Abstract
The study was conducted to find out the association of various naso-oro-pharyngeal structures with sleep macro-architecture in suspected obstructive sleep apnea subjects. Study included 51 subjects with suspected obstructive sleep apnea. Subjects with possible central apnea and those consuming any substance that can affect sleep architecture were excluded. Level I polysomnography was performed after thorough physical examination. Overnight study was scored in 30 s epochs to find out the polysomnographic variables. Surgical treatment was offered wherever indicated. Subjects with moderate to severe obstructive sleep apnea were manually titrated on CPAP with the polysomnogram. SPSS v 17.0 was used for statistical analysis. We did not find any difference in the sleep architecture between genders. Sleep Efficiency was better in subjects with dental overjet, dental attrition, high tongue base, macroglossia, lesser oral cavity volume, edematous uvula, increased submental fat, hypertrophied facial muscles and Mallampatti grade III–IV. Shorter Sleep Latency was seen in subjects with tender TMJ and Mallampatti Gr III–IV. REM latency was shorter in subjects with high tongue base, macroglossia and hypertrophied muscles of mastication. Increased REM was observed in subjects with high tongue base, edematous uvula and tender TMJ. Enlarged tonsils had reversed effect with poor sleep efficiency, increased REM latency and decreased REM. CPAP therapy (N = 20) lessened awake time, decreased N2 and increased REM. Oro-pharyngeal structures affect the sleep architecture in suspected OSA subjects. Nasal structures do not affect the sleep architecture in these subjects and enlarged tonsils have opposite effect. Sleep architecture changes on the titration night with CPAP.
Keywords: Sleep-architecture, Sleep-apnea, Oro-naso-pharyngeal anatomy
Introduction
Structures in the nose, oral cavity and pharynx are central to the development in obstructive sleep apnea. Nasal obstruction may lead to the snoring and sleep apnea [1]. Common causes of nasal obstruction are deviated nasal septum, hypertrophied turbinates, polyps and mucosal congestion from rhinitis [1]. Similarly other deformities e.g., macroglossia, high tongue base, high arched palate, enlarged tonsils, and adenoids, submental fat, etc. also adds to the risks of obstructive sleep apnea [2, 3]. Few studies suggest that craniofacial and nasopharyngeal abnormalities may be related to the severity of obstructive sleep apnea [4, 5]. On the other hand, it has been also reported that sleep macro-architecture varies with the severity of OSA and between genders [6, 7]. Hence, it is possible that sleep architecture changes seen with the anatomical defects in these structure are similar to those seen in OSA or any of these structures may have a more marked effect than the other. In the past a few studies have been conducted to assess the effect of nasal pathologies and adenotonsillar enlargement on sleep macro-architecture, however, studies assessing effects of other structures are probably never attempted [8–11]. Hence, present study attempts to find out the effect of each naso-oro-pharyngeal structure on the sleep architecture in subjects suspected of OSA. Other aim of the study was to see if CPAP therapy can change the sleep architecture in these subjects.
Methodology
Study was conducted at Sleep and Headache Care, Jaipur after approval of ethics committee. 51 consecutive patients attending the sleep clinic for suspected OSA were included in this study after obtaining a written informed consent. However, subjects with obesity hyperventilation syndrome, with major cardio-respiratory or neurological conditions that might cause OR aggravate sleep apnea, pregnant females, subjects consuming substance of abuse except tobacco, those taking psychotropic medicines were excluded from the study. Their demographic data was recorded in a semi-structured performa. Sleep history was taken in the presence of a reliable informant, preferably a bed-partner. Clinical examination was done that included visual analysis for nares asymmetry, deviated nasal septum, collapsed nasal valve, turbinate hypertrophy, retrognathia, dental over-jet, dental occlusion, overlapping teeth, dental attrition, tongue base, macroglossia, ascertainment of oral cavity volume, position of hard palate, enlargement of soft palate, uvula, tonsils, presence of submental fat, tenderness of temporo-mandibular joints, hypertrophy of muscles of mastication, enlargement of thyroid, neck circumference at the level of cricoids and BMI. Then they were subjected to complete neurological, psychiatric and cardio-respiratory examination.
