Abstract
OBJECTIVE.
The purpose of our study was to accurately estimate the radiation dose to skin and the eye lens from clinical CT brain perfusion studies, investigate how well scanner output (expressed as volume CT dose index [CTDIvol]) matches these estimated doses, and investigate the efficacy of eye lens dose reduction techniques.
MATERIALS AND METHODS.
Peak skin dose and eye lens dose were estimated using Monte Carlo simulation methods on a voxelized patient model and 64-MDCT scanners from four major manufacturers. A range of clinical protocols was evaluated. CTDIvol for each scanner was obtained from the scanner console. Dose reduction to the eye lens was evaluated for various gantry tilt angles as well as scan locations.
RESULTS.
Peak skin dose and eye lens dose ranged from 81 mGy to 348 mGy, depending on the scanner and protocol used. Peak skin dose and eye lens dose were observed to be 66–79% and 59–63%, respectively, of the CTDIvol values reported by the scanners. The eye lens dose was significantly reduced when the eye lenses were not directly irradiated.
CONCLUSION.
CTDIvol should not be interpreted as patient dose; this study has shown it to overestimate dose to the skin or eye lens. These results may be used to provide more accurate estimates of actual dose to ensure that protocols are operated safely below thresholds. Tilting the gantry or moving the scanning region further away from the eyes are effective for reducing lens dose in clinical practice. These actions should be considered when they are consistent with the clinical task and patient anatomy.
Keywords: CT perfusion, eye lens dose, Monte Carlo simulation, radiation dose, skin dose
With the increased z-axis coverage and improved temporal sampling rate of MDCT scanners, brain perfusion scanning has become a viable tool for evaluating cerebral perfusion defects in patients with a suspicion of stroke. CT perfusion plays an important role in determining the nature, age, mechanism, and potential reversibility of a stroke rapidly and within the critical therapeutic time window [1]. Brain perfusion examinations with MDCT are also an important tool in the evaluation of brain tumors. Applications include using differences in the intrinsic perfusion characteristics of brain neoplasms to determine the malignant potential and assessing response to therapy by monitoring changes of the integrity of the blood-brain barrier [2].
CT perfusion imaging requires repeatedly exposing one location of the head to monitor the uptake and washout of iodinated contrast. These images are then used as an input for postprocessing calculations that allow estimation of functional cerebral perfusion parameters such as mean transit time, cerebral blood flow, tissue permeability, and cerebral blood volume [3]. When repeatedly scanning the same volume of brain tissue, there is typically no table motion during the scan. However, some recent advances have enabled scanning modes in which the table is moved rapidly back and forth to increase z-axis coverage while maintaining a sufficient temporal sampling. In either approach, the accumulated radiation dose to the skin or eye lens can be high, leading to concerns about potential radiation injury from these scans. For example, high radiation doses to local tissues (skin, lens of eye) may be delivered that have the potential to cause deterministic effects such as erythema (skin reddening), epilation (hair loss), or cataractogenesis (if the eye lenses are exposed to the x-ray beam). According to some recent studies, the thresholds for these effects could be as low as 1 Gy or even lower [4-7]. Several approaches have been suggested to avoid direct exposure of the eye lens, such as tilting the gantry to avoid the lens or maximizing the distance between the scanning volume and the eyes. However, the effectiveness of these techniques in reducing lens dose has not been clearly shown using Monte Carlo simulations.
It is important for radiologists, medical physicists, and CT technologists to understand local radiation dose to skin and the eye lens from CT brain perfusion examinations to avoid unnecessary radiation hazards. The purpose of this study is to use Monte Carlo simulation methods to accurately estimate the radiation dose to the eye lens and skin from CT brain perfusion studies, investigate how closely the dose metric reported on the scanner console (volume CT dose index [CTDIvol]) matches actual eye lens and skin doses estimated using Monte Carlo methods, and investigate the efficacy of dose reduction techniques to exclude the globes from the primary scanning range.
