SUMMARY
In mammals, calcium influx is required for oocyte maturation and egg activation. The molecular identities of the calcium-permeant channels that underlie the initiation of embryonic development are not established. Here, we describe a Transient Receptor Potential (TRP) ion channel current activated by TRP agonists that is absent in TrpV3−/− eggs. TRPV3 current is differentially expressed during oocyte maturation, reaching a peak of maximum density and activity at metaphase of meiosis II (MII), the stage of fertilization. Selective activation of TRPV3 channels provokes egg activation by mediating massive calcium entry. Widely used to activate eggs, strontium application is known to yield normal offspring in combination with somatic cell nuclear transfer. We show that TRPV3 is required for strontium influx, as TrpV3−/− eggs failed to permeate Sr2+ or undergo strontium-induced activation. We propose that TRPV3 is the major mediator of calcium influx in mouse eggs and is a putative target for artificial egg activation.
INTRODUCTION
Increases in the intracellular concentration of calcium ([Ca2+]i initiate a myriad of physiological processes in all cell types, including oocytes and eggs (Berridge et al., 2000; Clapham, 2007). Fully-grown mammalian oocytes are arrested in prophase of meiosis I, also known as the germinal vesicle (GV) stage, until puberty. At this time, an increase in luteinizing hormone (LH) triggers resumption of meiosis (maturation) and progression to the metaphase stage of the second meiosis (MII). This process is known as oocyte maturation. Mature oocytes (eggs) are ovulated and arrested at the MII stage until fertilization. Oocyte maturation is accompanied by an increase in the content of Ca2+ stores ([Ca2+]ER) and Ca2+ influx from the extracellular milieu is required for this increase (Cheon et al., 2013). Oocytes deprived of external Ca2+ ([Ca2+]e) or chelation of [Ca2+]i do not complete meiosis I, suggesting that disruption of Ca2+ signaling uncouples the cell cycle machinery (MPF-MAPK) from nuclear maturation (Homa, 1995).
Spermatozoa deliver a male-specific phospholipase C, PLCζ, to the egg that triggers a series of [Ca2+]i responses that coordinate the exit of MII and progression to the interphase stage, inducing events known collectively as egg activation (Ducibella et al., 2002; Saunders et al., 2002; Schultz and Kopf, 1995). Thus, it is generally accepted that Ca2+ influx and intracellular Ca2+ release are necessary to complete maturation (Homa, 1995) and to sustain [Ca2+]i oscillations (Kline and Kline, 1992b) during egg activation. The channels that mediate Ca2+ influx during these stages have not been established.
The complement of Ca2+ channels expressed in mammalian oocytes has not been completely investigated. Voltage-gated Ca2+ channels (Cav), consistent with CaV3 (T type) Ca2+ channels, have been measured in mature mouse eggs (Peres, 1987). During mouse fertilization, changes in the membrane potential are small (Igusa et al., 1983; Jaffe and Cross, 1984) and the oocyte membrane potential is depolarized relative to CaV current activation thresholds. Thus, most CaV current should be inactivated. In contrast, the relatively voltage-insensitive TRP channels are non-selective, calcium-permeant, channels that function over a much larger range of potentials. In general, TRP channels are modulated by a variety of stimuli and ligands, including G-protein coupled receptors (Ramsey et al., 2006; Venkatachalam and Montell, 2007). TRPV3, a highly temperature-dependent channel with Q10>20 above 32 °C (Peier et al., 2002; Smith et al., 2002; Xu et al., 2002) is most highly expressed on skin and mucosal surfaces, but is also present in dorsal root ganglion, brain, and testis. Here we show that it is also expressed in mouse oocytes and eggs. We found that TRPV3 functional expression increased during oocyte maturation from GV to MII stages. Using mice in which TrpV3 had been deleted (TrpV3−/− or TrpV3-KO), we show that specific stimulation of TRPV3 promotes Ca2+ entry and induces egg activation.
Mammalian eggs can be parthenogenetically activated by a variety of artificial stimuli, but replacement of external Ca2+ by 5–10 mM strontium (Sr2+) has been extensively used to induce activation in rodents (Kline and Kline, 1992a; Liu et al., 2002). This procedure is not associated with chromosome abnormalities (O’Neill et al., 1991) and is capable of promoting full-term development when combined with somatic cell nuclear transplantation (Wakayama et al., 1998). We demonstrate that Sr2+ influx during strontium-induced egg activation occurs via TRPV3 channels.
RESULTS
Functional expression of TRPV3 channels in mouse eggs
We used whole-cell patch clamp methods to record from mouse eggs and determine their ion conductances. Prior work established the primary voltage-gated calcium, potassium, and chloride currents in oocytes (Bountra and Martin, 1987; Day et al., 1993; Kolajova et al., 2001; Okamoto et al., 1977; Peres, 1987; Seguin and Baltz, 1997). Here we focus on whether oocytes/eggs have TRP channels.
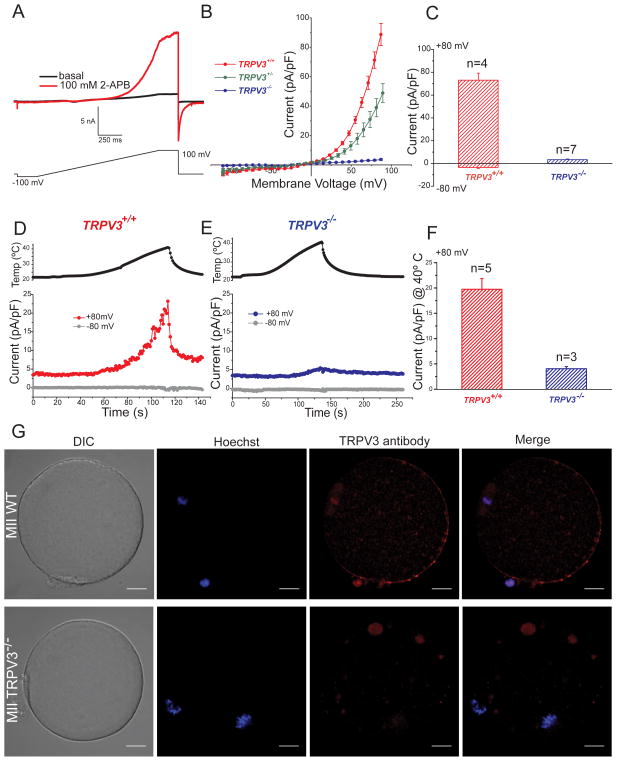
In response to a voltage ramp (Fig. 1A), addition of the nonselective agent 2-APB (2-aminoethoxydiphenyl borate, 100 μM) evoked an outwardly rectifying current with properties characteristic of TRPV3 (Hu et al., 2004). This current was present in TrpV3+/+ (WT, CD1 strain) and TrpV3+/− (heterozygous, V3-Het), but absent in TrpV3−/− (V3-KO) eggs (Fig 1B), confirming the identity of the channel as TRPV3. Fig. 1C summarizes the average current amplitudes. Because V3-KO animals used in the initial study were generated from a mixed strain background (Sv129EvTac/C57BALB6) and variations in behavioral responses can be strain-dependent (Huang et al., 2011), we tested responses to the aforementioned agonists in other mouse strains including C57BALB6, Sv129EvTac, CD1, CF1, and the WT mixed background Sv129EvTac/C57BALB6. All WT strains exhibited similar TRPV3 currents (data not shown). We compared reproductive parameters between V3-KO, V3-Het, and WT females, and found no differences in the number of eggs per superovulation (Fig. S1B) or fertility, as reflected by the number of pups/litter (7.4 ± 0.7 for V3-KO and V3-Het, Fig. S1A).
Figure 1.