Then they were subjected to Level I whole night diagnostic polysomnography following the procedure mentioned below.
PSG Protocol
The 24 channel polysomnography was started at usual bedtime for the respective subjects. They were prepared for the study well before and whole of the procedure was explained. They were given enough time to get familiarized with the room. Attended polysomnography was performed. Electrophysiological sleep parameters included: frontal (F4/A1 and F3/A2), central (C4/A1 and C3/A2) and occipital (O2/A1 and O1/A2) electroencephalogram (EEG); right and left electro-occulogram (ROG and LOG) and submentalis electromyogram (EMG). Limb movements were observed using anterior tibialis electromyogram. Cardiac rhythm was continuously recorded (ECG). The patient was tested while breathing room air. Airflow was detected using pressure transducers and respiratory motion was detected using chest and abdomen movements. Arterial pulse oximetry was measured by an oximeter set in the fast response mode using a finger probe. Apneas and hypopneas were scored on the basis of absence or reduction of airflow for continuous ten seconds, respectively. Obstructive and mixed events were defined by the presence of respiratory efforts and characteristics changes of inspiratory flow pattern.
Raw data was manually scored in 30 s epochs for sleep staging using American Academy of Sleep Medicine criteria by a trained sleep specialist (RG) who was blind to the ENT pathologies [12]. The raw data was reviewed for arousals using standard criteria by the scorer [12].
Surgery
Relevant surgeries according to prevailing guidelines were done for the deviated nasal septum, adenotonsillar enlargement, hypertrophied turbinates and nasal polyps by an ENT surgeon (PS).
Titration of CPAP
Manual CPAP titration according to AASM guidelines was done by a trained sleep specialist (RG) in subjects with moderate to severe OSA [13].
Subjects where AHI < 5 were operated for their ENT problem. Subjects with mild apnea (AHI 6–15) were operated and they were suggested sleep hygiene techniques and were enrolled under weight reduction program.
Statistical Analysis
Analysis was performed using SPSS 17.0. Chi-Square test was applied on the non-parametric variables. Two tailed t test was used to assess difference between continuous variables. Paired t test was used to find out the difference between pre and post CPAP sleep architecture. One way ANOVA with post hoc Tukey analysis was run on the continuous variables divided in more than 2 groups.
Results
This study included a total of 51 subjects out of which 40 were males and 11 were females. Mean age of the subjects was 38 years (range 8–60 years). There was no difference between the male and female members in this group based upon the frequency of craniofacial and otorhinolaryngological anatomy. There was no difference between the frequency of deviated nasal septum, overlapping teeth, macroglossia, high tongue base, high arched palate, edematous soft palate and uvula, tonsillar enlargement, submental fat, nares asymmetry, hypertrophied facial muscles and tenderness in the TMJs. However, more females were suffering from dental over-jet (P = 0.003), dental attrition (P = 0.001), poor dental occlusion (P = 0.04) and hypertrophied turbinates (P = 0.004). Similarly, the age and BMI did not differ significantly across genders. However, mean collar size was more in males as compared to females (Males: 39.94 + 2.97 cms; Females: 36.11 + 5.77; P = 0.007). Mallampatti grading was also not different between genders (X2 = 4.77; P = 0.9). Polysomnography related variables did not differ across genders except that females spent statistically but, not clinically, more time awake than males (male: 12.20 + 8.99 min; females: 20.8 + 12.88; P = 0.01). Desaturation Index, NREM AHI and REM AHI did not differ across genders. Hence, largely the group was homogenous.