Materials and Methods
Monte Carlo Simulation Methods and CT Scanner Models
Monte Carlo–based methods are widely used to simulate the transport of photons through tissue and are often used in radiation dosimetry [8-10]. A series of previously developed MDCT source models based on Monte Carlo methods were used in this study to estimate absorbed radiation dose [11]. The Monte Carlo N-Particle eXtended (MCNPX) code, which was created at the Los Alamos National Laboratory [12, 13], was used to develop MDCT source models to simulate scanners from all major manufacturers, including the Sensation 64 Siemens Healthcare), LightSpeed VCT (GE Healthcare), Brilliance 64 (Philips Healthcare), and Aquilion 64 (Toshiba). Each of these MDCT source models has been benchmarked against physical measurements made in phantoms under a variety of conditions, and each agreed to within 5% [14]. The simulations take into account various aspects of the individual CT scanner designs and CT perfusion protocol parameters, such as x-ray beam spectra, bowtie filtration, x-ray collimation characteristics, scan location, and other factors [15, 16]. The user is able to specify acquisition parameters, such as tube voltage (kVp), tube current-time product (mAs), collimation, and so on.
Patient Model
An adult female model (Irene, GSF [now Helmholtz Zentrum München]) was used to estimate dose to the skin and eye lens. This model was developed on the basis of whole-body CT images of a 32-year-old woman by individually segmenting radiosensitive organs and tissues, including the skin and eye lens [17]. The weight and the height of the patient model were 51 kg and 163 cm, respectively. The voxels in the model were 1.875 × 1.875 × 5 mm, with a resolution of 5 mm in the longitudinal direction. Each voxel in the patient phantom was assigned a tissue type, described by elemental composition and mass density, with values derived from the organ composition tables in the International Commission on Radiation Units Report 44 [18].
Peak Skin Dose and Eye Lens Dose Estimation
The radiation dose was assessed in each voxel of the patient model using the MCNPX mesh tally feature. The peak skin dose was computed by selecting the highest value of absorbed dose among the voxels identified as skin; the eye lens dose was computed by averaging the absorbed dose for voxels within the eye lens region.
Monte Carlo Simulation of Brain Perfusion CT Examinations
Simulations were performed using cerebral perfusion acquisition protocols posted on the Website of the American Association of Physicists in Medicine (AAPM) [19]. These included protocols for three of the four scanners used in this study and are summarized in Table 1. Because the published protocols do not include a protocol for the Aquilion 64 (only Aquilion One and Aquilion Premium, Toshiba), this scanner was excluded from this part of the study. It should be noted that all the protocols used lower tube potential (e.g., for quantitative studies) and relatively lower tube current-time product compared with noncerebral perfusion protocols because in brain perfusion CT examinations the image quality is not as crucial as in other examinations, e.g., a routine head examination. Because the pixels are routinely binned during postprocessing, hence smoothing the images, the acquisition parameters need not resemble a routine head examination [20]. In all simulations, there was no table motion, and repeated axial scans were simulated.
TABLE 1.
Posted Protocols From American Association of Physicists in Medicine (AAPM) [19] for the Scanners and Modes in This Study
| Scanner and Mode | Kilovoltage (kVp) | Bowtie | Nominal Collimation (Total) (mm) |
Milliampere Seconds (mAs)/Rotation |
No. of Rotations | Total mAs |
|---|---|---|---|---|---|---|
|
| ||||||
| Sensation 64 | 80 | General | 24 × 1.2 (28) | 270 | 40 | 10,800 |
| VCT axial mode | 80 | Head | 64 × 0.625 (40) | 150 | 22 | 3300 |
| VCT cine mode | 80 | Head | 64 × 0.625 (40) | 150 | 45 | 6750 |
| Brilliance 64 nonjog mode | 80 | General | 32 × 1.25 (40) | 125 | 30 | 3750 |
Note—Sensation 64 manufactured by Siemens Healthcare, VCT manufactured by GE Healthcare, and Brilliance 64 manufactured by Philips Healthcare. There is no table movement for all four protocols listed. For Sensation 64 and VCT cine mode, the x-ray beams are on continuously; for VCT axial mode and Brilliance 64 nonjog mode, the x-ray beams alternate between on and off, and the acquisitions are not continuous.
Clinically, the locations of perfusion defects may vary, requiring different anatomy to be scanned. However, to represent the maximum dose scenario, all of the simulated brain perfusion studies were performed at the location where the eye lenses were completely covered by the primary beam (Fig. 1). CTDIvol is a standardized quantity that measures the radiation output of the scanners using a specific measurement phantom; CTDIvol is not a measure of patient dose [21]. It depends on the CT scanner model and the beam quality. The values of CTDIvol that correspond to each of these specific protocols were from the AAPM website. These values were compared with the Monte Carlo method estimated doses to the eye lens and peak skin dose from each protocol to determine the differences between estimated patient dose and the scanner output (CTDIvol) associated with the protocol.
