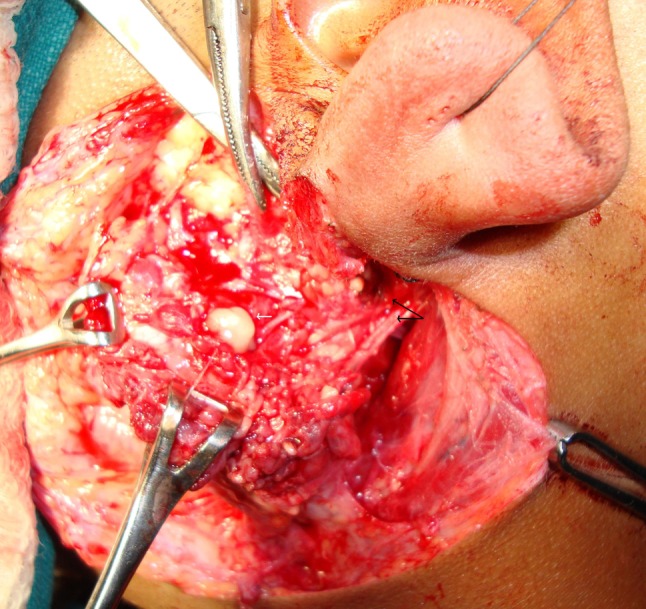
Pre-operative photograph depicting a branchial cyst in superficial lobe of parotid gland (white arrow) with aberrant facial (black arrow depicting two trunks of nerve coming out of stylomastoid foramen)
Case 2
A 9 year old boy presented to us with complaints of unilateral recurrent right ear discharge since 5 years. There were no complaints of reduced hearing, tinnitus, or vertigo. The child was diagnosed as a case of recurrent otitis externa by a private practitioner and was being treated for same since past 4 years. His examination revealed a pit in the floor of cartilaginous part of right external auditory canal (Fig. 3) and tympanic membrane had a myringeal web (Fig. 3) running from posterior quadrant of pars tensa to floor of external auditory canal. A sinus was seen in the right anterior triangle of neck lying just lateral to hyoid bone (Fig. 3). Sinogram revealed tract ascending upwards towards the ear. Thus a diagnosis of FBCA was established and after performing required investigations including CT scan surgical exploration was planned for the patient. Intraoperatively the tract extended upwards from neck passing through the superficial lobe of parotid gland, lying lateral to facial nerve terminating into cartilaginous part of ear canal. Thus whole of tract along with superficial lobe of parotid gland and a cuff of cartilaginous part of external auditory canal were removed. The biopsy report revealed Work’s type II FBCA.
Fig. 3.
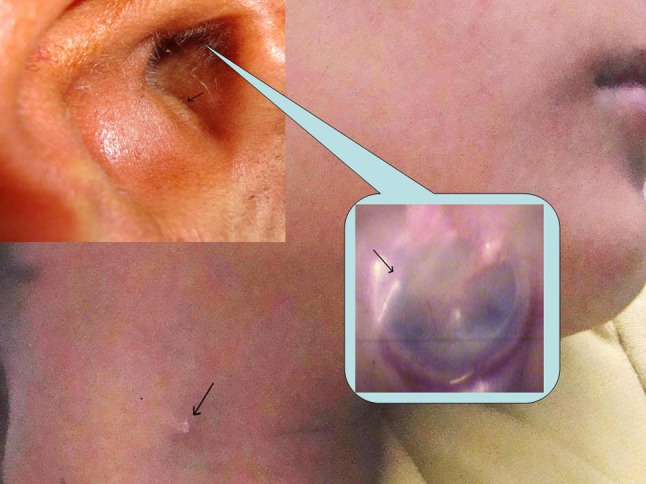
Photograph depicting pit in floor of right external auditory canal, myringeal web running from posterior quadrant of tympanic membrane to floor of external auditory canal and sinus in upper part of neck
Case 3
A boy 15 years of age came to us with complaints of recurrent painful swelling in right post auricular region since past 10 years. The child was being treated by a general surgeon. He had a history of multiple incision and drainage for the same. Despite treatment the swelling recurred and thus the patient was referred to us. On examination there was a sinus (Fig. 4) just anterior to mid of tragus and an inflamed swelling present in post auricular region with central fluctuations. On pressing the swelling purulent discharge could be seen coming out of the sinus. The external auditory canal and tympanic membrane were normal. Clinical diagnosis of FBCA was established and after aspirating the pus he was given a course of broad spectrum antibiotics and analgesics. The patient was subjected to surgery once the infection subsided. Post auricular incision was given and cyst was dissected free from its attachments a tract was found extending from it along the floor of cartilaginous part of external auditory canal ending up in the sinus in preauricular region. Cyst along with tract was excised. His histopathology report confirmed the diagnosis of FBCA.