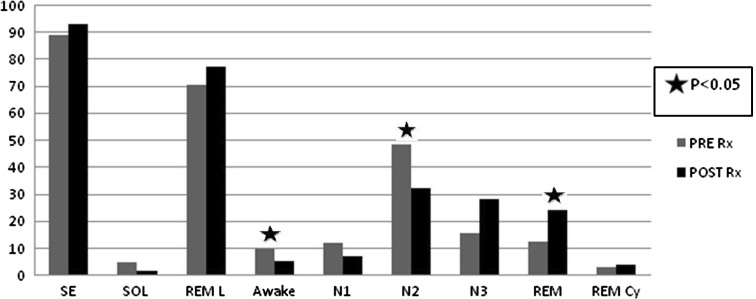
DNS correction was attempted in 16 cases, turbinectomy in ten subjects, tonsillectomy in nine, polyp removal in three (not shown in table). Few of these cases had more than one problem. Out of 51 subjects included in this study, 32 patients were found to have clinically significant obstructive sleep apnea. Because of moderate to severe sleep apnea, CPAP titration was done in 20 cases. Effect of anatomical variables on sleep macro-architecture is shown in Table 1; Table 2 shows effect of Mallampatti grading on polysomnographic variables. Fig. 1 shows the difference between the sleep architecture of test and titration night in 20 moderate to severe OSA subjects.
Table 1.
Effect of ENT pathology on sleep architecture (N = 51)
| Pathology | Present | Absent | P | SED |
|---|---|---|---|---|
| Dental overjet | N = 7 | N = 44 | ||
| Sleep efficiency | 89.0 + 3.25 | 83.23 + 11.61 | 0.01 | 2.19 |
| Dental attrition | N = 8 | N = 43 | ||
| Sleep efficiency | 88.55 + 3.99 | 83.04 + 11.8 | 0.02 | 2.3 |
| N1 | 6.75 + 4.8 | 12.28 + 7.45 | 0.05 | 2.75 |
| High tongue base | N = 34 | N = 17 | ||
| SE | 87.57 + 6.34 | 76.18 + 14.8 | 0.009 | 3.85 |
| REM latency | 108.88 + 61.86 | 154.81 + 78.55 | 0.03 | 20.47 |
| Awake | 10.52 + 6.10 | 21.12 + 13.64 | 0.008 | 3.56 |
| REM | 15.23 + 7.51 | 11.37 + 4.73 | 0.03 | 1.74 |
| Macroglossia | N = 32 | N = 19 | ||
| SE | 88.05 + 6.09 | 76.6 + 14.06 | 0.004 | 3.68 |
| REM latency | 103.84 + 61.62 | 158.66 + 72.54 | 0.007 | 19.35 |
| Awake | 10.06 + 5.09 | 20.77 + 13.32 | 0.004 | 3.28 |
| Decreased oral cavity volume | (N = 33) | (N = 18) | ||
| SE | 87.68 + 6.37 | 77.42 + 14.5 | 0.01 | 3.59 |
| Awake | 10.75 + 5.9 | 19.55 + 13.86 | 0.01 | 3.43 |
| High hard palate | N = 17 | N = 34 | ||
| Awake | 10.0 + 5.81 | 15.76 + 11.51 | 0.02 | 2.45 |
| REM cycling | 3.0 + 1.63 | 3.88 + 1.34 | 0.04 | 0.43 |
| Edematous soft palate | N = 6 | N = 45 | ||
| REM cycling | 2.33 + 0.51 | 3.77 + 1.49 | 0.02 | 0.61 |
| Edematous uvula | N = 11 | N = 40 | ||
| SE | 90.78 + 6.41 | 82.21 + 11.34 | 0.02 | 3.27 |
| Awake | 7.20 + 4.68 | 15.6 + 10.71 | 0.001 | 2.25 |
| REM | 19.6 + 2.71 | 12.6 + 6.98 | <0.001 | 1.39 |
| Hypertrophied tonsils | N = 9 | N = 42 | ||
| SE | 72.9 + 14.87 | 86.02 + 8.99 | 0.001 | 3.88 |
| REM latency | 186.75 + 45.72 | 111.54 + 67.92 | 0.004 | 25.13 |
| Awake | 23.25 + 13.76 | 12.14 + 8.68 | 0.004 | 3.69 |
| REM | 9 + 3.29 | 14.95 + 7.06 | 0.001 | 1.59 |
| Increased submental fat | N = 30 | N = 21 | ||
| SE | 86.96 + 6.07 | 79.38 + 14.98 | 0.04 | 3.52 |
| REM cycling | 3.2 + 1.44 | 4.2 + 1.26 | 0.01 | 0.4 |
| Tender TMJ | N = 6 | N = 45 | ||
| SOL | 3.83 + 3.23 | 10.88 + 15.23 | 0.01 | 2.64 |
| REM | 19.33 + 2.25 | 13.27 + 7.05 | <0.001 | 1.40 |
| Hypertrophied facial muscles | N = 26 | N = 25 | ||
| SE | 88.55 + 6.25 | 78.91 + 13.00 | 0.002 | 2.92 |
| REM latency | 96.84 + 59.86 | 152.54 + 70.61 | 0.004 | 18.41 |
| Awake | 10 + 5.64 | 18.16 + 12.53 | 0.006 | 2.78 |
| N2 | 46.61 + 13.7 | 38.58 + 13.91 | 0.045 | 3.9 |
Table 2.