Fig. 1.
Anterioposterior view of patient model (Irene, GSF [now Helmholtz Zentrum München]) shows scanning location. Shaded rectangular box indicates beam coverage (24 × 1.2 mm) at which eye lenses are completely included.
Although most manufacturers recommend the use of lower tube potential, to obtain more generalizable results that can be used for any acquisition protocol, simulations were also performed for all available tube potentials for each of the four scanners, including the Aquilion 64. Again, repeated axial scans were simulated with no table movement, and the scanning location was directly over the eyes of the patient. For the Aquilion 64, the 64 × 0.5 mm collimation and a small bowtie were used. The peak skin dose and eye lens dose were reported on a normalized basis of milligray (mGy) per 100 mAs.
Eye Lens Dose Reduction
For investigating the change in eye lens dose from tilting the gantry away from the structure, brain perfusion axial scans were simulated using the AAPM-posted protocol for an example scanner, the Sensation 64 MDCT, using a protocol of 24 × 1.2 mm, 80 kVp, 270 mAs/rotation, and 40 total rotations, with the gantry tilted by 5°, 10°, 15°, 20°, 25°, and 30°. Figure 2A illustrates graphically that by tilting the gantry angle, the primary x-ray beam covers a smaller volume of eye lens tissue.
Fig. 2.
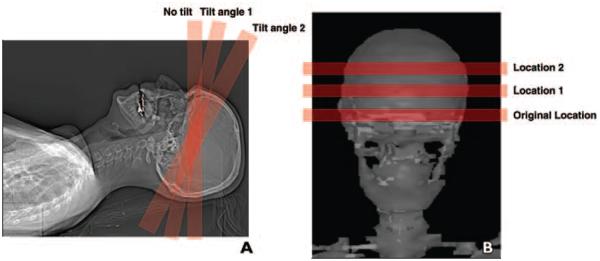
Illustrations of tilting CT scanner gantry angle to avoid direct exposure to eye lenses using patient model (Irene, GSF [now Helmholtz Zentrum München]).
A, CT image shows tilt angle 1 is not ideal because eye lenses may still be partially irradiated directly. Tilt angle 2 is preferred because eye lenses are completely out of x-ray beam.
B, CT image shows effect of moving scanning location further from eye lenses.
For investigating eye lens dose reduction when the location of the scanning volume was moved further away (i.e., superior) from the eye lens, brain perfusion axial scans were simulated with the Monte Carlo tool using the same AAPM-posted protocol for a Sensation 64 CT scanner, with varying imaging volume positions centered from 5.5 cm above the eye lens to 5.5 cm below the eye lens at 0.5-cm intervals. This is illustrated in Figure 2B.
Results
Peak Skin Dose and Eye Lens Dose Using AAPM Protocol
The peak skin dose and eye lens dose to the Irene model from a brain perfusion examination using the AAPM protocols for three of the four scanners were calculated as absorbed dose in units of mGy as shown in Table 2. For peak skin dose, the values ranged from 87 mGy to 348 mGy; for eye lens dose, the values ranged from 81 mGy to 279 mGy. There are significant dose differences between the scanners because the imaging protocols (mAs/rotation, temporal sampling interval, and so on) are different. Therefore, one cannot claim superiority of one scanner over another solely based on the dose information. CTDIvol values, which are a dose index and often used to approximate patient dose, are also listed in Table 2. This table shows that peak skin dose and eye lens dose are estimated to be only 66–79%, and 59–63% of the CTDIvol values, respectively, under this worst-case scenario (x-ray beam directly over the eye lens).
TABLE 2.
Monte Carlo–Based Estimates for Brain Perfusion CT
| Scanner and Mode | CTDIvol (mGy) | Skin |
Eye Lens |
||
|---|---|---|---|---|---|
| Dose (mGy) | % of CTDI | Dose (mGy) | % of CTDI | ||
|
| |||||
| Sensation 64 | 433 | 326 | 75 | 256 | 59 |
| VCT axial mode | 216 | 170 | 79 | 137 | 63 |
| VCT cine mode | 441 | 348 | 79 | 279 | 63 |
| Brilliance 64 | 132 | 87 | 66 | 81 | 61 |
Note—Data based on acquisition protocols of the American Association of Physicists in Medicine [19]. Sensation 64 manufactured by Siemens Healthcare, VCT manufactured by GE Healthcare, and Brilliance 64 manufactured by Philips Healthcare. CTDIvol = volume CT dose index.