Fig. 4.
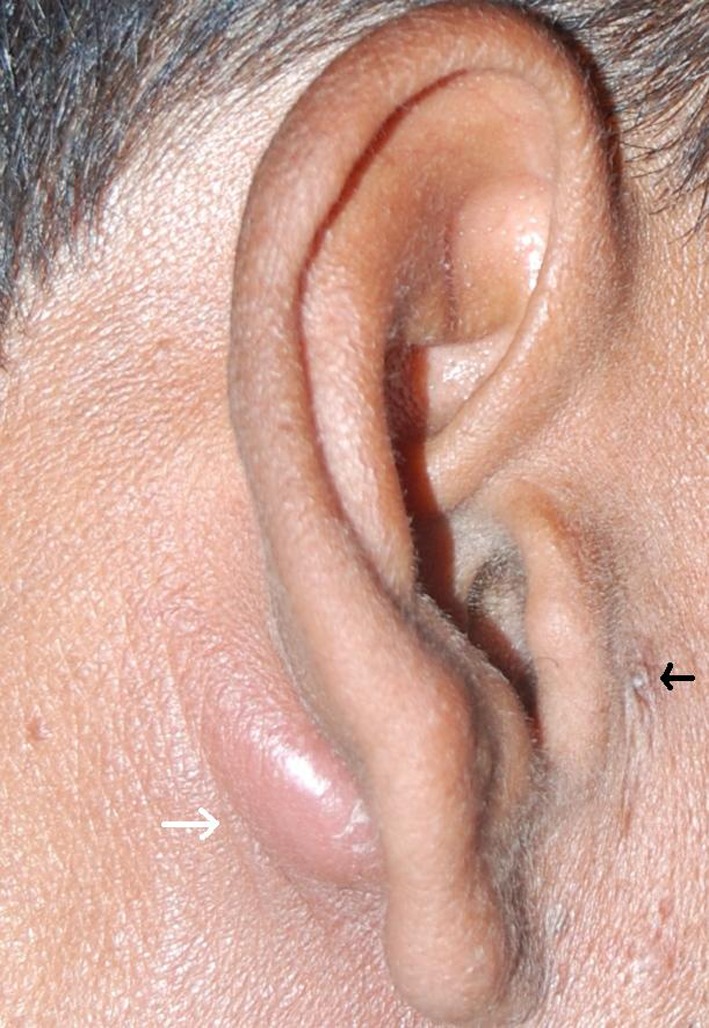
Depicting preauricular discharging sinus (black arrow) with post auricular infected cyst (white arrow)
All three patients are maintaining a regular follow-up and are disease free till date.
Conclusion
As otolaryngologists we deal commonly with second and third branchial cleft anomalies, occurrence of FBCA is relatively rare. Since these anomalies exhibit a wide spectrum of clinical presentation, their correct early diagnosis may be difficult. Due to their complex presentation and close relation with facial nerve they are challenging lesions for surgeons.
References
- 1.Wilson DB. Embryonic development of the head and neck. Part II: the branchial region. Head Neck Surg. 1979;2:59–66. doi: 10.1002/hed.2890020109. [DOI] [PubMed] [Google Scholar]
- 2.Chandler JR, Mitchell B. Branchial cleft cysts, sinuses and fistulas. Otolaryngol Clin North Am. 1981;14:175–186. [PubMed] [Google Scholar]
- 3.D’Souza AR, Uppal HS, De R, Zeitoun H. Updating concepts of the first branchial cleft defects: a literature review. Int J Pediatr Otorhinolaryngol. 2002;62:103–109. doi: 10.1016/S0165-5876(01)00612-7. [DOI] [PubMed] [Google Scholar]
- 4.Ford G, Balakrishnan A, Evans J, et al. Branchial cleft and pouch anomalies. J Laryngol Otol. 1992;106:137–143. doi: 10.1017/S0022215100118900. [DOI] [PubMed] [Google Scholar]
- 5.Arndal H, Bonding P. First branchial cleft anomaly. Clin Otolaryngol Allied Sci. 1996;21:203–207. doi: 10.1111/j.1365-2273.1996.tb01725.x. [DOI] [PubMed] [Google Scholar]
- 6.Finn DG, Buchalter IH, Sarti E, Romo T, Chodosh P. First branchial cleft cysts: clinical update. Laryngoscope. 1987;97:136–140. doi: 10.1288/00005537-198702000-00003. [DOI] [PubMed] [Google Scholar]
- 7.De PR, Mikhail T. A combined approach excision of branchial fistula. J Laryngol Otol. 1995;109:999–1000. doi: 10.1017/S002221510013186X. [DOI] [PubMed] [Google Scholar]
- 8.Drumm AJ, Chow JM. Congenital neck masses. Am Fam Physician. 1989;39:159–163. [PubMed] [Google Scholar]
- 9.Triglia JM, Nicollas R, Ducroz V, Koltai PJ, Garabedian EN. First branchial cleft anomalies: a study of 39 cases and review of the literature. Arch Otolaryngol Head Neck Surg. 1998;124:291–295. doi: 10.1001/archotol.124.3.291. [DOI] [PubMed] [Google Scholar]
- 10.Arnot RS. Defects of the first branchial cleft. S Afr J Surg. 1971;9:93–98. [PubMed] [Google Scholar]
- 11.Work WP. Newer concepts of first branchial cleft defects. Laryngoscope. 1972;82:1581–1593. doi: 10.1288/00005537-197209000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Tom L, Kenealy J, Torsiglieri A. First branchial cleft anomalies involving the tympanic membrane and middle ear. Otolaryngol Head Neck Surg. 1991;105:473–477. doi: 10.1177/019459989110500321. [DOI] [PubMed] [Google Scholar]
- 13.Cremers CW. Congenital pre-auricular fistula communicating with the tympanic cavity. J Laryngol Otol. 1983;97:749–753. doi: 10.1017/S0022215100094925. [DOI] [PubMed] [Google Scholar]