Sleep architecture based upon Mallampatti score
| N | Mean | SD | P | ||
|---|---|---|---|---|---|
| Sleep efficiency | <0.001 | ||||
| II | 10 | 68.98 | 13.56 | ||
| III | 10 | 88 | 7.77 | ||
| IV | 31 | 87.55 | 5.97 | ||
| SOL | 0.01 | ||||
| II | 10 | 21.70 | 21.0 | ||
| III | 10 | 4.00 | 1.97 | ||
| IV | 31 | 8.16 | 12.39 | ||
| REM latency | 0.02 | ||||
| II | 10 | 174.90 | 64.56 | ||
| III | 10 | 128.70 | 74.36 | ||
| IV | 31 | 104.76 | 63.60 | ||
| Awake | <0.001 | ||||
| II | 10 | 27.20 | 13.08 | ||
| III | 10 | 11.20 | 8.46 | ||
| IV | 31 | 10.40 | 5.40 | ||
| N2 | 0.002 | ||||
| II | 10 | 29.40 | 11.74 | ||
| III | 10 | 47.60 | 12.60 | ||
| IV | 31 | 45.60 | 13.16 | ||
| REM | 0.04 | ||||
| II | 10 | 10.00 | 5.03 | ||
| III | 10 | 17.60 | 7.13 | ||
| IV | 31 | 14.13 | 6.90 |
Post Hoc Tukey: Sleep efficiency- II is different from III, IV; Sleep onset latency- II is different from III, IV; REM latency- II is different from IV; Awake- II is different from III, IV; N2- II is different from III, IV; REM- II is different from III
Fig. 1.
Comparison of sleep macroarchitecture before and after CPAP therapy
Discussion
In short, to best of our knowledge, for the first time this kind of study has been conducted and data suggests that high tongue base, macroglossia, decreased volume of oral cavity, edematous uvula, edematous soft palate, hypertrophied facial muscles and submental fat were consistently related with the change in sleep architecture. Other features e.g., dental over-jet, dental attrition, tender TMJ, and tonsillar enlargement had effect on some of the sleep related variables. Of these, tonsillar enlargement had opposite effect as compared to other variables. Interestingly, nasal pathologies did not affect the sleep architecture at all. Hence, three assumptions can be made first, structures that make a person prone to develop obstructive events are involved in change in sleep architecture. However, it must be remembered that these structures did not show any consistent pattern and many of them influenced different parameters of sleep. Secondly, for unknown reasons, tonsillar hypertrophy had an opposite effect of the sleep macro-architecture as compared to other cranio-facial structures. Thirdly, nasal structures perhaps do not contribute to sleep apnea and consequently do not produce any change in sleep architecture. This study also suggests that structures that make a person prone to obstructive sleep apnea consistently increase the sleep efficiency, decrease REM latency, increase REM, increase REM cycles and lessen the wake time.