TRPV3 channels are expressed in MII mouse egg. A–F. Whole-cell voltage clamp recordings from an MII mouse egg. A. Current evoked from a voltage ramp from −100 to +100 mV in the absence (black trace) and presence (red trace) of 100 μM 2-APB. B. Current-voltage relations (I–Vs) in response to 100 μM 2-APB in WT (TrpV3+/+, CD-1 strain), heterozygous (TrpV3+/−) and eggs lacking TRPV3 (TrpV3-KO, TrpV3−/−). C. Averaged 2-APB evoked current recorded at +80 mV and −80 mV in WT and V3-KO eggs (73 ± 6 pA/pF for WT and 3.3 ± 0.5 pA/pF for V3-KO at +80 mV; −3.5 ± 0.8 pA/pF for WT and −0.2 ± 0.04 pA/pF for V3-KO eggs at −80 mV (± S.E.M). D–E. Temperature responses for WT and V3-KO eggs. Upper panels: Temperature (egg surface). Lower panels: Current responses at +80 mV (red trace) and −80 mV (gray trace) for WT and V3-KO eggs. F. Average TRPV3 current in response to 40°C recorded at +80 mV for WT and KO eggs. Current was 20 ± 2 pA/pF in WT eggs in contrast to only 4 ± 0.4 pA/pF in V3-KO eggs. The averaged inward current at −80 mV was −0.6 ± 0.2 pA/pF for WT and −0.4 ± 0.08 pA/pF for V3-KO eggs (data not shown). ± S.E.M; # of experiments are indicated over the bars. G. TRPV3 protein localization in mature mouse MII zona-free eggs; shown are differential interference contrast (DIC), DNA staining (Hoechst), and anti-TRPV3 staining. Upper panel: WT (129SvEvTac strain), Lower panel: TrpV3-KO egg. Most of the antibody-stained protein is at the membrane, except at the animal pole. Scale bar: 10 μm.
Since 2-APB is not a selective agent, we next investigated temperature-induced TRPV3 activation in MII eggs (Peier et al., 2002; Smith et al., 2002; Xu et al., 2002). Increasing bath temperature from 23°C to 40°C elicited a large outwardly rectifying whole-cell current (Fig. 1D), which was not present in V3-KO eggs (Fig. 1E). As is typical for TRPV3 current (Xu et al., 2002), it rapidly deactivated after removal of the heating stimulus (Fig. 1D). The average heat-activated currents at 40°C, again consistent with TRPV3 properties, were absent in V3-KO cells (Fig. 1F). To determine the molecular identity and distribution of TRPV3 on the mouse egg surface, we performed immunofluorescence studies using an anti-TRPV3 antibody. In WT eggs, TRPV3 protein was fairly evenly distributed throughout the plasma membrane, but was remarkably absent from the zone overlying the spindle (animal pole, Fig. 1G). V3-KO egg surface TRPV3 reactivity was absent, as expected (Fig. 1G). In summary, TRPV3 channels are preferentially located on the vegetal pole of the plasma membrane and are activated by temperature elevation in the physiological range and by the nonselective TRPV3 agonist, 2-APB.
Functional TRPV3 channels progressively increase during oocyte maturation
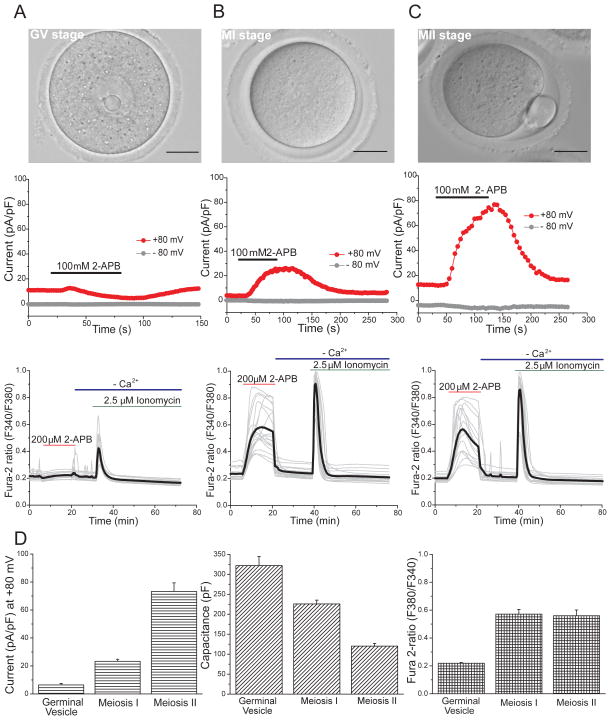
Since significant changes take place in Ca2+ signaling in both oocytes and eggs during maturation (Homa, 1995; Jones et al., 1995; Wakai and Fissore, 2013), we explored changes in TRPV3 functional expression at different oocyte stages. During the GV stage, addition of 100 μM 2-APB did not induce a measurable current (Fig. 2A). Similarly, Ca2+ imaging studies at this stage revealed that addition of 200 μM 2-APB failed to trigger an increase in [Ca2+]i (Fig. 2A, D). Once oocytes had undergone germinal vesicle breakdown (GVBD) and progressed to MI (Fig. 2B), application of 2-APB evoked a TRPV3 current (Fig. 2B) of 23 ± 2 pA/pF (Fig. 2D, left panel) and a [Ca2+]i increase (Fig. 2B, D). At MII (Fig. 2C), when eggs become competent to mount oscillations in response to fertilization, TRPV3 channels responded to 2-APB with a current of 73 ± 6 pA/pF (Fig. 2C, D), which is significantly higher than in MI eggs. Thus, TRPV3 more densely populates the MII membrane compared to the MI membrane (in channels/μm2). Despite their much smaller surface area (120 ± 7 pF vs. 226 ± 9.5 pF, Fig. 2D) and higher TRPV3 channel density, [Ca2+]i increases in MII eggs were similar in magnitude to those in MI (Fig. 2C), reflecting the lower sensitivity of Ca2+ measurements compared with direct voltage clamp recordings of current (due to native Ca2+ buffers and variations in resting membrane potential in unclamped oocytes). Addition of ionomycin, a Ca2+ ionophore that under Ca2+-free conditions causes Ca2+ release from the intracellular stores, induced a response in all stages of maturation (Fig. 2 A–C, lower panel).
Figure 2.
ITRPV3 in response to 2-APB during oocyte maturation. A–C. Upper panels: Germinal vesicle (GV) oocyte, Meiosis I (MI) and Meiosis II (MII) eggs. Scale bar: 50 μm. Middle panel: Whole-cell patch -clamp recording in response to 100 μM 2-APB (black bar). Lower panel: Changes in [Ca2+]i induced by 200 μM 2-APB (red bars; black= averaged trace. GV, n=19; MI, n=19; MII, n=16) and ionomycin (green bars) in nominal Ca2+-free solutions (blue bars). D. Summary of parameters measured at different stages of oocyte maturation. Left panel: peak current measured at +80 mV in response to 100 μM 2-APB (GV, n=4; MI, n=3; MII, n=4). Middle panel: Whole-cell capacitance measurements (pF) (GV, n=4; MI, n=5; MII, n=10). Right panel: Peak fura-2 ratio, reflecting relative [Ca2+]i. Data are averages ± S.E.M. Scale bar: 50 μm.
Our results show that the number of TRPV3 channels increases during oocyte maturation, being undetectable with electrophysiological recordings in the germinal vesicle (GV) stage and reaching maximal absolute numbers of functional channels in the MII stage (0.1 channels/μm2 in MII vs. 0.034 channels/μm2 in MI, assuming an equal open probability, Po =0.5 for both stages). They also show that ion channel density in oocytes and eggs is very low compared to excitable cells in which these values are typically 103–104 -fold larger (Hille, 2001).
