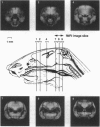
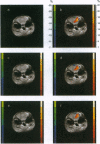
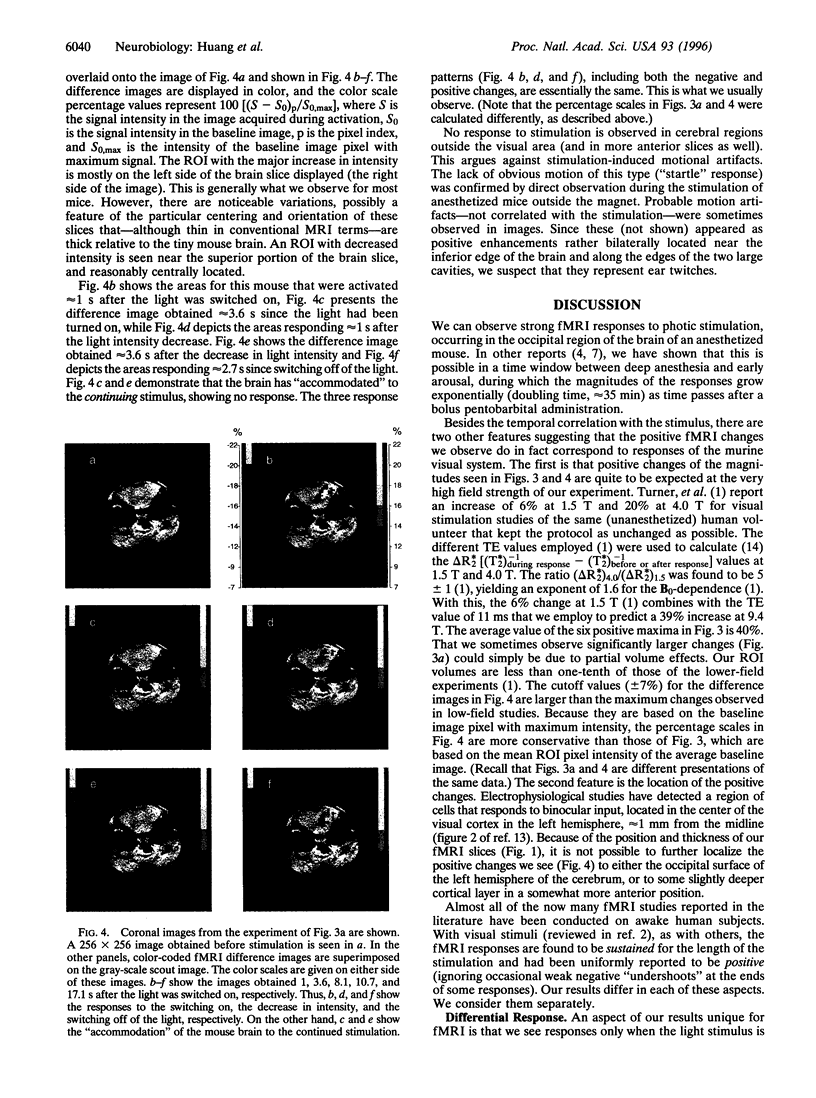
Abstract
Using a 9.4 T MRI instrument, we have obtained images of the mouse brain response to photic stimulation during a period between deep anesthesia and the early stages of arousal. The large image enhancements we observe (often >30%) are consistent with literature results extrapolated to 9.4 T. However, there are also two unusual aspects to our findings. (i) The visual area of the brain responds only to changes in stimulus intensity, suggesting that we directly detect operations of the M visual system pathway. Such a channel has been observed in mice by invasive electrophysiology, and described in detail for primates. (ii) Along with the typical positive response in the area of the occipital portion of the brain containing the visual cortex, another area displays decreased signal intensity upon stimulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert M. S., Huang W., Lee J. H., Patlak C. S., Springer C. S., Jr Susceptibility changes following bolus injections. Magn Reson Med. 1993 May;29(5):700–708. doi: 10.1002/mrm.1910290520. [DOI] [PubMed] [Google Scholar]
- Blamire A. M., Ogawa S., Ugurbil K., Rothman D., McCarthy G., Ellermann J. M., Hyder F., Rattner Z., Shulman R. G. Dynamic mapping of the human visual cortex by high-speed magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11069–11073. doi: 10.1073/pnas.89.22.11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr D. C., Morrone M. C., Ross J. Selective suppression of the magnocellular visual pathway during saccadic eye movements. Nature. 1994 Oct 6;371(6497):511–513. doi: 10.1038/371511a0. [DOI] [PubMed] [Google Scholar]
- Chen J. L., Wei L., Acuff V., Bereczki D., Hans F. J., Otsuka T., Finnegan W., Patlak C., Fenstermacher J. Slightly altered permeability-surface area products imply some cerebral capillary recruitment during hypercapnia. Microvasc Res. 1994 Sep;48(2):190–211. doi: 10.1006/mvre.1994.1049. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoe E. A., Van Essen D. C. Concurrent processing streams in monkey visual cortex. Trends Neurosci. 1988 May;11(5):219–226. doi: 10.1016/0166-2236(88)90130-0. [DOI] [PubMed] [Google Scholar]
- Frahm J., Merboldt K. D., Hänicke W., Kleinschmidt A., Boecker H. Brain or vein--oxygenation or flow? On signal physiology in functional MRI of human brain activation. NMR Biomed. 1994 Mar;7(1-2):45–53. doi: 10.1002/nbm.1940070108. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994 Feb 17;367(6464):607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Goodale M. A., Milner A. D. Separate visual pathways for perception and action. Trends Neurosci. 1992 Jan;15(1):20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Gyngell M. L. The application of steady-state free precession in rapid 2DFT NMR imaging: FAST and CE-FAST sequences. Magn Reson Imaging. 1988 Jul-Aug;6(4):415–419. doi: 10.1016/0730-725x(88)90478-x. [DOI] [PubMed] [Google Scholar]
- Hetherington H. P., Luney D. J., Vaughan J. T., Pan J. W., Ponder S. L., Tschendel O., Twieg D. B., Pohost G. M. 3D 31P spectroscopic imaging of the human heart at 4.1 T. Magn Reson Med. 1995 Mar;33(3):427–431. doi: 10.1002/mrm.1910330318. [DOI] [PubMed] [Google Scholar]