- 14.Karmody CS. A classification of the anomalies of the first branchial groove. Otolaryngol Head Neck Surg. 1979;87:334–338. doi: 10.1177/019459987908700310. [DOI] [PubMed] [Google Scholar]
- 15.Olsen KD, Maragos NE, Weiland LH. First branchial cleft anomalies. Laryngoscope. 1980;90:423–436. doi: 10.1002/lary.5540900309. [DOI] [PubMed] [Google Scholar]
- 16.Teleander R, Deane S. Thyroglossal and branchial cleft cysts and sinuses. Surg Clin N Am. 1977;57:779–791. doi: 10.1016/s0039-6109(16)41288-0. [DOI] [PubMed] [Google Scholar]
- 17.Graham MD, Kemink JL. First branchial cleft cyst presenting as a mass within the external auditory canal. Am J Otolaryngol. 1985;6:500–502. [PubMed] [Google Scholar]
- 18.Mukherji SK, Tart RP, Slattery WH, Stringer SP, Benson MT, Mancuso AA. Evaluation of the first branchial anomalies by CT and MR. J Comput Assist Tomogr. 1993;17:576–581. doi: 10.1097/00004728-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Shirley DM, Santos PM. An unusual first branchial cleft defect. Otolaryngol Head Neck Surg. 1995;113:829–832. doi: 10.1016/S0194-5998(95)70034-X. [DOI] [PubMed] [Google Scholar]
- 20.Shaw A, Santulli TV, Rankow R. Cysts and sinuses of the first branchial cleft and pouch. Surg Gynecol Obstet. 1962;115:671–676. [PubMed] [Google Scholar]
- 21.Sichel JY, Halperin D, Dano I, Dangoor E. Clinical update on type II first branchial cleft cysts. Laryngoscope. 1998;108:1524–1527. doi: 10.1097/00005537-199810000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Yalcin S, Karlidag T, Kaygusuz I, Demirbag E. First branchial cleft sinus presenting with cholesteatoma and external auditory canal atresia. Int J Pediatr Otorhinolaryngol. 2003;67:811–814. doi: 10.1016/S0165-5876(03)00074-0. [DOI] [PubMed] [Google Scholar]
- 23.Ku WY, Wang KJ, Jou YL, Chang YH, Chou CS. TypeII first branchial cleft anomaly—a case report. Tzu Chi Med J. 2005;17:357–360. [Google Scholar]
- 24.McRae RG, Lee KJ, Goertzen E. First branchial cleft anomalies and the facial nerve. Otolaryngol Head Neck Surg. 1983;91:197–201. doi: 10.1177/019459988309100216. [DOI] [PubMed] [Google Scholar]
- 25.Choi SS, Zalzal GH. Branchial anomalies: a review of 52 cases. Laryngoscope. 1995;105:909–913. doi: 10.1288/00005537-199509000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Trail ML, Lyons GD, Jr, Greely JJ., Jr Anomalies of the first branchial cleft. South Med J. 1972;65(6):716–720. doi: 10.1097/00007611-197206000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Jones PG. Branchial sinuses and cysts. In: Rob C, Smith R, editors. Operative surgery (head neck and lymph nodes) vol 6. 2. London: Butterworths; 1969. p. 19. [Google Scholar]
- 28.Murthy P, Shenoy P, Khan NA. First branchial fistula in a child-a modified surgical technique. J Laryngol Otol. 1994;108:1078–1080. doi: 10.1017/s0022215100128932. [DOI] [PubMed] [Google Scholar]