Abstract
Background
The relationship between cardiac autonomic nerve activity and blood pressure (BP) changes in ambulatory dogs is unclear.
Objective
To test the hypotheses that simultaneous termination of stellate ganglion nerve activity (SGNA) and vagal nerve activity (VNA) predisposes to spontaneous orthostatic hypotension and that specific β2 adrenoceptor blockade prevents the hypotensive episodes.
Methods
We used a radiotransmitter to record SGNA, VNA and blood pressure (BP) in 8 ambulatory dogs. Video imaging was used to document postural changes.
Results
Out of these 8 dogs, 5 showed simultaneous sympathovagal discharges in which the minute by minute integrated SGNA correlated with integrated VNA in a linear pattern (“Group 1”). In these dogs abrupt termination of simultaneous SGNA-VNA at the time of postural changes (as documented by video imaging) was followed by abrupt (>20 mmHg over 4 beats) drops in BP. Dogs without simultaneous on/off firing (“Group 2”) did not have drastic drops in pressure. ICI 118,551 (ICI, a specific β2-blocker) infused at 3.1 µg/kg/hr for 7 days significantly increased BP from 126 (95% confidence interval, CI: 118 to 133) mmHg to 133 (95% CI 125 to141) mmHg (p=0.0001). The duration of hypotension (mean systolic BP < 100 mmHg) during baseline accounted for 7.1% of the recording. The percentage was reduced by ICI to 1.3% (p = 0.01).
Conclusions
Abrupt simultaneous termination of SGNA-VNA was observed at the time of orthostatic hypotension in ambulatory dogs. Selective β2 adrenoceptor blockade increased BP and reduced the duration of hypotension in this model.
Keywords: Ambulatory nerve recording, orthostatic hypotension, beta blockade
It is well-established that the autonomic nervous system (ANS) is important in controlling not only heart rhythm and contraction, but also the vasomotor tone and systemic blood pressure (BP). Chronic stimulation of the stellate ganglion can cause hypertension in dogs,1 suggesting that elevated stellate ganglion nerve activity (SGNA) is a cause of hypertension. While sympathetic activation may cause vasoconstriction by activating α-1 adrenoreceptors, sympathetic activation can also activate β2 adrenoreceptors to cause vasodilation.2, 3 In addition to its effects on peripheral vascular resistance, the autonomic nervous system also controls heart rate that impacts diastolic filling, stroke volume, cardiac output and hence systemic BP. To better understand the ANS control of cardiac function, we have developed methods to simultaneously record stellate ganglion nerve activity (SGNA) and vagal nerve activity (VNA) in ambulatory dogs.4–6 Preliminary data demonstrated that the stellate ganglion and vagal nerve do not fire independently of each other. Rather, there is a high degree of coordination between these two nerve structures, and the patterns of correlation in part determine the spontaneous occurrences of atrial tachyarrhythmias.7, 8, 9 Whether or not the patterns of SGNA-VNA correlation play a role in BP control of ambulatory dogs remains unknown. We therefore performed a study to simultaneously record SGNA, VNA and brachial artery BP in ambulatory dogs to determine if the patterns of SGNA-VNA coordination influence BP control. We unexpectedly observed an association between simultaneous SGNA-VNA termination and orthostatic hypotension. This observation gave us the opportunity to test the effects of pharmacological intervention on the hypotensive episodes. We chose to first test the hypothesis that a non-selective β blocker and α1 blocker (carvedilol) can prevent these hypotensive episodes. This was followed by the infusion of ICI-118,551 (ICI), a specific β2 adrenoceptor blocker10, 11 in the same dogs.
Materials and Methods
The study protocols were approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine and the Methodist Research Institute, Indianapolis, Indiana.