Studies conducted in the past show that subject with sleep apnea show inconsistent changes in sleep architecture. A study done in Singapore on the OSA subjects, shows that REM sleep decreases consistently as the severity of sleep apnea measured by AHI progresses while the light sleep shows opposite trend [7]. Another study suggested that sleep efficiency, sleep latency, and N1 did not differ between control group, mild apnea and severe apnea. On the other hand, N2 and REM latency increased with the severity of AHI with the decrease in N3 and REM [14]. Other literature suggests that at least in children sleep architecture do not differ with the position dependent AHI [15]. Moreover, animal experiments suggest that sleep stages particularly, N2 decreases with the initial periods of intermittent hypoxia akin to sleep apnea, to again reach the baseline after few days. While the delta portion of NREM (N3 sleep) show a persistent decrement over days [16]. However, these studies do not provide any data regarding the naso-oro-pharyngeal anatomy. They have considered the either the presence of OSA or severity of AHI as the defining criteria for classifying the population. Upper airway anatomy plays an important role which is shown by the fact that subjects with upper airway resistance syndrome, although do not have clinically significant desaturation or apnic events, yet they have frequent arousals to disrupt their daytime wakefulness and probably altering sleep architecture [17]. Moreover, presence of major depressive disorder may also affect the sleep architecture in OSA subjects. OSA subjects without depression have short sleep latency while those with depression show higher REM period [18]. Previous studies are silent in this regard. In the present study we have excluded subjects with any kind of comorbidity or drugs that could alter sleep architecture except hypertension, diabetes and coronary artery disease.
First night effect must also be taken into account while analyzing the data of the present study. It has been shown that first night polysomnograms in OSA subjects shows reduced sleep efficiency, N3 and REM all of which increase on the second night [19]. Another study that compared the first night effect in OSA subjects studied either in sleep laboratory or a hotel room failed to find any difference of sleep architecture between two groups, concluding that first night effect may not be apparent [20]. First night effect was demonstrated in normal healthy volunteers in one study with increased alpha activity that diminished over consecutive nights [21]. In present study subjects were asked to attend the sleep laboratory 3–4 h before their usual bedtime and they were allowed to do their activities in the room to promote their familiarity with the room. This could have increased the sleep efficiency as well as the REM sleep [22]. Another reason for better sleep efficiency in subjects predisposed to OSA could be because of their sleepiness [23, 24]. Post hoc inspection of our data showed that these subjects were suffering from excessive daytime sleepiness. Hence, it is possible that first night effect is apparent only in those patients who are sleep deprived below a critical limit.
In this study, contrary to our assumptions, enlarged tonsils reduced the sleep efficiency, REM, and increased wake time and REM latency. Previous studies suggest that children with adenotonsillar hypertrophy but with or without OSA do not differ with respect to sleep architecture [9]. Adenotonsillar hypertrophy is associated with decrease in REM sleep but removal of lymphoid tissue does not improve the obstructive events [10]. Recent study suggests that adenotonsillectomy do not change the slow wave sleep duration but may change it’s dynamics [25]. On the contrary, Zhang et al. [26] found that adenoid enlargement increases the REM latency with decrease in REM sleep in children. Similarly, restless sleep with frequent arousals is also common in the presence of tonsillar hypertrophy [27].
Previous reports provide conflicting results with regards to the change in sleep architecture after nasal surgery. While one study suggested the improvement in nasal resistance after nasal surgery, it did not find any change in snoring and nocturnal breathing. However, REM sleep increased on the second polysomnographic study that was delayed for a mean of 113 days after surgery [8]. Another study conducted on the UARS patients found that application of nasal dilators decreased the time spent in N1 sleep. In these cases sleep studies were conducted 1–2 weeks apart [28]. McLean et al. [11] suggested that nasal surgery may improve the airway and decrease the resistance, but reduction in OSA severity was only modest. But, they reported an improvement in sleep architecture with a decrease in N1, N2 and increment of sleep efficiency, N3 and REM after surgery. However, in their study they considered only the turbinate hypertrophy excluding other anatomical variables included in present study. On the contrary two other studies failed to show any improvement in sleep architecture by nasal dilatation in subjects with mild sleep apnea [29, 30]. Similar results were reported by Li et al. [31] in a recent meta-analysis. Thus, it is possible that nasal structures do not cause obstructive apnea and in selected cases they may alter the sleep macro-architecture, especially in the presence of other contributing pathologies.