- Jezzard P., Heineman F., Taylor J., DesPres D., Wen H., Balaban R. S., Turner R. Comparison of EPI gradient-echo contrast changes in cat brain caused by respiratory challenges with direct simultaneous evaluation of cerebral oxygenation via a cranial window. NMR Biomed. 1994 Mar;7(1-2):35–44. doi: 10.1002/nbm.1940070107. [DOI] [PubMed] [Google Scholar]
- Labadie C., Lee J. H., Vétek G., Springer C. S., Jr Relaxographic imaging. J Magn Reson B. 1994 Oct;105(2):99–112. doi: 10.1006/jmrb.1994.1109. [DOI] [PubMed] [Google Scholar]
- Mangini N. J., Pearlman A. L. Laminar distribution of receptive field properties in the primary visual cortex of the mouse. J Comp Neurol. 1980 Sep 1;193(1):203–222. doi: 10.1002/cne.901930114. [DOI] [PubMed] [Google Scholar]
- Matin L., Picoult E., Stevens J. K., Edwards M. W., Jr, Young D., MacArthur R. Oculoparalytic illusion: visual-field dependent spatial mislocalizations by humans partially paralyzed with curare. Science. 1982 Apr 9;216(4542):198–201. doi: 10.1126/science.7063881. [DOI] [PubMed] [Google Scholar]
- Menon R. S., Ogawa S., Hu X., Strupp J. P., Anderson P., Uğurbil K. BOLD based functional MRI at 4 Tesla includes a capillary bed contribution: echo-planar imaging correlates with previous optical imaging using intrinsic signals. Magn Reson Med. 1995 Mar;33(3):453–459. doi: 10.1002/mrm.1910330323. [DOI] [PubMed] [Google Scholar]
- Morgan M. J. Vision. When it pays not to see. Nature. 1994 Oct 6;371(6497):473–473. doi: 10.1038/371473a0. [DOI] [PubMed] [Google Scholar]
- Ngai A. C., Ko K. R., Morii S., Winn H. R. Effect of sciatic nerve stimulation on pial arterioles in rats. Am J Physiol. 1988 Jan;254(1 Pt 2):H133–H139. doi: 10.1152/ajpheart.1988.254.1.H133. [DOI] [PubMed] [Google Scholar]
- Padmos P., Haaijman J. J., Spekreuse H. Visually evoked cortical potentials to patterned stimuli in monkey and man. Electroencephalogr Clin Neurophysiol. 1973 Aug;35(2):153–163. doi: 10.1016/0013-4694(73)90171-5. [DOI] [PubMed] [Google Scholar]
- Pang X. D., Bonds A. B. Visual evoked potential responses of the anesthetized cat to contrast modulation of grating patterns. Vision Res. 1991;31(9):1509–1516. doi: 10.1016/0042-6989(91)90128-r. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Logothetis N. K. The color-opponent and broad-band channels of the primate visual system. Trends Neurosci. 1990 Oct;13(10):392–398. doi: 10.1016/0166-2236(90)90117-s. [DOI] [PubMed] [Google Scholar]
- Schiller P. H. The ON and OFF channels of the visual system. Trends Neurosci. 1992 Mar;15(3):86–92. doi: 10.1016/0166-2236(92)90017-3. [DOI] [PubMed] [Google Scholar]
- Turner R., Jezzard P., Wen H., Kwong K. K., Le Bihan D., Zeffiro T., Balaban R. S. Functional mapping of the human visual cortex at 4 and 1.5 tesla using deoxygenation contrast EPI. Magn Reson Med. 1993 Feb;29(2):277–279. doi: 10.1002/mrm.1910290221. [DOI] [PubMed] [Google Scholar]
- Uğurbil K., Garwood M., Ellermann J., Hendrich K., Hinke R., Hu X., Kim S. G., Menon R., Merkle H., Ogawa S. Imaging at high magnetic fields: initial experiences at 4 T. Magn Reson Q. 1993 Dec;9(4):259–277. [PubMed] [Google Scholar]