Study Protocol
Left thoracotomy was performed through the first intercostal space for the implantation of a D70-CCTP radiotransmitter manufactured by the Data Sciences International (DSI, St Paul, MN in 8 mongrel dogs (4 male, weighing 20–30 kg). The first pair of bipolar electrodes was used to record from left stellate ganglion and a second pair was used to record from left thoracic vagal nerve at the level 4 to 5 cm above the aortic arch. A third lead (a catheter with a pressure sensor) was inserted directly into the subclavian artery for BP recording. The transmitter and ground wires were inserted into a subcutaneous pocket. The chest wall was then closed. After 2 weeks of recovery, the radiotransmitter was turned on to record nerve signals and BP at baseline. The pre-implantation BP was calibrated against the ambient pressure in the vivarium. There was 12 hr daylight cycle in the vivarium starting at 7 AM each day. After 24-hr baseline (SGNA, VNA, and BP) recordings, the dogs were given oral carvedilol, 12 mg twice daily for a week. The drugs were then washed out for a week and baseline recordings were re-collected at the end of the week. Sterile surgery was then performed for the subcutaneous implantation of a 2 ml osmotic pump (model 2ML1, Alzet Inc., Cupertino, CA). The pump contained 2 ml of ddH20 and 18 mg of ICI 118,551 (ICI), a specific inhibitor of β2 adrenoceptor.10 The pump is designed to empty its contents over 7 days at a rate of 10 µl/hr. Therefore, the infusion rate is 3.1 µg/kg/hr for a 30 kg dog. The infusion rate at which half the β2 receptors are saturated has been reported to be 2 µg/kg in beagles.10 The data collected at days 6–7 of the infusion were used for analyses. A week after completing this stage of drug infusion, the dogs were euthanized.
Data analyses
Nerve activity analyses
Nerve activities were analyzed both manually and with computerized methods.12 The nerve activities were integrated minute by minute. We manually reviewed the data of all dogs and selected for analyses the first 10 noise-free min of each hour. The mean BP was also analyzed minute by minute during the same period. The SGNA was plotted against the VNA on an XY graph to visualize the patterns of activation.12, 13
Statistical analyses
To determine the relationship between BP, heart rate and ANS activities, we computed 1-min and 4- hour average for each dog on each day of data collection starting from midnight. Pearson correlation coefficients between SGNA and VNA were also calculated within each 4-hour interval. To analyze data from all dogs and the 4-hour average data, a random effects model with dog as the random effect was used with the same dependent and independent variables, plus heart rate and the Pearson correlation coefficient as additional independent variables. Random effects models were also used to compare the effects of the drugs on the proportion of hypotensive episodes (average SAP<100mmHg) using data from all dogs, which includes the main effects of drug and the six 4-hour intervals and their interactions. In this model, the correlations of the multiple 4-hour data points from the same dog is accounted for by treating dog as random effect. A cubic smoothing spline was also used to fit BP versus hour for one dog. Statistical analysis was performed with IBM SPSS Statistics 19 and SAS version 9.3. A two –sided p value of ≤ 0.05 was considered statistically significant.