Carvacrol, a TRPV3 agonist, induces [Ca2+]i responses and activation in mouse eggs
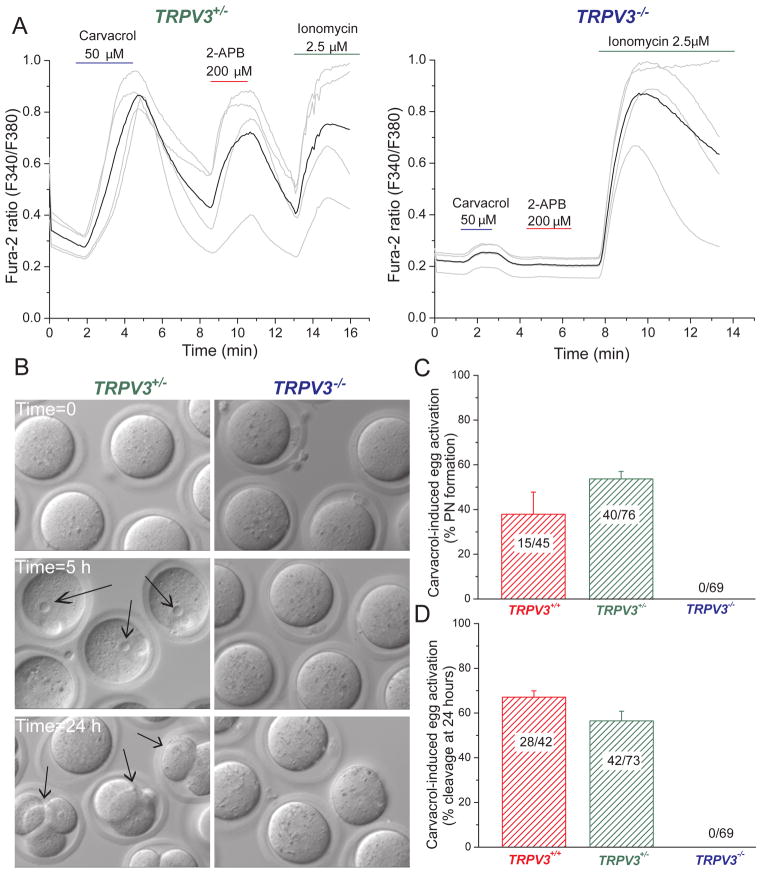
Carvacrol, the major ingredient in oregano, elicits TRPV3-mediated currents in heterologously expressing cells and in primary keratinocytes (Cheng et al., 2010; Xu et al., 2006). Because V3-Het eggs had robust TRPV3 currents in response to 2-APB, we performed functional studies in eggs isolated from heterozygous (V3-Het) and knockout (V3-KO) littermates. Addition of 50 μM carvacrol to V3-Het eggs evoked a substantial increase in [Ca2+]i (Fig. 3A, left panel) and responded with an additional [Ca2+]i increase following the addition of 200 μM 2-APB. In contrast, eggs from V3-KO mice failed to respond to either agonist (Fig. 3A, right panel). We next tested whether stimulation of TRPV3 channel activates mature eggs. Incubation of WT and V3-Het eggs with 50 μM carvacrol provoked parthenogenesis, as pronuclear (PN) formation was observed within 5 h after carvacrol treatment in roughly half of the mature eggs (Fig. 3B, C). Cleavage to the 2-cell stage was observed 24 h after treatment (67 ± 3% in WT eggs and 56 ± 4% in V3-Het eggs, Fig. 3B, D). Consistent with Ca2+ imaging data, carvacrol failed to activate V3-KO eggs (Fig. 3B – D), but did respond to the addition of the Ca2+ ionophore, ionomycin (Fig. 4B). Furthermore, incubation of V3-Het eggs with 200 μM 2-APB also induced high rates of egg activation, but had no effect on eggs from V3-KO mice (Fig. 4A).
Figure 3.
The TRPV3 channel agonist, carvacrol, induces Ca2+ responses and activation in MII mouse eggs. A. Changes in [Ca2+]i induced by TRPV3 channels activated by 50 μM carvacrol (violet), 200 μM 2-APB (red), and ionomycin (green) in TrpV3+/− and TrpV3−/− cells (V3-Het, n=4; V3-KO, n=4). B. Activation of TrpV3+/−, but not TrpV3−/− eggs, by treatment with 50 μM carvacrol (37°C, 10 min). Arrows indicate PN formation (5 h, left panel) and cell cleavage (24 h, left panel). C. Percentages of PN formation in WT (TrpV3+/+, CD1 strain), heterozygous (TrpV3+/−) and V3-KO (TrpV3−/−) eggs 5–6 h after of carvacrol activation. Numbers of eggs undergoing PN formation/total number of eggs is indicated. D. Percentage of eggs cleaved after 24 h exposure to carvacrol. Numbers of 2, 3, or 4-cell blastomeres over the total number of eggs is indicated. Data are averages ± S.E.M.
Figure 4.
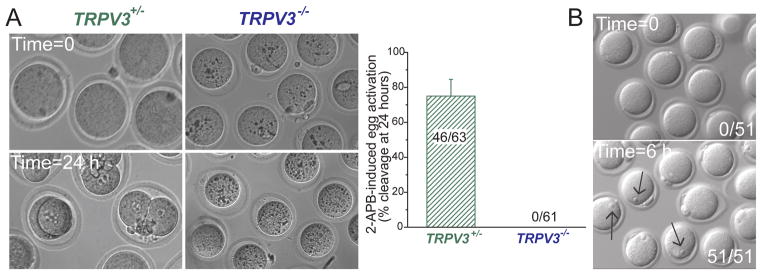
2-APB induces egg activation in TrpV3+/− eggs but not in TrpV3−/− MII eggs. A. Treatment with 2-APB (200 μM; 37°C) for 30 min activates eggs. Left panel: 2-APB induces cleavage, observed 24 h after 200 μM 2-APB treatment in TrpV3-Het MII eggs, but not in TrpV3-KO eggs. Right panel: Percentage of eggs undergoing cleavage at 24 h. Numbers of 2, 3, or 4-cell blastomeres over the total number of eggs is indicated. Data are averages ± S.E.M. B. Ionomycin (2.5 μM for 5 min) at 37°C induces activation of TrpV3-KO eggs. Numbers of eggs with PN formation/total number of eggs is indicated. Arrows indicate PN formation (right panel).
TRPV3 channels mediate Sr2+ influx and subsequent egg activation
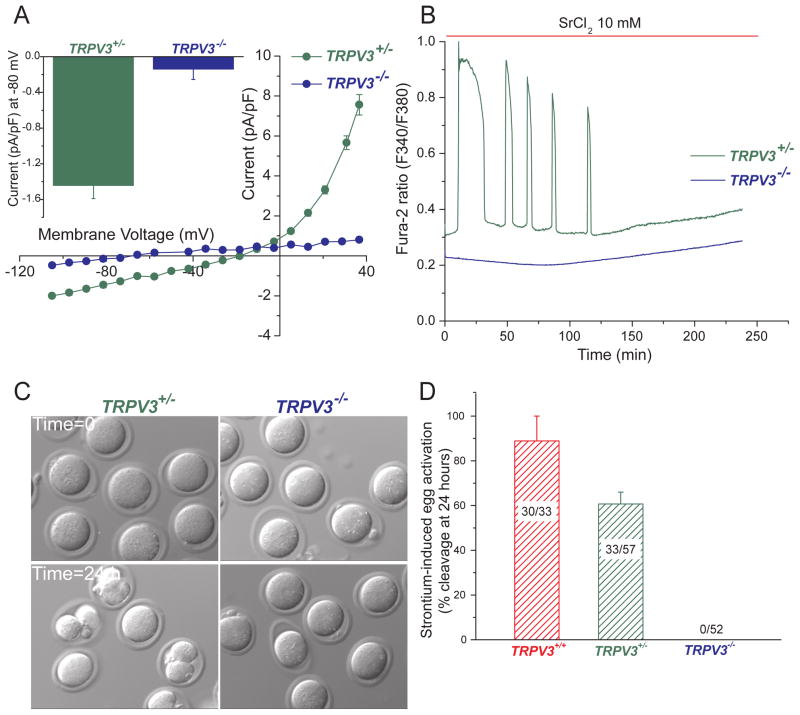
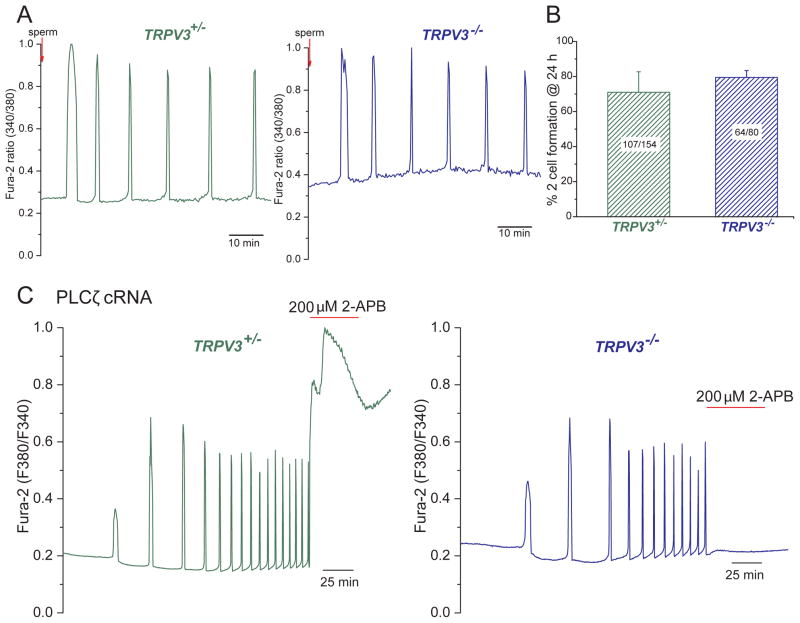
Sr2+ has been widely used to induce parthenogenesis in rodent eggs (Kono et al., 1996; Li et al., 2009; Tomashov-Matar et al., 2005). The channel that mediates Sr2+ influx has not been reported in any species, and whether Sr2+ and Ca2+ permeate the same channel(s) in mouse eggs is unknown. To ascertain whether the TRPV3 channel mediates Sr2+ influx, we recorded the heat-activated TRPV3 current in V3-Het and V3-KO cells in the presence of an extracellular solution containing 10 mM SrCl2 and nominal [Ca2+] (~10 μM) (Fig. 5A). At −80 mV, the current measured in V3-Het cells averaged −1.4 ± 0.1 pA/pF, while the current in V3-KO cells was 10-fold lower (−0.14 ± 0.1 pA/pF). Similarly, at +80 mV, V3-Het current averaged 36 ± 2 pA/pF, 36-fold larger than V3-KO current (1.0 ± 0.06 pA/pF; data not shown). Reversal potentials were consistent with Sr2+ and Na+ permeation in V3-Het eggs (Erev ~ −15 mV) and a lack of permeability in V3-KO eggs (Erev <−60 mV; Fig. 5A). The absence of inward Sr2+ current in V3-KO eggs correlates with the lack of SrCl2-induced oscillations in these eggs (Fig. 5B) and their inability to become activated in media supplemented with SrCl2, as assessed by 2-cell formation (Fig. 5C,D). Nearly all WT eggs (89 ± 11 %) and over half of V3-Het eggs (61 ± 5.3 %) became activated in these experiments.
