Abstract
Inflammatory infiltration with eosinophilia in or around the tumoral tissue varies among the cases with invasive squamous cell carcinoma of the larynx. The aim of this study was to investigate the possible role of the tumor-associated tissue eosinophilia (TATE) as a predictive factor for the metastatic status in laryngeal squamous cell carcinoma in patients who had neck dissections. One hundred consecutive specimens from the patients who had been treated surgically for invasive squamous cell carcinoma of the larynx were re-evaluated in terms of TATE. Based on the eosinophil counts per 10 high power field (HPF), the cases were grouped into three different categories (I, II, III) according to three different cut off values (A, B, C). The number of eosinophil cells per 10 HPF for the groups were defined as: IA: 0–10; IB: 11–29; IC: 30 and greater; IIA: 0–20; IIB: 21–39; IIC: 40 and greater; IIIA: 0–30; IIIB: 31–49; IIIC: 50 and greater. Statistical significance between tissue eosinophil counts of the metastatic and non-metastatic lymph node groups were evaluated. This study comprised 97 male and three female patients with squamous cell carcinoma of the larynx (mean age 59.9). Forty-five were well differentiated, 50 were moderately differentiated and five were poorly differentiated invasive squamous cell carcinoma. At least one lymph node metastasis was observed in 34 cases. Eosinophil counts varied between 1 and 138 per 10 HPF in the tumor and/or peritumoral areas. In the three distinct categories with three different cut off values of eosinophil cell counts among nonmetastatic cases and cases with lymph node metastasis, correlation of eosinophil counts with lymph node metastasis were statistically insignificant (Crosstabs, χ2). Although in the series, numerical values of the TATE seem to be increased in patients with laryngeal squamous cell carcinoma with lymph node metastasis, this fact has not been confirmed with statistical analysis.
Keywords: Squamous cell carcinoma, Larynx, Tumor-associated tissue eosinophilia
Introduction
Invasive squamous cell carcinoma of the larynx which accounts for 95% of all laryngeal carcinomas, is most commonly seen in males in the sixth and seventh decades of life [1, 2]. Various parameters have been examined as possible prognostic factors for squamous cell carcinoma. Clinically, the most significant prognostic parameter is the TNM classification whereas the lymph node metastasis component of the classification is of primary importance. The predictive histopathologic parameters are resection borders, proliferation indices, lymphovascular and perineural invasion, pericapsular invasion in the lymph node and DNA diploidy [2, 3].
There are several studies regarding prediction of prognosis with tumor-associated tissue eosinophilia (TATE) in epithelial carcinomas in various sites [4–6]. Recently, eosinophilia has been investigated in head and neck carcinomas, especially the oral cavity carcinomas [7–11]. Although it has been shown that the relationship between eosinophil count and prognosis is statistically significant, it still remains controversial [12–15].
Materials and Methods
Pathology specimens of 100 patients with invasive squamous carcinoma of the larynx who were treated surgically either with partial or total laryngectomy and neck dissection in two tertiary hospitals, were evaluated. Tumors were divided into well, moderately and poorly differentiated carcinoma based on degree of differentiation. Neck dissection specimens were fixed in 10% formalin and lymph node status of the cases were determined by the absence or presence of lymph node metastasis in hematoxylin and eosine stained preparations. Eosinophil polymorphs were counted in randomly chosen 10 high power fields (HPFs) of intratumoral and peritumoral areas (×40 objective lens). Three categories (I, II, III) were determined based on three distinct random cut off values of eosinophil counts. Each category was further subdivided into three groups as A, B, C with cut off values in increasing order. Eosinophil count limit in 10 HPF for IA was 0–10, and it was 11–29 for IB, 30 and greater for IC. For IIA, IIB, IIC the cut off values were 0–20, 21–39 and 40 and greater, respectively. For IIIA, IIIB and IIIC, they were determined as 0–30, 31–49 and 50 and greater, respectively. The association between lymph node metastasis and TATE in determined categories was analyzed statistically with cross tables and χ2 tests. Phi coefficient ≤0.05 was significant.