Results
Relationship between autonomic nerve activity and BP
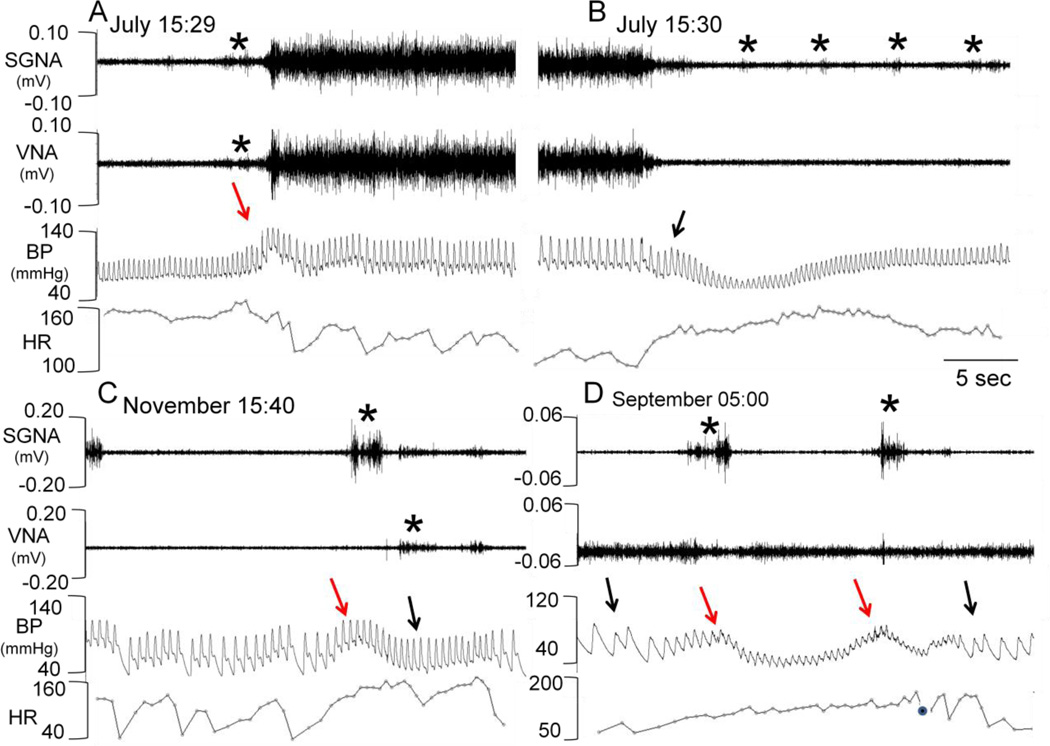
Each dog was monitored for an average of 10 weeks (range 6 to 21 weeks) during which SGNA, VNA and BP were intermittently collected. There are two basic patterns of nerve activity.8 In the first pattern (termed “Group 1” in that report), the SGNA and VNA activate simultaneously (Figure 1A and 1B). A plot of integrated stellate ganglion nerve activity (iSGNA) vs. integrated vagal nerve activity (iVNA) gives a linear pattern (Figure 2A). Five of the eight dogs initially demonstrated Group 1 firing at baseline. We found that the SGNA-VNA correlation is important in BP control. In general, simultaneous sympathovagal firing was associated with a higher mean BP, while there were more extreme drops in BP at the time of simultaneous sympathovagal termination. Figure 1A shows a Group 1 dog with a baseline BP ranging from 75 to 100 mmHg and heart rate averaging 162 bpm. At the start of the simultaneous low amplitude sympathovagal discharge (asterisk), the blood pressure increased rapidly. An abrupt increase in BP (red arrow) is followed by sustained and large amplitude simultaneous sympathovagal discharges without an apparent initial increase in heart rate. Figure 1B shows that after 68 seconds of continuous sympathovagal discharge, the nerve activity abruptly and simultaneously terminates. This is followed by a rapid drop in BP to as low as 65 mmHg systolic and 45 mmHg diastolic (black arrow) and tachycardia. The BP then gradually recovers, assisted by low amplitude intermittent SGNA (asterisks).
Figure 1.
Representative autonomic nerve activity. Panel A: examples of simultaneous sympathovagal co-activation in a Group 1 dog. Asterisk indicates the start of nerve firing which occurs a few seconds prior to increase in blood pressure. Panel B: Same dog as panel A; termination of sympathovagal firing after 68 seconds (not shown) of continuous firing. Note that the end of firing is followed by a sharp decrease in blood pressure. The VNA is quiescent but intermittent small bursts of SGNA (asterisks) were present. Panel C: Group 2 dog; note the separate sympathetic and vagal firing, as marked by asterisks. SGNA was associated with increased heart rate and BP (red arrow). VNA was associated with reduced heart rate and BP. Panel D. Solitary sympathetic nerve firing associated with transient increases in blood pressure (red arrows) followed by tachycardia and reduction of BP. Black arrows indicate bradycardia, which are associated with VNA at times when SGNA was inactive. A filled black circle marked the time when the amplitude of BP was too small for heart rate determination. HR, heart rate.