Figure 5.
TRPV3 channels mediate Sr2+ influx and subsequent egg activation. A. Results of whole-cell patch -clamp recording (37°C) of MII eggs from heterozygous (TrpV3+/−, green symbols, n=4) and TrpV3-KO (TrpV3−/−, blue symbols, n=4) mice. Current-voltage (I–V) relation in 10 mM SrCl2. Inset: Averaged current at −80 mV. B. Oscillations induced by Sr2+ in a TrpV3+/− egg (green line, n=6), but not in a TrpV3-KO egg (blue line, n=8). C. Sr2+ induces cleavage in V3-Het (left) but not in V3-KO (right) eggs. D. Percentage of cleaved eggs after 24 h of 10 mM Sr2+ treatment. Numbers of 2, 3, or 4-cell blastomeres/total number of eggs is indicated. Data are averages ± S.E.M.
TRPV3-mediated Ca2+ influx is not required for maintenance of calcium oscillations
The fertilization-associated [Ca2+]i oscillations that underlie activation of mammalian eggs are thought to be triggered by the sperm-specific PLCζ. PLCζ activates the egg’s phosphoinositide pathway to generate IP3, which in turn gates the endoplasmic reticular calcium-permeant IP3R (Miyazaki and Ito, 2006; Saunders et al., 2002). While the first few oscillations of Ca2+ rely on internal Ca2+ stores, the persistence of the Ca2+oscillations requires Ca2+ influx (Igusa and Miyazaki, 1983; Kline and Kline, 1992b; Winston et al., 1995). To ascertain whether TRPV3 channels are required for these oscillations in response to fertilization, we tested sperm-induced calcium oscillations in V3-KO and V3-Het MII eggs. We observed the first [Ca2+]i transients 5–10 min after addition of sperm (Fig. 6A; see (Swann, 2013)). No difference was observed between the two groups during 2.5 h of recording. Confirming the in vivo data (Fig. S1A), no differences were found for in vitro fertilization success, assessed as the rate of 2-cell formation between V3-KO and V3-Het eggs (Fig. 6B). We next examined whether the activity of TRPV3 channels was required to support oscillations induced by injection of PLCζ cRNA. These oscillations, which were monitored for 6 h, were similar in V3-KO and V3-Het MII eggs with respect to time to initiation, amplitude, frequency and duration (Fig. 6C). These data suggest that PLCζ1 does not substantially potentiate TRPV3 as a mechanism of its action on Ca2+ oscillations. However, addition of 200 μM 2-APB to V3-Het eggs that were undergoing oscillations at 37°C dramatically increased Ca2+ influx and prevented [Ca2+]i from returning to baseline, immediately blocking oscillations in V3-KO eggs (Fig. 6C). Finally and as expected, the addition of the TRPV3 agonist, carvacrol (50 μM), to oscillating WT eggs increased Ca2+ influx and immediately halted the oscillations. Addition of carvacrol to oscillating V3-KO eggs had no effect in the pattern or frequency of the Ca2+ oscillations (Fig. S2A).
Figure 6.
Calcium influx through TRPV3 channels does not contribute to early development in mouse eggs. A. Oscillations induced by sperm fertilization in heterozygous (left, TrpV3+/−, green line, n=12/24 have 5–6 oscillations in 60 min) and V3-KO (right) eggs (blue line, n=9/18 have 5–6 oscillations in 60 min). B. In vitro fertilization (IVF) rates measured by 2-cell formation after 24 h of fertilization showed no difference between V3-Het and V3-KO eggs (71 ± 12 for V3-Het vs. 80 ± 4 for V3-KO eggs, p > 0.05). Numbers of 2-cell blastomeres/total number of eggs is indicated. Data are averages ± S.E.M. C. [Ca2+]i responses were induced by injection of 0.01μg/μl mPLCζ cRNA (similar responses were obtained by 0.05 μg/μl mPLCζ cRNA injection). [Ca2+]i oscillations (37°C) in a control egg (TrpV3+/−, left panel, n=8) and KO egg (TrpV3−/−, right panel, n=3). 200 μM 2-APB (red bar) was applied at the end of the experiment.
Examination of Compensatory Mechanisms for loss of TrpV3
The lack of an effect of TRPV3 deletion on fertilization-induced oscillations could be due to compensatory Ca2+ influx by other channels. One possibility is that other TRP channels were upregulated in V3-KO mice. If this were the case, we should have observed them in our recordings. Small CaV3 (T type) currents (~5 pA/pF) are present in all the oocyte stages studied here, and previously identified as Cav3.2 (Kang et al., 2007). Cav3.2 KO animals do not display a fertility phenotype (Chen et al., 2003) and following in vitro fertilization, Cav3.2-KO eggs [Ca2+] oscillated at normal frequencies and durations (M. Bernhardt et al., 2013, Soc. for the study of Reproduction (SSR), abstract #405). In agreement with these data, the calcium channel blocker, mibefradil (20 μM) did not prevent in vitro oocyte maturation in WT and V3-KO oocytes, and PLCζ injection still activated these eggs (data not shown).
When [Ca2+]ER is severely depleted, the Stim/Orai complex is assembled to mediate CRAC current in many cells (Lewis, 2011). The contribution of CRAC/SOCE channels to Ca2+ homeostasis in mouse oocytes and eggs is controversial. Thapsigargin, which blocks SERCA pumps and thus activates CRAC, prematurely terminated oscillations, and was assumed to reflect CRAC activation in this process (Kline and Kline, 1992b). However, Ca2+ overload alone, as we observed with TRPV3 stimulation, also terminates Ca2+ oscillations. Recent studies suggest that CRAC channels are not functional in mouse eggs (Miao et al., 2012; Takahashi et al., 2013). We evaluated the pattern of PLCζ-induced calcium oscillations in V3-KO and Het-V3 eggs in the presence of the CRAC blocker STA-12-5775 (RO2959; 3μM (Chen et al., 2013)). In both groups of eggs, the frequency of calcium oscillations after the addition of the blocker was unaltered, showing no compensation of TRPV3 channel function by CRAC channels (data not shown).
DISCUSSION
Here we examined the contribution of a TRP channel to Ca2+ influx in mouse oocytes and eggs during maturation and fertilization. Using voltage clamp and calcium imaging measurements of WT and V3-KO mouse oocytes, we show that TRPV3 becomes the major calcium entry pathway in these cells as they mature. Calcium permeation via TRPV3, as induced by temperature elevation, and the TRPV3 agonists 2-APB and carvacrol, were confirmed using TRPV3 knockout mice. Notably, TRPV3 channels are the major mediators of SrCl2 influx during strontium-induced activation in mouse eggs. SrCl2 failed to induce oscillations or egg activation in TRPV3-null eggs.