Results
The pathology specimens of 100 patients were evaluated (97 males, 3 females). The ages of the patients included in the study ranged from 37 to 78 years (mean 59.9). The diagnoses of the specimens were 45 well differentiated, 50 moderately differentiated and 5 poorly differentiated invasive carcinoma of the larynx. The average number of lymph nodes dissected were 25.9 (5–74). In 66 patients, lymph node invasion was not evident, whereas at least one lymph node metastasis was established in 34 patients. In the evaluation of the laryngectomy specimens, 1–138 eosinophils were counted per 10 HPF in hematoxylin and eosin stained preparations from the tumor and tumor’s periphery (Figs. 1, 2). The distribution of TATE in the groups with and without lymph node metastases was evaluated independently. Sixty-six patients without metastasis were evaluated in three different categories: IA, IB, IC consisted of 16, 18, 32 patients, respectively. IIA, IIB, IIC consisted of 27, 16, 23 cases, respectively, whereas IIIA, IIIB and IIIC had 34, 11 and 21 cases, respectively (Tables 1, 2, 3). In the group of 34 patients with lymph node metastasis IA, IB and IC comprised 7, 12 and 15 patients; IIA, IIB, IIC included 14, 8, 12 cases; IIIA, IIIB and IIIC comprised 19, 7 and 8 patients, respectively (Tables 4, 5, 6). Cross tables and χ2 tests which were performed to analyze the variables for significant relationships revealed phi coefficient in Group I as 0.841 for the group of patients without metastasis and 0.426 for patients with metastasis. In Group II phi coefficient was 0.08 for the group without metastasis and 0.351 for the group with metastasis (Tables 7, 8). The phi coefficients for Group III were 0.150 and 0.382 for the patients without and with metastasis, respectively.
Fig. 1.
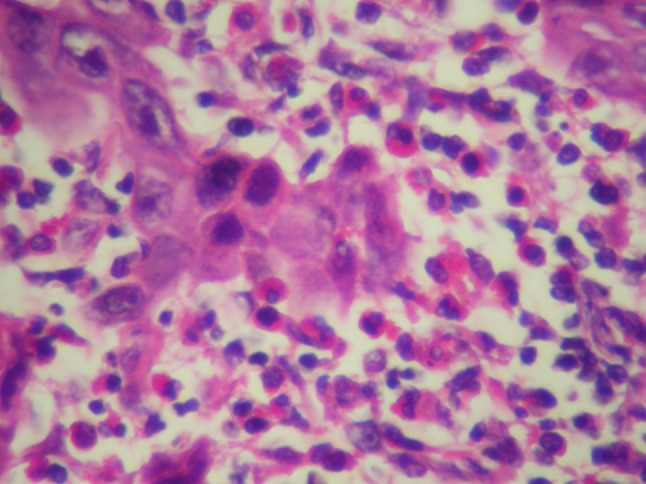
Severe eosinophilic infiltration in tumoral tissue HEX40
Fig. 2.
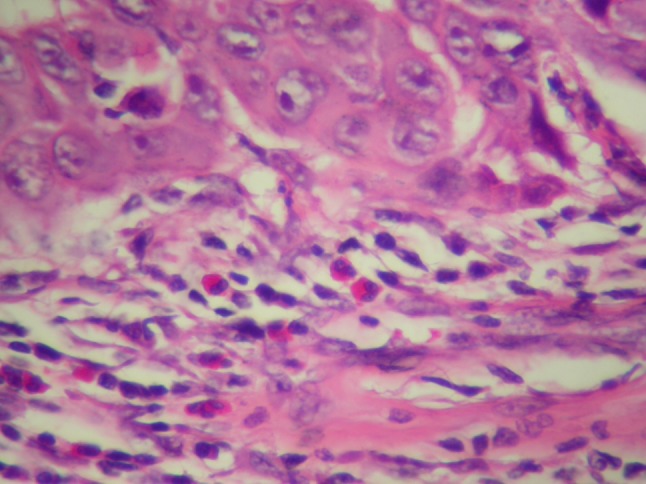
Mild infiltration of the eosinophilic leukocytes around a squamous cell carcinoma island HEX40
Table 1.
The distribution of the Group I: the cases with no metastasis (n = 66)
| Group I | Differentiation | ||
|---|---|---|---|
| Well | Moderate | Poor | |
| IA (0–10) n = 16 | 6 | 9 | 1 |
| IB (11–29) n = 18 | 8 | 10 | |
| IC (30 and greater) n = 32 | 15 | 16 | 1 |
Table 2.