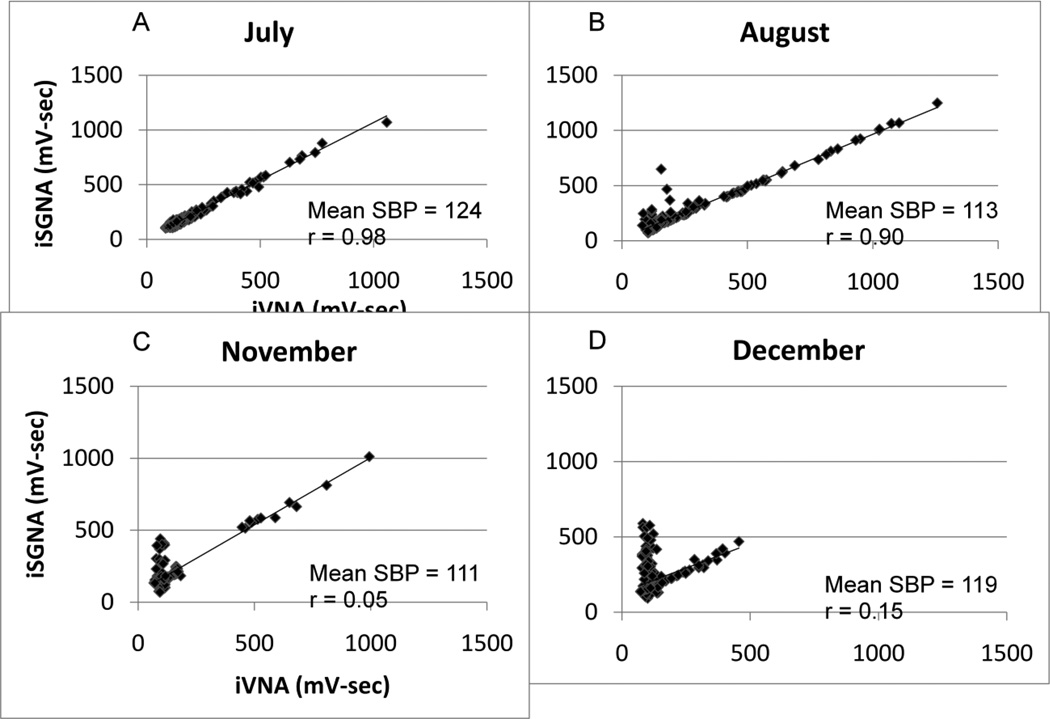
Figure 2.
Evolution of the SGNA-VNA correlation over time in dog #1, which has been monitored for over 6 months. Panel A shows that the dog had a linear SGNA-VNA correlation in July. Each dot represents SGNA and VNA activity integrated over 60s. The patterns gradually changed to L-shaped SGNA-VNA correlation in December. Mean systolic arterial blood pressure and correlation are shown for each graph. There is no apparent relationship between the correlation patterns and the mean Systolic blood pressure (SBP).
The second pattern of nerve activity (Group 2) where SGNA and VNA activate separately is shown in Figure 1C. Note that the independent activation of SGNA (asterisk, first line) is associated with elevated BP (red arrow) while VNA (asterisk, second line) is associated with a lower mean arterial blood pressure and heart rate. Figure 1D shows a second dog in which SGNA and VNA activate separately - here the vagus nerve activates alone during the first eight seconds of the recording. This activation is associated with bradycardia (black arrow). The sympathetic bursts were low amplitude (asterisks) in the beginning, which are associated with brief increases in heart rate (red arrows). This was then followed by large increase of SGNA and a simultaneous drop of BP. Complete cessation of SGNA and continuous VNA was associated with bradycardia (second black arrow).