The small T-type, CaV3.2, currents in eggs do not appear to be necessary or sufficient for calcium oscillations. In any case, the normally depolarized egg membrane potential insures that they are primarily inactivated (see also (Okamoto et al., 1977; Peres, 1987). Thus, Cav channels would be most likely be important during an acute depolarizing stimulus, such as fertilization in some species (Whitaker, 2006). Interestingly, commonly used Cav1 (L-type) and Cav3 (T-type) channel-blocking drugs are not known to be associated with human female infertility.
Another potential source of calcium entry, CRAC currents, might be active at the GV stage of mouse oocytes, but their function decreases as maturation progresses (Cheon et al., 2013; Lee et al., 2013). Expression of Stim1 and Orai1 was detected in mouse oocytes and eggs (Cheon et al., 2013) but to date, no Stim1 or Orai1 subtype female heterozygous or KO mice are reported to be subfertile. Since Orai1−/− and Stim1−/− mice died perinatally, tissue- and age-specific genetic deletion would clarify this issue. Direct measurements of CRAC current under voltage clamp would likely clear up these inconsistencies, but the size of eggs makes this task difficult due to the size of background currents. In our studies, if CRAC currents are present, they are ≤ 3 pA/pF (in 20 mM [Ca2+]e,), but still large enough to be significant in maintaining cytoplasmic and ER levels. We found that addition of a specific CRAC (Orai1, Orai3) channel blocker during in vitro maturation of Het-V3 and V3-KO oocytes did not affect GVBD, although we observed a slight decrease in the percentage of polar body extrusion in both groups. Further experiments, such as tissue-specific deletion of Orai subunits, are necessary to clarify the function of CRAC channels during maturation.
Functional expression of TRPV3 channels during mouse oocyte maturation
At the germinal vesicle stage of oocyte development, TRPV3 currents were undetectable and, consistent with this observation; conditions that activate TRPV3 fail to induce Ca2+ influx. By the MI stage, however, small TRPV3 currents were recorded and these became significantly larger (in both net amplitude and density) in MII oocytes. Thus during maturation, increases in TRPV3 functional expression coincides with meiotic progression during maturation. [Ca2+]e is required for progression beyond GVBD during oocyte maturation (Wakai and Fissore, 2013); without [Ca2+]e the formation of the polar body is inhibited and oocytes fail to progress past MI (Tombes et al., 1992). In vivo, the immature GV stage oocyte maintains direct communication with the surrounding cumulus and granulosa cells through gap junctions, permitting heterologous metabolic and electrical coupling (Homa, 1995). However, spontaneous calcium oscillations recorded in GV stage oocytes are not dependent on the surrounding follicle cells (Carroll and Swann, 1992).
Although TRPV3 currents coincide with meiotic progression during maturation, the TRPV3 channel function is not required for this process (data not shown). The striking fact is that V3-KO females mice have normal fertility, number of offspring per litter, and the mice have only minor phenotypes related to skin and hair rather than mating or reproduction (Cheng et al., 2010). Constitutively active TRPV3 mutations cause hair loss and increase susceptibility to dermatitis and inflammatory skin lesions in rodents (Asakawa et al., 2006; Yoshioka et al., 2009), although without apparent defects in female fertility. The Olmsted syndrome, a rare human congenital disorder producing palmoplantar keratoderma, alopecia, and severe itching, is associated with TRPV3 gain-of-function mutations (Lin et al., 2012). However, it is unclear whether female fertility is affected.
Stimulation of TRV3 channels leads to egg activation
Activation of TRPV3 by carvacrol and 2-APB induces calcium entry, which can parthenogenetically activate eggs. Treatment with calcium ionophores such as ionomycin or A213187 can result in embryos with decreased or impaired developmental competence. The long-term ramifications on offspring are unknown (Swain and Pool, 2008), even though these manipulations are used in patients who have low fertilization potential (Heindryckx et al., 2005; Nasr-Esfahani et al., 2010). Thus, the understanding of TRPV3 expression and function in human eggs may be an opportunity to improve artificial oocyte activation (AOA) via specific activation of TRPV3 channels rather than the use of nonspecific ionophores.
Sr2-induced egg activation and TRPV3 channels
Strontium induces egg activation by promoting oscillations in [Ca2+]i/[Sr2+], perhaps by sensitizing the egg IP3R1 receptors. These SrCl2-induced calcium oscillations are distinct from those provoked by the sperm (or by adenophostin, a nonhydrolyzable agonist of the IP3R) and do not induce down regulation of the IP3R (Brind et al., 2000; Jellerette et al., 2000). The mechanism of Sr2+ gating of the IP3R1 is thus the TRPV3 channel, since it mediates Sr2+ influx into rodent eggs. It has been suggested that Sr2+ could sensitize IP3Rs and facilitate calcium oscillations (Zhang et al., 2005), perhaps by substituting for calcium in the potentiation of IP3Rs (Bezprozvanny et al., 1991; Girard and Clapham, 1993; Marshall and Taylor, 1994; Stehno-Bittel et al., 1995). Strontium-induced egg activation is mediated by CaMKIIγ as CaMKIIγ−/− eggs failed to respond to Sr2+ treatment, likely due to a failure to resume meiosis II (Backs et al., 2010).
The fact that V3-KO eggs do not to respond to Sr2+ is clear evidence that under the conditions in which Sr2+-induced activation was measured (37°C, 10 mM SrCl2 in the extracellular media, ~10 μM [Ca]), TRPV3 is required for measurable strontium permeation. This does not mean that TRPV3 is the only strontium-permeable channel in the egg plasma membrane. In fact, CaV channels, present in the egg plasma membrane, are permeable to Sr2+ as shown by Hirano et al. (Hirano et al., 1989a; Hirano et al., 1989b), but CaV3 channels are largely inactivated at egg resting membrane potentials. Since the egg membrane potential remains stable at potentials in which CaVs are inactivated (Igusa et al., 1983), we did not observe measurable Sr2+ permeation via CaV, positioning TRPV3 as the major mechanism for Sr2+-induced egg activation in mice.
Regulation and function of TRPV3 channels in mouse oocytes and eggs
Muscarinic receptors couple to the Gq/11 proteins in mouse eggs to activate PLCβ (Igarashi et al., 2007; Williams et al., 1998) and initiate PI(4,5)P2 hydrolysis. This, in turn, generates IP3 and the PKC activator, diacylglycerol (Clapham, 2007). Muscarinic agonists induce membrane potential changes (Eusebi et al., 1979) and a series of small [Ca2+]i oscillations in mouse eggs (Swann, 1992). Stimulation of PKC activity greatly enhances the frequency of these oscillations (Halet et al., 2004). These same pathways also modify TRPV3 channels: in somatic cells, carbachol (Xu et al., 2006), diacylglycerol, phorbol-12-myristate-13-acetate (Hu et al., 2006), and PLC-coupled receptor-catalyzed PI(4,5)P2 hydrolysis (Doerner et al., 2011) all modulate TRPV3 activity. Ca2+, via calmodulin, also activates CaMKII to modulate oscillatory changes during fertilization (Markoulaki et al., 2003) and govern exit from MII (Ducibella and Fissore, 2008). Ca2+/calmodulin initially inhibits TRPV3 activity, perhaps explaining sensitization of TRPV3 to repetitive stimulation by mediating Ca2+-dependent initial channel inhibition (Xiao et al., 2008). In summary, there is good evidence that PLC activation regulates both oocyte and egg TRPV3 and [Ca2+]i.
In order to test modulation of TRPV3 activity by hormones, we patch-clamped MII eggs in the presence of progesterone (30 μM), estradiol (100 μM), and prostaglandin E2 (1 μM). None of these had an effect on TRPV3 current at RT (data not shown).
PLCζ, cloned from a mouse spermatid cDNA library, is unlike PLCβ in that it is soluble, has no pleckstrin homology domain, and is active at resting (100 nM) [Ca2+] (Kouchi et al., 2004). PLCζ is expressed only in males. Injection of PLCζ cRNA results in egg Ca2+ oscillations that are prevented by inhibition of protein synthesis (Saunders et al., 2002). Since male-specific PLCζ more effectively generates PI(4,5)P2 production in eggs (Swann and Lai, 2013), we would expect that PLCζ overexpression would potentiate endogenous egg TRPV3 current. In spite of the expression of TRPV3 in MII mouse eggs and its predicted nearly constitutive function at 37°C, TRPV3-mediated Ca2+ influx is seemingly dispensable for maintaining PLCζ-induced [Ca2+]i oscillations or for fertility, as V3-KO females show normal oscillatory responses in eggs and after fertilization.