On the night of manual titration of CPAP a change in sleep architecture was seen. Wake time and N2 decreased with the improvement of REM duration and REM cycling. Earlier studies report decrement of N1/N2 stages with consequent rebound of N3 and REM sleep period during the first CPAP night [32, 33]. Kondo et al. [34] found that beneficial effects of CPAP treatment on sleep architecture lasts till the device is used for controlling apneas. Macro-architecture of sleep bounces back to that of pretreatment stage even if it is not used for single night. This data suggests that change in sleep architecture is related to presence of sleep apneas and consequent arousals rather than any permanent change in brain morphology.
However, this study had some methodological limitations. Firstly, sample size was small as compared to prevalence of illness and factors studied. Secondly, we did not study the change in sleep study dynamics. Thirdly, because of the technical limitations we could not perform the spectral analysis and sleep stages were reported on visual scoring only. Fourth, the study included subjects who were not found to suffer from clinically significant apnea. Fifth, some part of the night was spent in finding an optimum pressure for CPAP, hence below that the sleep was not normal. This could have affected the comparison between diagnostic and titration night and consequently could have affected the results. However, despite these limitations, this is the first study of its kind and shows that different anatomical structures may have different effects on sleep stages.
In conclusion, this study suggests that nasal pathologies do not change sleep stages significantly, while oro-pharyngeal structures alter the sleep architecture except that tonsillar enlargement have an opposite effect. This study also shows that first night effect must be considered when analyzing the sleep architecture.
Acknowledgments
Conflict of interest
None.
References
- 1.Kohler Bloch KE, Stardling JR. The role of nose in pathogenesis of obstructive sleep apnea and snoring. Eur Respi J. 2007;30:1208–1215. doi: 10.1183/09031936.00032007. [DOI] [PubMed] [Google Scholar]
- 2.Bausmer U, Gouveris H, Selivanova O, Goepel B, Mann W. Correlation of the epworth sleepiness scale with respiratory sleep parameters in patients with sleep-related breathing disorders and upper airway pathology. Eur Arch Otorhinolaryngol. 2010;267:1645–1648. doi: 10.1007/s00405-010-1250-y. [DOI] [PubMed] [Google Scholar]
- 3.Zonato AI, Martinho FL, Bittencourt LR, de Oliveira Camponês BrasilO, Gregório LC, Tufik S. Head and neck physical examination: comparison between nonapneic and obstructive sleep apnea patients. Laryngoscope. 2005;115:1030–1034. doi: 10.1097/01.MLG.0000163494.19965.DC. [DOI] [PubMed] [Google Scholar]
- 4.Brown DL, Bapuraj JR, Mukherji SK, Chervin RD, Concannon M, Helman JI, Lisabeth LD. MRI of the pharynx in ischemic stroke patients with and without obstructive sleep apnea. Sleep Med. 2010;11:540–544. doi: 10.1016/j.sleep.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong ML, Sandham A, Ang PK, Wong DC, Tan WC, Huggare J. Craniofacial morphology, head posture, and nasal respiratory resistance in obstructive sleep apnoea: an inter-ethnic comparison. Eur J Orthod. 2005;27:91–97. doi: 10.1093/ejo/cjh077. [DOI] [PubMed] [Google Scholar]