Changing sympathovagal correlation
A previous study that monitored canines for approximately 2 months8 suggested that the sympathovagal correlation is static. However, recordings done in the present study over a longer time frame (5–6 months) demonstrate that patterns of sympathovagal correlation evolve over time. This is best illustrated by dog #1 that was monitored over 6 months, as shown in Figure 2. This dog started out during the monitoring period (July) as a Group 1 dog (Figure 2A). At that time, the dog was 6 months old and weighed 30.1 Kg. Slowly, over months, he evolved into a Group 2 dog (Figures 2B–D). The weight increased to 40.3 Kg in December. The iSGNA and iVNA have drastically dropped in December. There were increasing SGNA at the time when VNA was silent. This nonlinear relationship between SGNA and VNA indicates a complex interaction between SGNA and VNA. Other dogs stayed in the same group throughout the recording, although the patterns of correlation changed slightly over time. While Figure 2A suggests that a high r value between iSGNA and iVNA is associated with high systolic BP, statistical analyses of all dogs studied failed to support this correlation.
ANS and BP
The ANS activity in all dogs studied is significantly associated with BP changes. Each mV-s increase of iSGNA is associated with 0.04580 mmHg increase in BP (p=0.0478). Each mV-s increase of iVNA is associated with an insignificant decrease in BP of −0.05484 mmHg (p=0.0648). Heart rate (p=0.9991) and the SGNA-VNA correlation coefficient (p=0.1294) are not associated with BP changes using analyses summarized by 4 hour intervals.
Abrupt hypotensive episodes
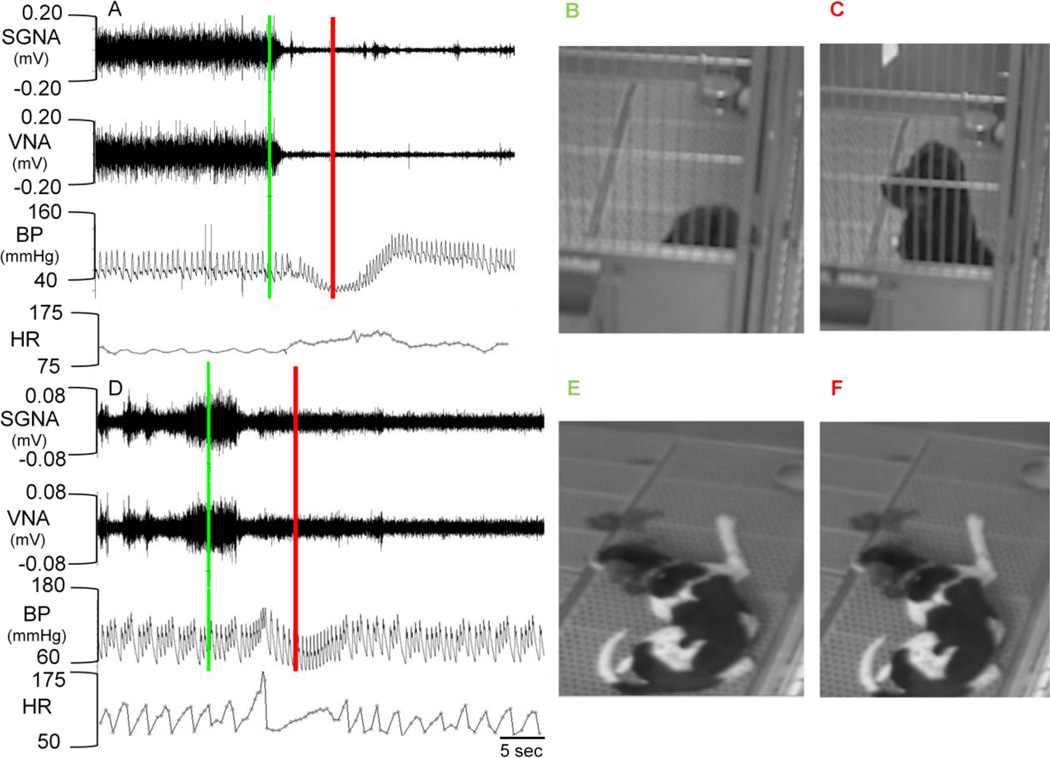
In Figure 1B, abrupt cessation of simultaneous sympathovagal discharges seen in Group 1 dogs was associated with an abrupt drop in BP. To determine if these episodes were related to postural changes, we installed video cameras in the dog kennel and monitored nerve activity, BP and video recordings simultaneously. Several patterns of behavior emerged as is shown in Figure 3. The first pattern is illustrated in Figures 3A–C. Figure 3A shows simultaneous firing of the