The distribution of the Group II: the cases with no metastasis (n = 66)
| Group II | Differentiation | ||
|---|---|---|---|
| Well | Moderate | Poor | |
| IIA (0–20) n = 27 | 12 | 14 | 1 |
| IIB (21–39) n = 16 | 3 | 13 | |
| IIC (40 and greater) n = 23 | 14 | 8 | 1 |
Table 3.
The distribution of the Group II: the cases with no metastasis (n = 66)
| Group III | Differentiation | ||
|---|---|---|---|
| Well | Moderate | Poor | |
| IIIA (0–30) n = 34 | 14 | 19 | 1 |
| IIIB (31–49) n = 11 | 1 | 10 | |
| IIIC (50 and greater) n = 21 | 14 | 6 | 1 |
Table 4.
The distribution of the Group I: the cases with metastasis (n = 34)
| Group I | Differentiation | ||
|---|---|---|---|
| Well | Moderate | Poor | |
| IA (0–10) n = 7 | 2 | 4 | 1 |
| IB (11–29) n = 12 | 5 | 5 | 2 |
| IB (30 and greater) n = 15 | 9 | 6 | |
Table 5.
The distribution of the Group II: the cases with metastasis
| Group II | Differentiation | ||
|---|---|---|---|
| Well | Moderate | Poor | |
| IIA (0–20) n = 14 | 6 | 7 | 1 |
| IIB (21–39) n = 8 | 4 | 2 | 2 |
| IIIC (40 and greater) n = 12 | 6 | 6 | |
Table 6.
The distribution of the Group III: the cases with metastasis
| Group III | Differentiation | ||
|---|---|---|---|
| Well | Moderate | Poor | |
| IIIA (0–30) n = 19 | 7 | 9 | 3 |
| IIIB (31–49) n = 7 | 5 | 2 | |
| IIIC (50 and greater) n = 8 | 4 | 4 | |
Table 7.
Cross table analysis for the Group II
| Group | Group II | Total | ||
|---|---|---|---|---|
| A | B | C | ||
| No metastasis | ||||
| Tumor differentiation | ||||
| Well | ||||
| Number of the cases | 12 | 3 | 14 | 29 |
| Tumor differentiation (%) | 41.4 | 10.3 | 48.3 | 100.0 |
| Distribution in the group (%) | 44.4 | 18.8 | 60.9 | 43.9 |
| Moderate | ||||
| Number of the cases | 14 | 13 | 8 | 35 |
| Tumor differentiation (%) | 40.0 | 37.1 | 22.9 | 100.0 |
| Distribution in the group (%) | 51.9 | 81.3 | 34.8 | 53.0 |
| Poor | ||||
| Number of the cases | 1 | 0 | 1 | 2 |
| Tumor differentiation (%) | 50.0 | 0 | 50.0 | 100.0 |
| Distribution in the group (%) | 3.7 | 0 | 4.3 | 3.0 |
| Total | ||||
| Number of the cases | 27 | 16 | 23 | 66 |
| Tumor differentiation (%) | 40.9 | 24.2 | 34.8 | 100.0 |
| Distribution in the group (%) | 100.0 | 100.0 | 100.0 | 100.0 |
| With metastasis | ||||
| Tumor differentiation | ||||
| Well | ||||
| Number of the cases | 6 | 4 | 6 | 16 |
| Tumor differentiation (%) | 37.5 | 25.0 | 37.5 | 100.0 |
| Distribution in the group (%) | 42.9 | 50.0 | 50.0 | 47.1 |
| Moderate | ||||
| Number of the cases | 7 | 2 | 6 | 15 |
| Tumor differentiation (%) | 46.7 | 13.3 | 40.0 | 100.0 |
| Distribution in the group (%) | 50.0 | 25.0 | 50.0 | 44.1 |
| Poor | ||||
| Number of the cases | 1 | 2 | 0 | 3 |
| Tumor differentiation (%) | 33.3 | 66.7 | 0 | 100.0 |
| Distribution in the group (%) | 7.1 | 25.0 | 0 | 8.8 |
| Total | ||||
| Number of the cases | 14 | 8 | 12 | 34 |
| Tumor differentiation (%) | 41.2 | 23.5 | 35.3 | 100.0 |
| Distribution in the group (%) | 100.0 | 100.0 | 100.0 | 100.0 |
Table 8.