- 6.Vagiakis E, Kapsimalis F, Lagogianni I, Perraki H, Minaritzoglou A, Alexandropoulou K, Roussos C, Kryger M. Gender differences on polysomnographic findings in Greek subjects with obstructive sleep apnea syndrome. Sleep Med. 2006;7:424–430. doi: 10.1016/j.sleep.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Lim LL, Tham KW, Fook-Choong SMC. Obstructive sleep apnea in Singapore: polysomnographic data from a tertiary sleep disorder unit. Ann Acad Med Singapore. 2008;37:629–636. [PubMed] [Google Scholar]
- 8.Virkkula P, Bachour A, Hytönen M, Salmi T, Malmberg H, Hurmerinta K, Maasilta P. Snoring is not relieved by nasal surgery despite improvement in nasal resistance. Chest. 2006;129:81–87. doi: 10.1378/chest.129.1.81. [DOI] [PubMed] [Google Scholar]
- 9.Zhang XW, Li Y, Zhou F, Guo CK, Huang ZT. Comparison of polygraphic parameters in children with adenotonsillar hypertrophy with vs without obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2007;133:122–126. doi: 10.1001/archotol.133.2.122. [DOI] [PubMed] [Google Scholar]
- 10.Nixon GM, Kermack AS, McGregor CD, Davis GM, Manoukian JJ, Brown KA, Brouillette RT. Sleep and breathing on the first night after adenotonsillectomy for obstructive sleep apnea. Pediatr Pulmonol. 2005;39:332–338. doi: 10.1002/ppul.20195. [DOI] [PubMed] [Google Scholar]
- 11.McLean HA, Urton AM, Driver HS, Tan AK, Day AG, Munt PW, Fitzpatrick MF. Effect of treating severe nasal obstruction on the severity of obstructive sleep apnoea. Eur Respir J. 2005;25:521–527. doi: 10.1183/09031936.05.00045004. [DOI] [PubMed] [Google Scholar]
- 12.Iber C, Ancoli-Israel S, Chesson A, Quan SF, American Academy of Sleep Medicine . The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1. Westchester: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 13.Kushida CA, Chediak A, Berry RB, Brown LK, Gozal D, Iber C, Parthasarathy S, Quan SF, Rowley JA, Positive Airway Pressure Titration Task Force. American Academy of Sleep Medicine Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4:157–171. [PMC free article] [PubMed] [Google Scholar]
- 14.Bianchi MT, Cash SS, Mietus J, Peng C-K, Thomas R. Obstructive sleep apnea alters sleep stage transition dynamics. PLoS ONE. 2010;5(6):e11356. doi: 10.1371/journal.pone.0011356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang XW, Li Y, Zhou F, Guo CK, Huang ZT. Association of body position with sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Acta Otolaryngol. 2007;127:1316–1321. doi: 10.1080/00016480701283745. [DOI] [PubMed] [Google Scholar]
- 16.Polotsky VY, Rubin AE, Balbir A, Dean T, Smith PL, Schwartz AR, O’Donnell CP. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6 J mouse. Sleep Med. 2006;7:7–16. doi: 10.1016/j.sleep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Guilleminault C, Lopes MC, Hagen CC, da Rosa A. The cyclic alternating pattern demonstrates increased sleep instability and correlates with fatigue and sleepiness in adults with upper airway resistance syndrome. Sleep. 2007;30:641–647. doi: 10.1093/sleep/30.5.641. [DOI] [PubMed] [Google Scholar]
- 18.Bardwell WA, Moore P, Ancoli-Israel S, Dimsdale JE. Does obstructive sleep apnea confound sleep architecture findings in subjects with depressive symptoms? Biol Psychiatry. 2000;48:1001–1009. doi: 10.1016/S0006-3223(00)00887-8. [DOI] [PubMed] [Google Scholar]
- 19.Selwa LM, Marzec ML, Chervin RD, Weatherwax KJ, Vaughn BV, Foldvary-Schaefer N, Wang L, Song Y, Malow BA. Sleep staging and respiratory events in refractory epilepsy patients: is there a first night effect? Epilepsia. 2008;49:2063–2068. doi: 10.1111/j.1528-1167.2008.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchison KN, Song Y, Wang L, Malow BA. Analysis of sleep parameters in patients with obstructive sleep apnea studied in a hospital vs. a hotel-based sleep center. J Clin Sleep Med. 2008;4:119–122. [PMC free article] [PubMed] [Google Scholar]