canine’s sympathetic and vagal nerves. The green bar indicates the time the video in Figure 3B was captured; red bar is the time Figure C was captured. Note that the dog is lying down during simultaneous sympathovagal discharge (Figure 3A). Abrupt termination of the nerve discharges coincides with a change in posture from lying to sitting up (Figure 3B–C). This was accompanied by an abrupt drop in the mean arterial pressure from 80 mmHg to 60 mmHg, a dramatic narrowing of the pulse pressure and an acceleration of heart rate. We have reviewed the video imaging collected in 3 dogs and analyzed a total of 1000 episodes of abrupt simultaneous termination of sympathovagal discharges. Among these episodes, 390 were associated with sudden movement, such as standing up or starting to jump. In some episodes it appeared that the dog was aroused (Figure 3A). The second pattern of behavior is illustrated in Figures 3D–E from a different dog. The dog was lying quietly on the floor and remains lying down during both the simultaneous sympathovagal discharges (green line) and after the abrupt termination of the firing (red line). Note in Panel D there is only mild drop in BP (from 110 mmHg to 100 mmHg) without narrowing of the pulse pressure. There is tachycardia after the termination of sympathovagal discharges.
Figure 3.
Autonomic nerve activity and postural hypotension. Panels A–C: Orthostatic hypotension in dog #1. A shows simultaneous SGNA, VNA and BP recordings; video recordings were taken during the same time (Panels B and C). The green line marks the time in the recording that corresponds to Panel B and the red line marks the time for C. Note the simultaneous sympathovagal discharge while the dog is lying quietly (B). The termination of sympathovagal discharge occurs when the dog stands up (C) and resulted in a large (>20 mmHg) change in BP. Panels D–F: Dog #2 shows no change in position from E to F. Note the cessation of sympathovagal discharge at the same time. However, there was only a small drop in BP (~10mmHg) without changes in position. HR, heart rate.
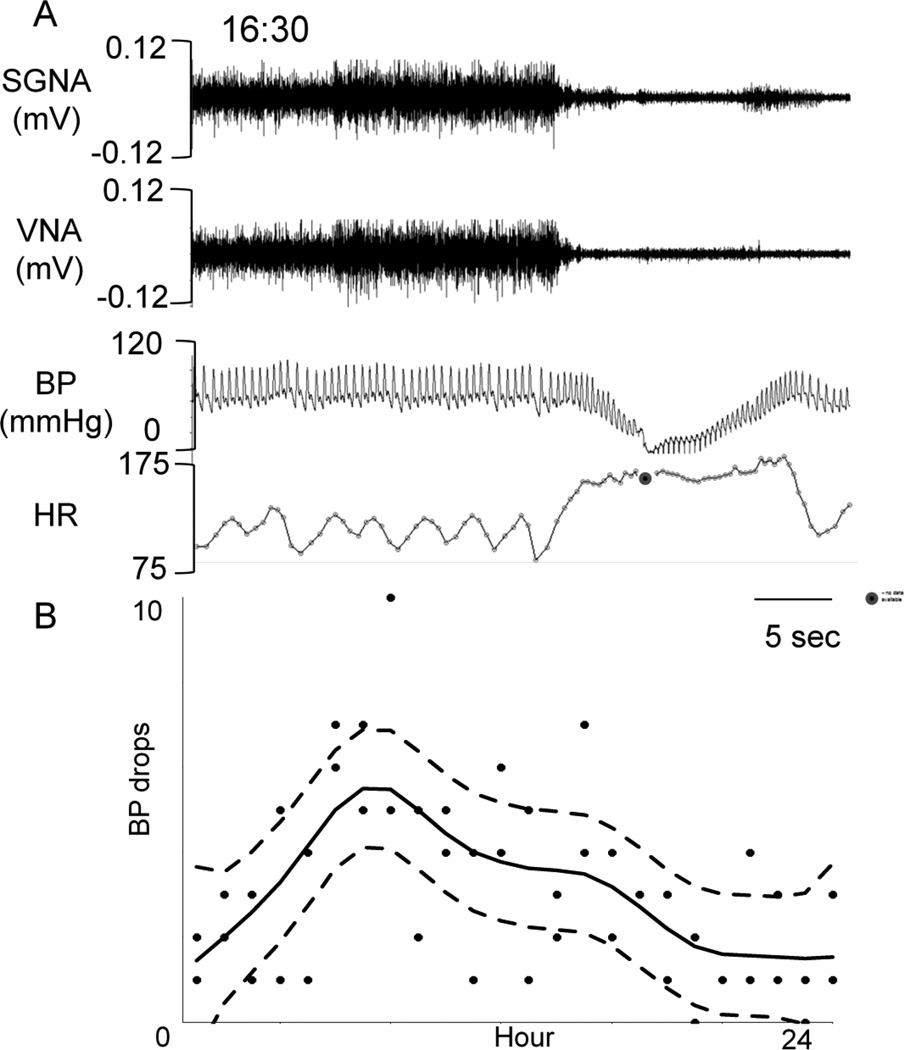