What is the function of TRPV3 in oocytes and eggs? One possibility is that TRPV3 channels constitutively conduct low levels of Ca2+ to maintain Ca2+ homeostasis. Nevertheless, given the importance of these functions, eggs may have a redundant system that may explain the unchanged oscillation pattern and fertility in V3-KO eggs. It is likely that an unknown G protein receptor may be activated to induce PLCβ activation and modulate TRPV3 activity. Less probably, but more intriguing, is that highly temperature-sensitive TRPV3 may function to ‘reawaken’ egg maturation/activation in mammals that significantly lower their body temperatures during hibernation, and that TRPV3 is thus not functionally relevant in mammals that do not hibernate. Increasing ambient temperatures above 32°C would increase TRPV3 activity and prime progression to MII. Finally, it is possible that there are unknown endogenous direct activators of oocyte and egg TRPV3. Future studies will examine the expression of TRPV3 in other species, such as humans, as well as the regulation of TRPV3 function.
EXPERIMENTAL PROCEDURES
Oocyte collection
Six-to-ten-week-old females (CD1, TrpV3−/− colony (Cheng et al., 2010)) were superovulated with intraperitoneal (i.p.) injection of 5 IU pregnant mare’s serum gonadotropin (PSMG, Calbiochem, EMD Biosciences), followed 48 h later by i.p. injection of 5 IU of human chorionic gonadotropin (hCG, Calbiochem, EMD Biosciences). Ovulated eggs (cumulus masses) were obtained by pulling the oviducts open with fine forceps in a HEPES-buffered culture medium (M2 medium, Millipore) 13–16 h after administration of hCG. Cumulus cells were removed using hyaluronidase (Calbiochem, EMD Biosciences) and gentle aspiration through a pipette. The zona pellucidae (ZP) were removed by exposure to Tyrode’s acid solution (pH 2.5) for a few seconds followed by thorough washing in M2 medium. GV oocytes were collected from the ovaries of 5- to 12-week-old CD-1 female mice. Females were injected with 5 IU PMSG and cumulus cell-enclosed oocytes were recovered 42–46 h later into HEPES-buffered culture medium (M2 medium) and 100 μM isobutyl-1-methylxanthine (IBMX). Cumulus cells were removed by repeated pipetting and denuded oocytes were matured in HTF medium at 37°C in 5% CO2. After 5–6 hours of in vitro maturation, eggs were in MI. All animal experiments were approved by the Boston Children’s Hospital Institutional Animal Use and Care Committee (Protocols #13-03-2380R and 11-01-1879).
Electrophysiology
Whole-cell currents were measured at 22–24°C using an Axopatch 200B amplifier digitized at 20 kHz (Digidata 1320A) and filtered at 5 kHz Electrophysiology recordings were performed on the same day of egg isolation 8 hours post-surgery. Eggs were maintained in human tubal fluid medium (HTF, EMD Millipore) at 37°C and 5% CO2. For temperature ramps, the perfusate was heated using a TC-324B temperature controller and SH-27B solution heater (Warner Instrument Corporation). Data were analyzed using Clampfit (Molecular Devices) and Origin 7.0 (OriginLab). Pipettes of 1–3 MΩ resistance were made from glass capillaries (593600, A–M systems). Series resistance was compensated by 60–80%. The intracellular solution contained (in mM): 152 Cs-Methanesulfonate, 1 mM Cs-BAPTA, 10 HEPES, 2 MgATP, 8 NaCl, 0.3 NaGTP, pH: 7.3–7.4. The external solution contained (in mM): 125 NaCl, 6 KCl, 20 mM CaCl2, 1.2 MgCl2, 20 mM HEPES-NaOH, pH: 7.3–7.4. The response to 2-aminoethoxydiphenyl borate (2-APB, Sigma) were measured in an external solution containing (in mM): 140 mM NaCl, 10 mM HEPES, 10 mM glucose, 4 mM KCl, 1 mM MgCl2, and 2 mM CaCl2. In order to avoid chloride currents (Qu and Hartzell, 2000) a solution of Na-gluconate was used in the temperature response experiments and in the Sr2+ permeability experiments. The osmolarity of all solutions was 290–300 mOsm. All voltages were corrected for measured junction potentials present between the internal and external solution before seal formation. TRPV3 currents were activated by voltage ramps from −100 mV to 100 mV (600 ms, every 2 s), in the presence of 2-APB or temperature ramps. The holding potential (HP) was −80 mV. For Sr2+ permeability recordings and to avoid T-type voltage gated Ca2+ channels, ramps were from +100 mV to −100 mV with a HP of 0 mV.
[Ca2+]i imaging
Intracellular Ca2+ ([Ca2+]i) was estimated using the Ca2+ sensitive dye Fura-2AM (Life Technologies). Briefly, oocytes/eggs were loaded with 1.25 μM Fura-2AM supplemented with 0.02% pluronic acid (Life Technologies) for 30 min at room temperature. To estimate [Ca2+]i, oocytes/eggs were thoroughly washed and attached to glass-bottom chambers. Responses to TRPV3 agonists were recorded in FCS-free (serum free) HEPES-buffered Chatot, Ziomek, and Bavister (HCZB) medium. For Figures 2, 5B, 6C, and S2A., the Ca2+ measurements were performed on a Nikon Diaphot microscope fitted for fluorescence measurements. The objective used was a 20x Nikon Fluor and the excitation lamp was a 75 W Xenon lamp. Emitted light >510 nm was collected by a cooled Photometrics SenSys CCD camera (Roper Scienti c) using SimplePCI (C-Imaging Systems). In Fig. 3A and 6A, cells were alternatively illuminated with 340 and 380 nm light (Lambda DG-4; Sutter Instrument Co.) for 75–100ms, emission light >510 nm was captured by CCD camera. The setup was equipped with a 175 W Xenon lamp; objective 20x UApo/340. Data was analyzed with Slidebook software (Intelligent Imaging Innovations) after background subtraction. All fura-2AM ratios were normalized.
Parthenogenetic egg activation
For TRPV3-mediated egg activation, eggs were incubated at 37°C for 10 min in 50 μM carvacrol in CZB medium or for 30 min in 200 μM 2-APB in modified Krebs–Ringer bicarbonate medium. In both cases, the media was FCS-free 0.1% PVA (Poly-vinyl alcohol, Sigma). In studies using V3-KO eggs, activation was induced by exposure to 2.5 μM ionomycin (A.G. Scientific) for 5 min in Ca2+-free CZB media followed by incubation for 4h in CZB (0.1% PVA) supplemented with cycloheximide (CHX, 20 μg/ml, EMD Biosciences). All procedures were in humidified 5% CO2 at 37°C. For Sr2+ activation, eggs were incubated eggs for 2 h in Ca2+-free-CZB medium supplemented with 10 mM SrCl2. Eggs were then washed and transferred to HTF medium and cultured to the 2-cell stage. Eggs were evaluated for signs of activation 5 and 24 h after treatment under phase contrast microscopy. Activated eggs were classified according to the following criteria: (1) PN group, consisted of zygotes forming a single PN with first and second polar bodies (5 h post-treatment); (2) cleaved group; eggs undergoing immediate cleavage after 24 h. Eggs without 2nd polar bodies, PN formation, or those failing to cleave were considered as unactivated (MII egg). Fragmented eggs were excluded from analysis.
Microinjections
Oocytes were microinjected as described (Kurokawa et al., 2005). Reagents were loaded onto glass micropipettes and 7–12 pL (1–3% of the total volume of the egg) delivered by pneumatic pressure (PLI-100 picoinjector, Harvard Apparatus). The full-length of mouse PLCζ, cDNA, a kind gift from Dr. K. Fukami (Tokyo University of Pharmacy and Life Science, Japan) was subcloned into pcDNA6/ myc-His (Invitrogen) for in vitro transcription. Plasmids were linearized and the cDNA was in vitro transcribed using the T7 mMESSAGEmMACHINEKit (Ambion).
See Supplemental Methods for Sperm isolation, IVF, and Immunofluorescence staining
Supplementary Material
HIGHLIGHTS.
TRPV3 channels are functionally expressed in mouse oocytes and eggs.
TRPV3 channels are progressively expressed during oocyte maturation.
TRPV3 channels can mediate Ca2+ influx and parthenogenesis in MII eggs.
Strontium influx and subsequent egg activation in mice requires TRPV3 channels.