- 21.Tamaki M, Nittono H, Hayashi M, Hori T. Examination of the first-night effect during the sleep-onset period. Sleep. 2005;28:195–202. doi: 10.1093/sleep/28.2.195. [DOI] [PubMed] [Google Scholar]
- 22.Bruyneel M, Sanida C, Art G, Libert W, Cuvelier L, Paesmans M, Sergysels R, Ninane V. Sleep efficiency during sleep studies: results of a prospective study comparing home-based and in-hospital polysomnography. J Sleep Res. 2010;20(1 Pt 2):201–206. doi: 10.1111/j.1365-2869.2010.00859.x. [DOI] [PubMed] [Google Scholar]
- 23.Ghoshal AG, Sarkar S, Roy DJ, Das RK, Ray M. Polysomnographic profile in a sleep laboratory in Kolkata: a retrospective analysis of 714 cases. J Assoc Physicians India. 2010;58:415–419. [PubMed] [Google Scholar]
- 24.Skaer TL, Sclar DA. Economic implications of sleep disorders. Pharmacoeconomics. 2010;28:1015–1023. doi: 10.2165/11537390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Israel N, Zigel Y, Tal A, Segev Y, Tarasiuk A. Adenotonsillectomy improves slow wave activity in children with obstructive sleep apnoea. Eur Respir J. 2010;37:1144–1150. doi: 10.1183/09031936.00106710. [DOI] [PubMed] [Google Scholar]
- 26.Zhang XW, Zhou F, Li Y, Huang ZT, Wang ZL. Sleep architecture in children with adenoidal hypertrophy. J Paediatr Child Health. 2006;42:625–629. doi: 10.1111/j.1440-1754.2006.00939.x. [DOI] [PubMed] [Google Scholar]
- 27.Ramos RT, da Cunha Daltro CH, Gregório PB, de Freitas Souza LS, de Andrade NA, de Souza Andrade Filho A, de Souza Machado A., Jr OSAS in children: clinical and polysomnographic respiratory profile. Braz J Otorhinolaryngol. 2006;72:355–361. doi: 10.1016/S1808-8694(15)30968-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahammam AS, Tate R, Manfreda J, Kryger MH. Upper airway resistance syndrome: effect of nasal dilation, sleep stage, and sleep position. Sleep. 1999;22:592–598. [PubMed] [Google Scholar]
- 29.Pevernagie D, Hamans E, Van Cauwenberge P, Pauwels R. External nasal dilation reduces snoring in chronic rhinitis patients: a randomized controlled trial. Eur Respir J. 2000;15:996–1000. doi: 10.1034/j.1399-3003.2000.01504.x. [DOI] [PubMed] [Google Scholar]
- 30.Djupesland PG, Skatvedt O, Borgersen AK. Dichotomous physiological effects of nocturnal external nasal dilation in heavy snorers: the answer to a rhinologic controversy? Am J Rhinol. 2001;15:95–103. doi: 10.2500/105065801781543745. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Wang PC, Chen YP, Lee LA, Fang TJ, Lin HC. Critical appraisal and meta-analysis of nasal surgery for obstructive sleep apnea. Am J Rhinol Allergy. 2010;24:1108–1111. doi: 10.2500/194589210791012416. [DOI] [PubMed] [Google Scholar]
- 32.Issa FG, Sullivan CE. The immediate effects of nasal continuous positive airway pressure treatment on sleep patterns in patients with obstructive sleep apnea. Electroencephalogrph Clin Neurophysiology. 1986;63:10–17. doi: 10.1016/0013-4694(86)90056-8. [DOI] [PubMed] [Google Scholar]
- 33.Aldrich M, Eiser A, Lee M, Shipley JE. Effects of continuous positive airway pressure on phasic events of REM sleep in patients with obstructive sleep apnea. Sleep. 1989;12:413–419. doi: 10.1093/sleep/12.5.413. [DOI] [PubMed] [Google Scholar]
- 34.Kondo T, Ishii H, Iga T, Nishiya K, Kobayashi I. Changes in sleep architecture by resumption of CPAP in patients with sleep apnea syndrome. Nihon Kokyuki Gakkai Zasshi. 2005;43:578–582. [PubMed] [Google Scholar]