Figure 4 shows the circadian variation of the hypotensive episodes. Figure 4A shows an example of extreme hypotension in the same dog as in Figure 1. The BP dipped to as low as 20 mmHg. We manually analyzed the distribution of similar episodes (drop in pressure of 20 mmHg over 4 beats) throughout the day and found that there is a significant (p<0.0001) circadian variation of the hypotensive episodes associated with sympathovagal termination (Figure 4B).
Figure 4.
Circadian variation of the hypotensive (< 70 mmHg) episodes. Panel A shows a representative hypotensive sample. Note that the hypotensive episode occurs after abrupt termination of sympathovagal discharge. A filled black circle marked the time when the amplitude of BP was too small for heart rate determination. HR, heart rate. Panel B shows a catalogue of all hypotensive episodes in two different days, one week apart, fitted with a cubic smoothing spline (solid line). The hypotensive episodes were defined as a drop of 20 mmHg in BP over no more than 4 heart beats. The dashed lines are the point-wise 95% confidence intervals. There is strong evidence of circadian variation. P < 0.001.
Effects of drugs on BP
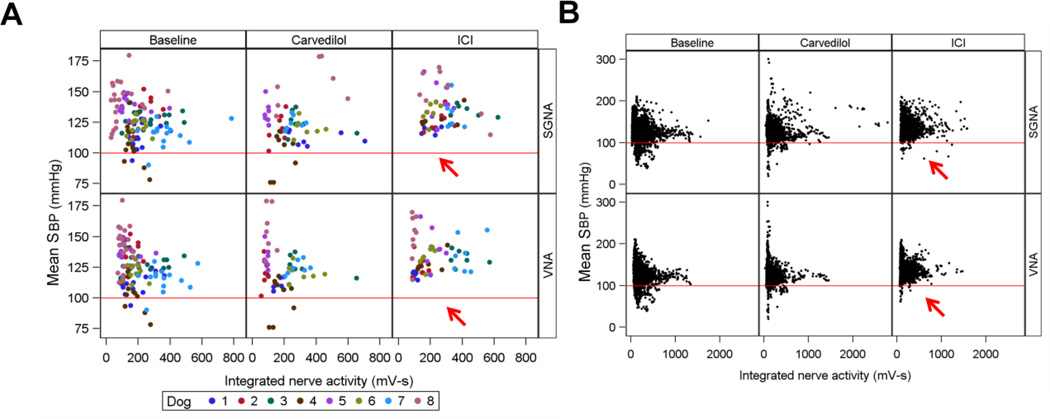
Figure 5 illustrates the relationship between SGNA, VNA and BP during baseline, carvedilol and ICI infusion across all eight dogs. In panel A, each dot represents the average of 4 hours of integrated nerve activity; in panel B, each dot is one minute of integrated nerve activity. The red line is drawn to mark 100 mmHg. Note the substantial increase in mean systolic BP between baseline and ICI recordings, but not between baseline and carvedilol in Panel A. The mean BP (calculated over 4 hour intervals) during ICI infusion does not dip lower than 110 mmHg in any dog (red arrows). In Panel B, each dot represents the mean systolic BP over one minute. The number of hypotensive episodes (defined as mean systolic pressure <100mmHg in that one minute) were calculated for each dog. The percentage of hypotensive episodes during baseline was 7.1%; after carvedilol, 7.8% (p = 0.7475) and after ICI, 1.3% (p = 0.0110). These data show that ICI, but not carvedilol, significantly reduces hypotensive episodes.