Acknowledgments
This work was supported by NIH grants U01 HD045857 to D.E.C. and HD051872 to R.A.F. We thank Dr. Jie Jin in establishing and maintenance of the TRPV3 mouse colony and the Clapham laboratory and Dr. Matthias Piesche for comments.
Footnotes
The authors declare no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES and CITATIONS
- Asakawa M, Yoshioka T, Matsutani T, Hikita I, Suzuki M, Oshima I, Tsukahara K, Arimura A, Horikawa T, Hirasawa T, et al. Association of a mutation in TRPV3 with defective hair growth in rodents. J Invest Dermatol. 2006;126:2664–72. doi: 10.1038/sj.jid.5700468. [DOI] [PubMed] [Google Scholar]
- Backs J, Stein P, Backs T, Duncan FE, Grueter CE, McAnally J, Qi X, Schultz RM, Olson EN. The gamma isoform of CaM kinase II controls mouse egg activation by regulating cell cycle resumption. Proc Natl Acad Sci U S A. 2010;107:81–6. doi: 10.1073/pnas.0912658106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–4. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bountra C, Martin RJ. Single-channel currents from zona-free mouse eggs. Q J Exp Physiol. 1987;72:483–92. doi: 10.1113/expphysiol.1987.sp003090. [DOI] [PubMed] [Google Scholar]
- Brind S, Swann K, Carroll J. Inositol 1,4,5-trisphosphate receptors are downregulated in mouse oocytes in response to sperm or adenophostin A but not to increases in intracellular Ca(2+) or egg activation. Dev Biol. 2000;223:251–65. doi: 10.1006/dbio.2000.9728. [DOI] [PubMed] [Google Scholar]
- Carroll J, Swann K. Spontaneous cytosolic calcium oscillations driven by inositol trisphosphate occur during in vitro maturation of mouse oocytes. J Biol Chem. 1992;267:11196–201. [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, et al. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science. 2003;302:1416–8. doi: 10.1126/science.1089268. [DOI] [PubMed] [Google Scholar]
- Chen G, Panicker S, Lau KY, Apparsundaram S, Patel VA, Chen SL, Soto R, Jung JK, Ravindran P, Okuhara D, et al. Characterization of a novel CRAC inhibitor that potently blocks human T cell activation and effector functions. Mol Immunol. 2013;54:355–67. doi: 10.1016/j.molimm.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Cheng X, Jin J, Hu L, Shen D, Dong XP, Samie MA, Knoff J, Eisinger B, Liu ML, Huang SM, et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell. 2010;141:331–43. doi: 10.1016/j.cell.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon B, Lee HC, Wakai T, Fissore RA. Ca2+ influx and the store-operated Ca2+ entry pathway undergo regulation during mouse oocyte maturation. Mol Biol Cell. 2013;24:1396–410. doi: 10.1091/mbc.E13-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day ML, Pickering SJ, Johnson MH, Cook DI. Cell-cycle control of a large-conductance K+ channel in mouse early embryos. Nature. 1993;365:560–2. doi: 10.1038/365560a0. [DOI] [PubMed] [Google Scholar]
- Doerner JF, Hatt H, Ramsey IS. Voltage- and temperature-dependent activation of TRPV3 channels is potentiated by receptor-mediated PI(4,5)P2 hydrolysis. J Gen Physiol. 2011;137:271–88. doi: 10.1085/jgp.200910388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducibella T, Fissore R. The roles of Ca2+, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol. 2008;315:257–79. doi: 10.1016/j.ydbio.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil JP. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Dev Biol. 2002;250:280–91. [PubMed] [Google Scholar]
- Eusebi F, Mangia F, Alfei L. Acetylcholine-elicited responses in primary and secondary mammalian oocytes disappear after fertilisation. Nature. 1979;277:651–3. doi: 10.1038/277651a0. [DOI] [PubMed] [Google Scholar]
- Girard S, Clapham D. Acceleration of intracellular calcium waves in Xenopus oocytes by calcium influx. Science. 1993;260:229–32. doi: 10.1126/science.8385801. [DOI] [PubMed] [Google Scholar]
- Halet G, Tunwell R, Parkinson SJ, Carroll J. Conventional PKCs regulate the temporal pattern of Ca2+ oscillations at fertilization in mouse eggs. J Cell Biol. 2004;164:1033–44. doi: 10.1083/jcb.200311023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindryckx B, Van der Elst J, De Sutter P, Dhont M. Treatment option for sperm- or oocyte-related fertilization failure: assisted oocyte activation following diagnostic heterologous ICSI. Hum Reprod. 2005;20:2237–41. doi: 10.1093/humrep/dei029. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland, Massachusetts: Sinauer Associates, Inc; 2001. [Google Scholar]
- Hirano Y, Fozzard HA, January CT. Characteristics of L- and T-type Ca2+ currents in canine cardiac Purkinje cells. Am J Physiol. 1989a;256:H1478–92. doi: 10.1152/ajpheart.1989.256.5.H1478. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Fozzard HA, January CT. Inactivation properties of T-type calcium current in canine cardiac Purkinje cells. Biophys J. 1989b;56:1007–16. doi: 10.1016/S0006-3495(89)82745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homa ST. Calcium and meiotic maturation of the mammalian oocyte. Mol Reprod Dev. 1995;40:122–34. doi: 10.1002/mrd.1080400116. [DOI] [PubMed] [Google Scholar]
- Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, Lee LY, Wood JD, Zhu MX. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–8. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- Hu HZ, Xiao R, Wang C, Gao N, Colton CK, Wood JD, Zhu MX. Potentiation of TRPV3 channel function by unsaturated fatty acids. J Cell Physiol. 2006;208:201–12. doi: 10.1002/jcp.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Li X, Yu Y, Wang J, Caterina MJ. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol Pain. 2011;7:37. doi: 10.1186/1744-8069-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi H, Knott JG, Schultz RM, Williams CJ. Alterations of PLCbeta1 in mouse eggs change calcium oscillatory behavior following fertilization. Dev Biol. 2007;312:321–30. doi: 10.1016/j.ydbio.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igusa Y, Miyazaki S. Effects of altered extracellular and intracellular calcium concentration on hyperpolarizing responses of the hamster egg. J Physiol. 1983;340:611–32. doi: 10.1113/jphysiol.1983.sp014783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igusa Y, Miyazaki S, Yamashita N. Periodic hyperpolarizing responses in hamster and mouse eggs fertilized with mouse sperm. J Physiol. 1983;340:633–47. doi: 10.1113/jphysiol.1983.sp014784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe LA, Cross NL. Electrical properties of vertebrate oocyte membranes. Biol Reprod. 1984;30:50–4. doi: 10.1095/biolreprod30.1.50. [DOI] [PubMed] [Google Scholar]
- Jellerette T, He CL, Wu H, Parys JB, Fissore RA. Down-regulation of the inositol 1,4,5-trisphosphate receptor in mouse eggs following fertilization or parthenogenetic activation. Dev Biol. 2000;223:238–50. doi: 10.1006/dbio.2000.9675. [DOI] [PubMed] [Google Scholar]
- Jones KT, Carroll J, Whittingham DG. Ionomycin, thapsigargin, ryanodine, and sperm induced Ca2+ release increase during meiotic maturation of mouse oocytes. J Biol Chem. 1995;270:6671–7. doi: 10.1074/jbc.270.12.6671. [DOI] [PubMed] [Google Scholar]
- Kang D, Hur CG, Park JY, Han J, Hong SG. Acetylcholine increases Ca2+ influx by activation of CaMKII in mouse oocytes. Biochem Biophys Res Commun. 2007;360:476–82. doi: 10.1016/j.bbrc.2007.06.083. [DOI] [PubMed] [Google Scholar]
- Kline D, Kline JT. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev Biol. 1992a;149:80–9. doi: 10.1016/0012-1606(92)90265-i. [DOI] [PubMed] [Google Scholar]
- Kline D, Kline JT. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J Biol Chem. 1992b;267:17624–30. [PubMed] [Google Scholar]
- Kolajova M, Hammer MA, Collins JL, Baltz JM. Developmentally regulated cell cycle dependence of swelling-activated anion channel activity in the mouse embryo. Development. 2001;128:3427–34. doi: 10.1242/dev.128.18.3427. [DOI] [PubMed] [Google Scholar]