Peak Skin Dose and Eye Lens Dose at All Tube Potentials for Four Scanners
The peak skin doses from brain perfusion examinations estimated for all available tube potentials on all four scanners were normalized to mGy/100 mAs and are shown in Table 3. The similar information for the eye lens dose is shown in Table 4. Because organ dose at a given tube potential is proportional to total mAs, the dose from any arbitrary user-specific protocol using the collimation provided in Table 1 can be estimated using these tables. For example, to calculate the dose from the AAPM protocol (80 kVp) for the Sensation 64 scanner, the user simply needs to multiply the mGy dose values from this table by the ratio of the total mAs ([(270 mAs/rotation × 40 rotations)] / [100 mAs]), which is 3 mGy × 270 × 40 / 100 = 324 mGy.
TABLE 3.
Estimated Peak Skin Dose to the Patient From Brain Perfusion CT
| Scanner | Tube Potential Setting (kVp) |
|||
|---|---|---|---|---|
| 80 | 100 | 120 | 140 | |
|
| ||||
| Sensation 64 | 3.0 | 6.2 | 10.5 | 16.4 |
| VCT (axial or cine) | 5.2 | 8.8 | 13.2 | 18.2 |
| Brilliance 64 | 2.3 | NA | 7.2 | 11.1 |
| Aquilion 64 | 5.4 | 9.5 | 14.1 | 18.1a |
Note—The peak skin dose is normalized to the unit of mGy/100 mAs. Sensation 64 manufactured by Siemens Healthcare, VCT manufactured by GE Healthcare, Brilliance 64 manufactured by Philips Healthcare, and Aquilion 64 manufactured by Toshiba. NA = not applicable.
Aquilion 64 provides a tube potential setting of 135 instead of 140 kVp.
TABLE 4.
Estimated Eye Lens Dose to the Patient From Brain Perfusion CT
| Scanner | Tube Potential Setting (kVp) |
|||
|---|---|---|---|---|
| 80 | 100 | 120 | 140 | |
|
| ||||
| Sensation 64 | 2.4 | 5.2 | 8.9 | 14.5 |
| VCT | 4.1 | 7.1 | 10.7 | 14.7 |
| Brilliance 64 | 2.2 | NA | 6.7 | 10.4 |
| Aquilion 64 | 4.4 | 7.7 | 11.5 | 14.7a |
Note—The eye lens dose is normalized to the unit of mGy/100 mAs. Sensation 64 manufactured by Siemens Healthcare, VCT manufactured by GE Healthcare, Brilliance 64 manufactured by Philips Healthcare, and Aquilion 64 manufactured by Toshiba. NA = not applicable.
Aquilion 64 provides a tube potential setting of 135 instead of 140 kVp.
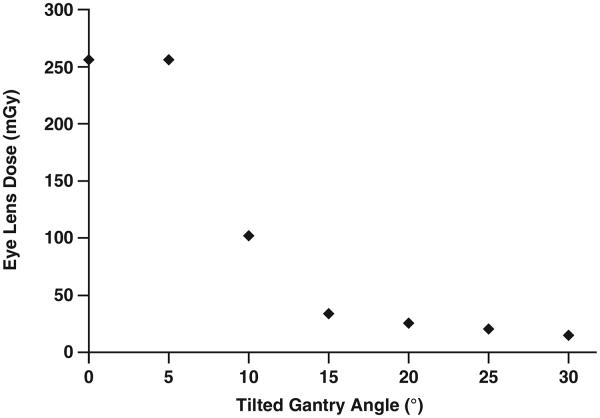
Eye Lens Dose Reduction by Tilting the Gantry Angle
Figure 3 shows the eye lens dose (in mGy) as a function of the tilted gantry angle from a brain perfusion CT examination using the AAPM protocol parameters for a Sensation 64 scanner. When the eye lens is completely covered, the absorbed dose is about 256 mGy. When the gantry was tilted by 15° away from the eye lens, the dose to that structure was decreased by 87%.
Fig. 3.
Graph shows eye lens dose as function of tilted gantry angle from protocol of American Association of Physicists in Medicine (80 kVp, 24 × 1.2-mm collimation, 270 mAs/rotation, 40 rotations) for brain perfusion examination using Sensation 64 scanner (Siemens Healthcare).