Phi co-efficient for the Group II
| Symmetric measurement | ||
|---|---|---|
| Group | Co-efficient | Approximate value |
| No metastasis (n = 66) | ||
| Numeric | ||
| Phi | 0.355 | 0.080 |
| Cramer value | 0.251 | 0.080 |
| With metastasis (n = 34) | ||
| Numeric | ||
| Phi | 0.361 | 0.351 |
| Cramer value | 0.255 | 0.351 |
Discussion
TATE has been described for the first time in 1896 [16]. The preliminary study on TATE in head and neck has been reported by Lowe et al. [17]. Despite the long period of time which has elapsed, the mechanisms related to TATE have not been clarified thoroughly. Although in some experimental studies the eosinophilic peroxidase system has been shown to prohibit tumor development, this has not been proved clearly [18].
In a series of 422 patients with nasopharyngeal carcinoma, the TATE ratio was 21%. The overall ratio of TATE for the head and neck cancers was 40% in which laryngeal cancers have not been considered separately [13]. A concrete consensus on qualifying the TATE categories is not available yet. While some authors specify eosinophil count greater than 10 in 10 HPF as intermediate, a count exceeding 100 is considered as severe [17, 19]. In the present study, cases were divided quantitatively into three groups based on the eosinophil count. The patients were evaluated with regard to three distinct cut off values for eosinophil counts in the group of patients with and without metastasis. The objective of performing the evaluation with different threshold values in both groups is the lack of a common threshold value in this subject and to analyze statistical significance in various numerical categories in comparative groups. At least one tissue eosinophil has been seen microscopically in all categories of invasive squamous cell carcinomas (100%). Although it has been shown that tumor differentiation does not significantly alter the recurrence rate and disease-free survival, it is generally known that poorly differentiated carcinomas tend to metastasize more frequently than well differentiated carcinomas [20, 21]. In a study where association between prognostic markers and TATE has been investigated, including the metastatic disease, a significant relevance has not been established [3]. In the present study in Group I, although the concomitant eosinophil count seems to increase as the differentiation worsens, this is not statistically significant (Phi co-efficient is 0.841 in the group without metastasis, and 0.426 in the group with metastasis). Considering the statistical relationships between lymph node metastasis and TATE, the most significant yet not statistically significant outcome was found in Group II. The other categories in this study revealed no rational or statistical significance. In conclusion, TATE is not a favourable predicting parameter for the lymph node metastasis which is a marker of poor prognosis in laryngeal carcinoma.
References
- 1.Crissman JD, Zarbo RJ. Dysplasia, in situ and progression to invasive squamous cell carcinoma of the upper aerodigestive tract. Am J Surg Pathol. 1989;13:5–16. [PubMed] [Google Scholar]
- 2.Barnes L, Everson JW, Reichart P, Sidransky D. WHO classification of tumours. Pathology and genetics of head and neck tumors. Lyon: IARC Press; 2005. pp. 109–121. [Google Scholar]
- 3.Ercan I, Cakir B, Başak T, Ozdemir T, Sayin I, Turgut S. Prognostic significance of stromal eosinophilic infiltration in cancer of the larynx. Otolaryngol Head Neck Surg. 2005;132(6):869–873. doi: 10.1016/j.otohns.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 4.Pasternak A, Jansa P. Local eosinophilia in stroma of tumors related to prognosis. Neoplasma. 1984;31:323–326. [PubMed] [Google Scholar]
- 5.Lowe D, Fletcher CDM. Eosinophilia in squamous cell carcinoma of the oral cavity, external genitalia and anus-clinical correlations. Histopathology. 1984;8:627–632. doi: 10.1111/j.1365-2559.1984.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 6.Caruso RA, Giuffre G, Inferrea C. Minute and small early gastric carcinoma with special reference to eosinophil infiltration. Histopathology. 1993;8:155–156. [PubMed] [Google Scholar]