- Kono T, Jones KT, Bos-Mikich A, Whittingham DG, Carroll J. A cell cycle-associated change in Ca2+ releasing activity leads to the generation of Ca2+ transients in mouse embryos during the first mitotic division. J Cell Biol. 1996;132:915–23. doi: 10.1083/jcb.132.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, Takenawa T, Miyazaki S. Recombinant phospholipase Czeta has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J Biol Chem. 2004;279:10408–12. doi: 10.1074/jbc.M313801200. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Sato K, Wu H, He C, Malcuit C, Black SJ, Fukami K, Fissore RA. Functional, biochemical, and chromatographic characterization of the complete [Ca2+]i oscillation-inducing activity of porcine sperm. Dev Biol. 2005;285:376–92. doi: 10.1016/j.ydbio.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Lee B, Palermo G, Machaca K. Down-regulation of store-operated Ca2+ entry during mammalian meiosis is required for the egg-to-embryo transition. J Cell Sci. 2013 doi: 10.1242/jcs.121335. [DOI] [PubMed] [Google Scholar]
- Lewis RS. Store-operated calcium channels: new perspectives on mechanism and function. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Mizutani E, Ono T, Wakayama T. Production of normal mice from spermatozoa denatured with high alkali treatment before ICSI. Reproduction. 2009;137:779–92. doi: 10.1530/REP-08-0476. [DOI] [PubMed] [Google Scholar]
- Lin Z, Chen Q, Lee M, Cao X, Zhang J, Ma D, Chen L, Hu X, Wang H, Wang X, et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet. 2012;90:558–64. doi: 10.1016/j.ajhg.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Trimarchi JR, Keefe DL. Haploidy but not parthenogenetic activation leads to increased incidence of apoptosis in mouse embryos. Biol Reprod. 2002;66:204–10. doi: 10.1095/biolreprod66.1.204. [DOI] [PubMed] [Google Scholar]
- Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–25. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markoulaki S, Matson S, Abbott AL, Ducibella T. Oscillatory CaMKII activity in mouse egg activation. Dev Biol. 2003;258:464–74. doi: 10.1016/s0012-1606(03)00133-7. [DOI] [PubMed] [Google Scholar]
- Marshall IC, Taylor CW. Two calcium-binding sites mediate the interconversion of liver inositol 1,4,5-trisphosphate receptors between three conformational states. Biochem J. 1994;301 (Pt 2):591–8. doi: 10.1042/bj3010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao YL, Stein P, Jefferson WN, Padilla-Banks E, Williams CJ. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc Natl Acad Sci U S A. 2012;109:4169–74. doi: 10.1073/pnas.1112333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S, Ito M. Calcium signals for egg activation in mammals. J Pharmacol Sci. 2006;100:545–52. doi: 10.1254/jphs.cpj06003x. [DOI] [PubMed] [Google Scholar]
- Nasr-Esfahani MH, Deemeh MR, Tavalaee M. Artificial oocyte activation and intracytoplasmic sperm injection. Fertil Steril. 2010;94:520–6. doi: 10.1016/j.fertnstert.2009.03.061. [DOI] [PubMed] [Google Scholar]
- O’Neill GT, Rolfe LR, Kaufman MH. Developmental potential and chromosome constitution of strontium-induced mouse parthenogenones. Mol Reprod Dev. 1991;30:214–9. doi: 10.1002/mrd.1080300308. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Takahashi K, Yamashita N. Ionic currents through the membrane of the mammalian oocyte and their comparison with those in the tunicate and sea urchin. J Physiol. 1977;267:465–95. doi: 10.1113/jphysiol.1977.sp011822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–9. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- Peres A. The calcium current of mouse egg measured in physiological calcium and temperature conditions. J Physiol. 1987;391:573–88. doi: 10.1113/jphysiol.1987.sp016757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E, Van Deusen AL, Vitko I. Molecular pharmacology of human Cav3.2 T-type Ca2+ channels: block by antihypertensives, antiarrhythmics, and their analogs. J Pharmacol Exp Ther. 2009;328:621–7. doi: 10.1124/jpet.108.145672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z, Hartzell HC. Anion permeation in Ca(2+)-activated Cl(-) channels. J Gen Physiol. 2000;116:825–44. doi: 10.1085/jgp.116.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–47. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129:3533–44. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Kopf GS. Molecular basis of mammalian egg activation. Curr Top Dev Biol. 1995;30:21–62. doi: 10.1016/s0070-2153(08)60563-3. [DOI] [PubMed] [Google Scholar]
- Seguin DG, Baltz JM. Cell volume regulation by the mouse zygote: mechanism of recovery from a volume increase. Am J Physiol. 1997;272:C1854–61. doi: 10.1152/ajpcell.1997.272.6.C1854. [DOI] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–90. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L, Luckhoff A, Clapham DE. Calcium release from the nucleus by InsP3 receptor channels. Neuron. 1995;14:163–7. doi: 10.1016/0896-6273(95)90250-3. [DOI] [PubMed] [Google Scholar]
- Swain JE, Pool TB. ART failure: oocyte contributions to unsuccessful fertilization. Hum Reprod Update. 2008;14:431–46. doi: 10.1093/humupd/dmn025. [DOI] [PubMed] [Google Scholar]
- Swann K. Different triggers for calcium oscillations in mouse eggs involve a ryanodine-sensitive calcium store. Biochem J. 1992;287 (Pt 1):79–84. doi: 10.1042/bj2870079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann K. Measuring Ca2+ oscillations in mammalian eggs. Methods Mol Biol. 2013;957:231–48. doi: 10.1007/978-1-62703-191-2_16. [DOI] [PubMed] [Google Scholar]
- Swann K, Lai FA. PLCzeta and the initiation of Ca(2+) oscillations in fertilizing mammalian eggs. Cell Calcium. 2013;53:55–62. doi: 10.1016/j.ceca.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kikuchi T, Kidokoro Y, Shirakawa H. Ca(2+) influx-dependent refilling of intracellular Ca(2+) stores determines the frequency of Ca(2+) oscillations in fertilized mouse eggs. Biochem Biophys Res Commun. 2013;430:60–5. doi: 10.1016/j.bbrc.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Tomashov-Matar R, Tchetchik D, Eldar A, Kaplan-Kraicer R, Oron Y, Shalgi R. Strontium-induced rat egg activation. Reproduction. 2005;130:467–74. doi: 10.1530/rep.1.00746. [DOI] [PubMed] [Google Scholar]
- Tombes RM, Simerly C, Borisy GG, Schatten G. Meiosis, egg activation, and nuclear envelope breakdown are differentially reliant on Ca2+, whereas germinal vesicle breakdown is Ca2+ independent in the mouse oocyte. J Cell Biol. 1992;117:799–811. doi: 10.1083/jcb.117.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakai T, Fissore RA. Ca(2+) homeostasis and regulation of ER Ca(2+) in mammalian oocytes/eggs. Cell Calcium. 2013;53:63–7. doi: 10.1016/j.ceca.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–74. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- Whitaker M. Calcium at fertilization and in early development. Physiol Rev. 2006;86:25–88. doi: 10.1152/physrev.00023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CJ, Mehlmann LM, Jaffe LA, Kopf GS, Schultz RM. Evidence that Gq family G proteins do not function in mouse egg activation at fertilization. Dev Biol. 1998;198:116–27. [PubMed] [Google Scholar]
- Winston NJ, McGuinness O, Johnson MH, Maro B. The exit of mouse oocytes from meiotic M-phase requires an intact spindle during intracellular calcium release. J Cell Sci. 1995;108 (Pt 1):143–51. doi: 10.1242/jcs.108.1.143. [DOI] [PubMed] [Google Scholar]
- Xiao R, Tang J, Wang C, Colton CK, Tian J, Zhu MX. Calcium plays a central role in the sensitization of TRPV3 channel to repetitive stimulations. J Biol Chem. 2008;283:6162–74. doi: 10.1074/jbc.M706535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–35. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–6. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Imura K, Asakawa M, Suzuki M, Oshima I, Hirasawa T, Sakata T, Horikawa T, Arimura A. Impact of the Gly573Ser substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J Invest Dermatol. 2009;129:714–22. doi: 10.1038/jid.2008.245. [DOI] [PubMed] [Google Scholar]
- Zhang D, Pan L, Yang LH, He XK, Huang XY, Sun FZ. Strontium promotes calcium oscillations in mouse meiotic oocytes and early embryos through InsP3 receptors, and requires activation of phospholipase and the synergistic action of InsP3. Hum Reprod. 2005;20:3053–61. doi: 10.1093/humrep/dei215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.