Eye Lens Dose Reduction by Moving Scanning Location Away From the Eye Lens
Figure 4 shows the eye lens dose as a function of the scanning location from a brain perfusion CT examination for a Sensation 64 scanner. The eye lens dose was decreased by approximately 50% when the scan location was displaced just 1.5-cm away from the eye lens and 86% when the scanning location was displaced a full 2 cm away (superior or inferior) from the center of the eye lens. It should be noted that moving the scanning location 2 cm resulted in geometry in which the eye lenses are just out of the x-ray primary beam. This is a function of both the simulated beam width for this scanner (24 × 1.2 mm = 28.8 mm nominal beam width; actual beam width, 32.2 mm) and the anatomy of the Irene patient model.
Fig. 4.
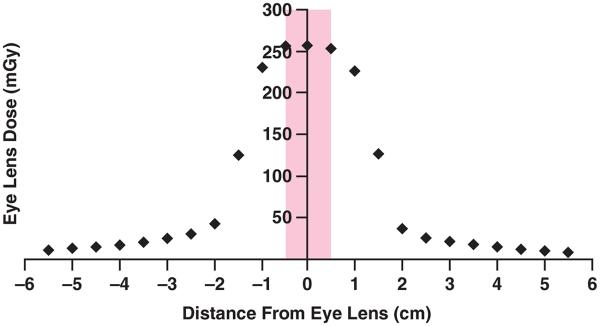
Graph shows eye lens dose as function of scanning location from protocol of American Association of Physicists in Medicine (80 kVp, 24 × 1.2-mm collimation, 270 mAs/rotation, 40 rotations) for brain perfusion CT examination using Sensation 64 scanner (Siemens Healthcare). Width of eye lens in z direction for this patient model (Irene, GSF [now Helmholtz Zentrum München]) is 1 cm. Shaded box indicates eye lens range in longitudinal direction. Therefore, when scanning location is 2 cm from center of eyes, eye lenses are completely out of x-ray beam (larger than half of beam width plus half of eye lens width).
Discussion
This study used Monte Carlo–based simulations to provide estimations of peak skin dose and eye lens dose from brain perfusion CT examinations for specific acquisition protocols for scanners from the four major manufacturers. Depending on the scanners used in the examination, the peak skin and the eye lens dose ranged from 81 to 348 mGy for selected acquisition protocols posted on the AAPM Website.
These results indicate that, although there are wide variations among scanners, the dose from brain perfusion CT performed on these scanners is well below 1000 mGy. Whereas International Commission on Radiological Protection guidelines suggest thresholds of 2000, 3000, and 2000 mGy for transient erythema, temporary epilation, and cataract formation, respectively [22, 23], some newer studies have proposed that the threshold for cataractogenesis is actually much lower if it exists at all [4-7, 24]. Therefore, the radiation doses from CT brain perfusion should be carefully monitored, and dose reduction schemes should be used whenever feasible.
In a clinical environment, multiple brain perfusion studies may be performed within a short period for various reasons, such as therapeutic repeat; change of medical status of the patient; repeat studies because of technical inadequacies, including motion artifacts; contrast bolus monitoring errors; or patient malpositioning. Furthermore, CT perfusion protocols are not regulated among different clinical sites; hence, some may use lower kVp and mAs protocols and others may use higher protocols.
As described previously, in brain perfusion CT examinations, the image quality can afford to be lower than in routine head examinations. Therefore, it is important for each clinical site to optimize scanning protocols according to the characteristics of the CT scanner to ensure the lowest possible radiation dose delivery to patients. For example, lower tube potential should be used, which is also appropriate for quantitative CT studies; mAs should be lower than diagnostic head examinations; and the number of acquisitions should be minimized to achieve lower total mAs without sacrificing the sampling rate. To assist clinicians in accounting for site-to-site variations in the applied protocols, Table 3 and Table 4 provide tools for calculating the peak skin dose and the eye lens dose from any acquisition protocol. In some sites, only processed perfusion map images are provided to the radiologists. In these cases, the acquisition and dose parameters (kVp, mAs, and CTDIvol) should still be monitored by the radiologists (e.g., through review of the patient protocol page or dose report summaries).
As an illustrative example: an arbitrary brain perfusion scanning protocol is considered using 100 kVp, 200 mAs/rotation, and 40 rotations on a VCT scanner. The total mAs is 8000 (200 mAs/rotation × 40 rotations). This requires that the normalized dose value of 8.8 mGy/100 mAs (obtained from Table 3, VCT scanner at 100 kVp) has to be multiplied by a factor of 80 (total mAs of 8000 divided by 100) to obtain the peak skin dose. The result is an estimated peak skin dose of approximately 704 mGy. It should be noted that, in these tables, the dose to eye lens represents the worst-case scenario. Therefore, if the x-ray beam does not irradiate the eye lens directly (e.g., by controlling the longitudinal and angular positions of the images), the eye lens will receive lower radiation dose, as shown in Figures 3 and 4.