- 7.Tadbir AA, Ashraf MJ, Sardari Y. Prognostic significance of stromal eosinophilic infiltration in oral squamous cell carcinoma. J Craniofac Surg. 2009;20(2):287–289. doi: 10.1097/SCS.0b013e318199219b. [DOI] [PubMed] [Google Scholar]
- 8.Tostes Oliveira D, Tjioe KC, Assao A, Sita Faustino SE, Lopes Carvalho A, Landman G, et al. Tissue eosinophilia and its association with tumoral invasion of oral cancer. Int J Surg Pathol. 2009;17(3):244–249. doi: 10.1177/1066896909333778. [DOI] [PubMed] [Google Scholar]
- 9.Falconieri G, Luna MA, Pizzolitto S, DeMaglio G, Angione V, Rocco M. Eosinophil-rich squamous carcinoma of the oral cavity: a study of 13 cases and delineation of a possible new microscopic entity. Ann Diagn Pathol. 2008;12:322–327. doi: 10.1016/j.anndiagpath.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Horie N, Shimoyama T, Kaneko T, Ide F. Multiple oral squamous cell carcinomas with blood and tissue eosinophilia. J Oral Maxillofac Surg. 2007;65:1648–1650. doi: 10.1016/j.joms.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Alkhabuli JO, High AS. Significance of eosinophil counting in tumor associated tissue eosinophilia (TATE) Oral Oncol. 2006;42:849–850. doi: 10.1016/j.oraloncology.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Said M, Wiseman S, Yang J, Alrawi S, Douglas W, Cheney R, et al. Tissue eosinophilia: a morphologic marker for assessing stromal invasion in laryngeal squamous neoplasms. BMC Clin Pathol. 2005;5(1):1. doi: 10.1186/1472-6890-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deron P, Goossens A, Halama AR. Tumour-associated tissue eosinophilia in head and neck squamous-cell carcinoma. ORL J Otorhinolaryngol Relat Spec. 1996;58:167–170. doi: 10.1159/000276819. [DOI] [PubMed] [Google Scholar]
- 14.Thompson AC, Bradley PJ, Griffin NR. Tumour-associated tissue eosinophilia and long term prognosis for carcinoma of the larynx. Am J Surg. 1994;168:469–471. doi: 10.1016/S0002-9610(05)80102-3. [DOI] [PubMed] [Google Scholar]
- 15.Sassler AM, McClatchey KD, Wolf GT, Fisher SG. Eosinophilic infiltration in advanced laryngeal squamous cell carcinoma. Laryngoscope. 1995;105:413–416. doi: 10.1288/00005537-199504000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Przeworski E. Über die lokale Eosinophilie beim Krebs nebst Bemerkungen über die bedeutung der eosinophilen Zellen im allgemeinen. Zentralbl Allg Pathol Anat. 1896;5:177–191. [Google Scholar]
- 17.Lowe D, Fletcher CD, Shaw MP, McKee PH. Eosinophil infiltration in keratoacanthoma and squamous cell carcinoma of the skin. Histopathology. 1984;8(4):619–625. doi: 10.1111/j.1365-2559.1984.tb02374.x. [DOI] [PubMed] [Google Scholar]
- 18.Jong EC, Klebanoff SJ. Eosinophil-mediated mammalian tumor cell cytotoxicity: role of the peroxidase system. J Immunol. 1980;124:1949–1953. [PubMed] [Google Scholar]
- 19.Looi L. Tumor associated tissue eosinophilia in nasopharyngeal carcinoma. A pathologic study of 422 primary and 138 metastatic tumors. Cancer. 1987;59:466–470. doi: 10.1002/1097-0142(19870201)59:3<466::AID-CNCR2820590319>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 20.McGavran MH, Bauer WC, Ogura JH. The incidence of cervical lymph node metastasis from epidermoid carcinoma of the larynx and their relationship to certain characteristics of the primary tumor. A study based on the clinical and pathological findings for 96 patients treated by primary en bloc laryngectomy and radical neck dissection. Cancer. 1961;14:55–66. doi: 10.1002/1097-0142(196101/02)14:1<55::AID-CNCR2820140109>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Yılmaz T, Hosal AS, Gedikoglu G, Kaya S. Prognostic significance of histopathological parameters in cancer of the larynx. Eur Arch Otolaryngol. 1999;256:139–144. doi: 10.1007/s004050050127. [DOI] [PubMed] [Google Scholar]