Although the CTDIvol is the value reported on the scanner console and in all dose reports on the scanner, it is not the patient dose [21]. Rather, CTDIvol is defined as the average dose to a homogeneous 16-cm-diameter acrylic phantom for a 100-mm-long scan. CTDIvol is an index that describes the amount of radiation being emitted by the scanner. This study showed the overestimation of CTDIvol to both the skin dose and the eye lens dose. This occurs because in CTDI determinations the scattered radiation generated in the 100-mm-long ion chamber is included, which represents contributions from adjacent scanning positions; however, in brain perfusion studies, there is no scatter from adjacent tube positions (or very little if there are two adjacent acquisitions). Overall, CTDIvol provides a conservative estimate (higher by at least 25%) of the peak skin and eye lens doses, especially for the eye lens dose because the values provided in Table 2 represent the worst-case scenario with direct irradiation of the eyes. This conservatism is recognized in international CT safety standards, which acknowledge that CTDIvol will overestimate surface dose for perfusion scans [25].
Radiation dose to the eye lens can be effectively reduced by avoiding direct exposure of this tissue. The reduction potential depends on the anatomy of the patient and the beam, so the absolute gantry tilt angle or scanning location do not necessarily result in the percentage of dose reduction shown in Figures 3 and 4. However, the dose drop-off is obvious once the eye lens is outside the primary beam. This can be achieved by tilting the gantry angle, tilting the patient’s head, or adjusting the scanning location. The sharp drop-off in Figures 3 and 4 suggests that the contribution from scattered radiation to the eye lens dose is small, probably because the eye lenses are located at the body surface where there is less scatter build-up than at locations at depth within the body.
Although these two techniques (tilting the gantry angle and moving the scanning location away from the eye lens) will not reduce the peak skin dose, they may be used in clinical practice to ensure lower eye lens doses and, presumably, a lower risk of developing cataracts. Needless to say, the exact tilt gantry angle and scanning location should be determined by a neuroradiologist to ensure that the region of interest (mid cerebral area including the basal ganglia nuclei for suspected stroke patients) is completely within the imaged volume. This helps ensure that the clinical objectives of the examination are not compromised in an effort to minimize dose to the lens of the eye.
That only one scanner from each of the four major manufacturers was modeled is a limitation of this study. These did not include some new scanners (e.g., Discovery 750HD [GE Healthcare] and Definition Flash [Siemens Healthcare]), which have a scanning acquisition mode (sometimes referred to as a shuttle mode or a short helical scan) to move the patient in and out of the gantry during the brain perfusion examination to image a greater volume of brain tissue. Some of these scanners do not support tilting of the gantry. Therefore, in the setup of the patient, the patient’s chin should be tilted toward the chest (if possible) to help avoid the exposure of the eyes. In addition, some new scanners provide coverage that is wide enough to include the entire brain anatomy (e.g., Aquilion One with 160-mm longitudinal coverage [Toshiba]). For such scanners, the eye lenses are inevitably within the beam during the whole examination, and therefore the possibility of dose reduction to the eye lens by moving the scanning location or tilting the gantry does not exist. However, manufacturers offering whole-brain imaging have introduced methods to assist in dealing with dose concerns by performing all required imaging functions in a reduced number of scans, such as an initial unenhanced scan, followed by a second contrast-enhanced scan from which the arterial, venous, and brain perfusion data are extracted.
Only one adult patient model was studied in this work, and it may not represent the entire patient population. Usually, patients with smaller size receive higher organ dose when the same scanning technique is used [26-28]. However, there is only a small variation in terms of the head size for adult patients. Therefore, the dose variations among patients should be small. Pediatric patients will receive higher organ doses for the same scanning protocols, but brain perfusion examinations are performed primarily in adult patients, and therefore adult patients are more relevant. Additionally, for very young pediatric patients in whom the calvaria is less calcified than for adults, a lower mAs can be used. Tube current modulation was not simulated in this study because this approach is typically not used for brain perfusion CT examination of the head. The size and the shape of the head do not vary sufficiently to justify the need for this application. Furthermore, the setting associated with tube current modulation can easily be misunderstood in perfusion mode and potentially cause overexposure to patients [20].
In summary, the radiation dose from CT perfusion studies should be carefully controlled to minimize patient dose and maximize the benefit-to-risk ratio of the examination. Clinical institutions can use the results from this study to ensure that their brain perfusion protocols (for any of the four scanners at any selected tube potential) operate below the limits at which deterministic effects may be seen from radiation dose to the eye lens and skin. In addition, it was shown that the CTDIvol value reported on the scanner consoles overestimates the peak skin and eye lens doses from brain perfusion studies. Therefore, CTDIvol should serve as only a conservative predictor. Tilting the gantry angle and moving the scanning location further from structures vulnerable to deterministic effects, such as the eye lens, could effectively reduce the dose to these structures. It is suggested that these dose reduction techniques be used in clinical practices whenever possible.
Acknowledgments
Supported by grant R01EB004898 from the National Institute of Biomedical Imaging and Bioengineering.
C. H. McCollough has research support from Siemans. D. D. Cody is a paid speaker for Medical Technology Management Institute workshops. M. F. McNitt-Gray holds a research grant from Siemens Healthcare.
Footnotes
CME
This article is available for CME credit.
References
- 1.Wintermark M. Brain perfusion-CT in acute stroke patients. Eur Radiol. 2005;15(suppl 4):D28–D31. doi: 10.1007/s10406-005-0112-y. [DOI] [PubMed] [Google Scholar]
- 2.Miles KA. Perfusion imaging with computed tomography: brain and beyond. Eur Radiol. 2006;16(suppl 7):M37–M43. doi: 10.1007/s10406-006-0194-1. [DOI] [PubMed] [Google Scholar]
- 3.Miles KA, Eastwood JD, König M. Multidetector computed tomography in cerebrovascular disease: CT perfusion imaging. xiv. Informa Healthcare; Oxon, United Kingdom: 2007. p. 175. [Google Scholar]
- 4.Chodick G, Bekiroglu N, Hauptmann M, et al. Risk of cataract after exposure to low doses of ionizing radiation: a 20-year prospective cohort study among US radiologic technologists. Am J Epidemiol. 2008;168:620–631. doi: 10.1093/aje/kwn171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minamoto A, Taniguchi H, Yoshitani N, et al. Cataract in atomic bomb survivors. Int J Radiat Biol. 2004;80:339–345. doi: 10.1080/09553000410001680332. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima E, Neriishi K, Minamoto A. A reanalysis of atomic-bomb cataract data, 2000–2002: a threshold analysis. Health Phys. 2006;90:154–160. doi: 10.1097/01.hp.0000175442.03596.63. [DOI] [PubMed] [Google Scholar]
- 7.Neriishi K, Nakashima E, Minamoto A, et al. Postoperative cataract cases among atomic bomb survivors: radiation dose response and threshold. Radiat Res. 2007;168:404–408. doi: 10.1667/RR0928.1. [DOI] [PubMed] [Google Scholar]
- 8.Huda W, Nickoloff EL, Boone JM. Overview of patient dosimetry in diagnostic radiology in the USA for the past 50 years. Med Phys. 2008;35:5713–5728. doi: 10.1118/1.3013604. [DOI] [PubMed] [Google Scholar]
- 9.Zaidi H, Ay MR. Current status and new horizons in Monte Carlo simulation of x-ray CT scanners. Med Biol Eng Comput. 2007;45:809–817. doi: 10.1007/s11517-007-0207-9. [DOI] [PubMed] [Google Scholar]
- 10.DeMarco JJ, Chetty IJ, Solberg TD. A Monte Carlo tutorial and the application for radiotherapy treatment planning. Med Dosim. 2002;27:43–50. doi: 10.1016/s0958-3947(02)00087-0. [DOI] [PubMed] [Google Scholar]
- 11.Turner AC, Zankl M, DeMarco JJ, et al. The feasibility of a scanner-independent technique to estimate organ dose from MDCT scans: using CTDIvol to account for differences between scanners. Med Phys. 2010;37:1816–1825. doi: 10.1118/1.3368596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waters LS. MCNPX User’s Manual, Version 2.4.0. Los Alamos National Laboratory; Los Alamos, NM: 2002. Los Alamos National Laboratory report. LA-CP-02-408. [Google Scholar]
- 13.Waters LS. MCNPX User’s Manual, Version 2.4.0. Los Alamos National Laboratory; Los Alamos, NM: 2003. Los Alamos National Laboratory report. LA-UR-03-2202. [Google Scholar]
- 14.Turner AC, Zhang D, Kim HJ, et al. A method to generate equivalent energy spectra and filtration models based on measurement for multidetector CT Monte Carlo dosimetry simulations. Med Phys. 2009;36:2154–2164. doi: 10.1118/1.3117683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarry G, DeMarco JJ, Beifuss U, Cagnon CH, McNitt-Gray MF. A Monte Carlo-based method to estimate radiation dose from spiral CT: from phantom testing to patient-specific models. Phys Med Biol. 2003;48:2645–2663. doi: 10.1088/0031-9155/48/16/306. [DOI] [PubMed] [Google Scholar]
- 16.DeMarco JJ, Cagnon CH, Cody DD, et al. A Monte Carlo based method to estimate radiation dose from multidetector CT (MDCT): cylindrical and anthropomorphic phantoms. Phys Med Biol. 2005;50:3989–4004. doi: 10.1088/0031-9155/50/17/005. [DOI] [PubMed] [Google Scholar]
- 17.Petoussi-Henss N, Zankl M, Fill U, Regulla D. The GSF family of voxel phantoms. Phys Med Biol. 2002;47:89–106. doi: 10.1088/0031-9155/47/1/307. [DOI] [PubMed] [Google Scholar]
- 18.International Commission on Radiation Units and Measurements (ICRU) Tissue substitutes in radiation dosimetry and measurement. 2011 ICRU Report No 44. ICRU Website. www.icru.org/index.php?option=com_content&task=view&id=80. Accessed October 4.
- 19.American Association of Physicists in Medicine (AAPM) Adult brain perfusion CT. 2011 AAPM Website. www.aapm.org/pubs/CTProtocols/documents/AdultBrainPerfusionCT_2011-01-11.pdf. Published January 11. Accessed October 4, 2011.
- 20.Shuren J. Letter to the Medical Imaging Technology Alliance regarding CT recommendations. 2010 U.S. Food and Drug Administration Website. www.fda.gov/Radiation-EmittingProducts/RadiationSafety/RadiationDoseReduction/ucm232551.htm. Published November 8. Accessed October 4, 2011.
- 21.McCollough CH, Leng S, Yu L, et al. CT dose index (CTDI) and patient dose: they are not the same thing. Radiology. 2011;259:311–316. doi: 10.1148/radiol.11101800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.International Commission on Radiological Protection (ICRP) Recommendations of the International Commission on Radiological Protection: ICRP publication 60. Ann ICRP. 1991;21:1–201. [PubMed] [Google Scholar]
- 23.International Commission on Radiological Protection (ICRP) Avoidance of radiation injuries from medical interventional procedures: ICRP Publication 85. Ann ICRP. 2000;30:7–67. doi: 10.1016/S0146-6453(01)00004-5. [DOI] [PubMed] [Google Scholar]
- 24.Worgul BV, Kundiyev YI, Sergiyenko NM, et al. Cataracts among Chernobyl clean-up workers: implications regarding permissible eye exposures. Radiat Res. 2007;167:233–243. doi: 10.1667/rr0298.1. [DOI] [PubMed] [Google Scholar]
- 25.International Electrotechnical Commission (IEC) Medical electrical equipment. Part 2-44. Particular requirements for the basic safety and essential performance of x-ray equipment for computed tomography: IEC 60601-2-44. 3rd IEC; Geneva, Switzerland: 2009. [Google Scholar]
- 26.Angel E, Yaghmai N, Jude CM, et al. Monte Carlo simulations to assess the effects of tube current modulation on breast dose for multidetector CT. Phys Med Biol. 2009;54:497–512. doi: 10.1088/0031-9155/54/3/003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeMarco JJ, Cagnon CH, Cody DD, et al. Estimating radiation doses from multidetector CT using Monte Carlo simulations: effects of different size voxelized patient models on magnitudes of organ and effective dose. Phys Med Biol. 2007;52:2583–2597. doi: 10.1088/0031-9155/52/9/017. [DOI] [PubMed] [Google Scholar]
- 28.Lee C, Lee C, Staton RJ, et al. Organ and effective doses in pediatric patients undergoing helical multislice computed tomography examination. Med Phys. 2007;34:1858–1873. doi: 10.1118/1.2723885. [DOI] [PubMed] [Google Scholar]