Figure 5.
Effects of drugs on BP distribution. In Panel A, each dot represents an averaged systolic BP over 4 hr recording period. The dogs are color coded, showing heterogeneous BP distribution among dogs. The BP did not drop below 100 mmHg during ICI infusion (red arrows). Each dot in Panel B shows the average systolic BP over a 1-min period. Data from all dogs are shown with the same color. ICI reduced the probability of BP dropping below 100 mmHg (red arrows).
Discussion
Autonomic Nerve Activity and Postural Hypotension
Dogs are quadruped while humans are biped. Dogs may not be optimal models for human orthostatic intolerance. However, in spite of this limitation, there are significant similarities between the results of this study and that found in human muscle sympathetic nerve activity (MSNA) studies during tilt table testing.14–19 Typically, upright tilt immediately increases the MSNA. An acute reduction of MSNA and BP is observed in patients with vasovagal syncope but not in the controls.14 These findings are similar to that observed in Group 1 dogs, when abrupt termination of SGNA is associated with abrupt drop in BP. They support the hypothesis that the final trigger for orthostatic hypotension is sympathetic nervous system inhibition. However, in a more recent study,15 the authors found that efferent sympathetic nerve traffic to the skeletal muscle vasculature (i.e., MSNA) is nearly always maintained through the faint. The authors of the latter study propose an alternative viewpoint, that vasodilator mechanisms underlie the blood pressure fall in vasovagal syncope. Similar findings were also reported by Fu et al.20 Because of these new findings, it has been proposed that vasovagal syncope is a heterogeneous condition and that there are different drivers in different group of patients.21 In our canine model, the Group 2 dogs also have intermittent reduction of BP, although not as severe as in Group 1 dogs. Typically elevated BP triggers a large increase of SGNA and a reduction of BP. The continuation of SGNA during BP reduction is similar to that found in some human patients with vasovagal syncope.15, 20
The contribution of vagal nerve
In our study, the VNA (or lack thereof) seems to be important in BP control. For example, simultaneous activation of VNA in Group 1 dogs may counterbalance the effects of SGNA and help maintain a relatively normal heart rate. These findings suggest that SGNA is the primary phenomenon while VNA is the secondary phenomenon that counterbalanced the SGNA for BP and heart rate control. Abrupt termination of VNA allowed heart rate to increase, which leads to an increased cardiac output and normalization of the BP after transient orthostatic hypotension. Absence of VNA in Group 2 dogs implies that sympathetic tone alone controls the BP changes through vasoconstriction, vasodilation and heart rate modulation. The differential patterns of VNA in these two groups of dogs suggest that more than one mechanism play a role in the development of intermittent hypotensive episodes in dogs. The importance of VNA in hypotensive episodes in each dog is complicated by the fact that the grouping of dogs may change over time. In addition, due to the complex physiology associated with vagal nerve activation, quantitative analyses using integrated vagal nerve activity may not be an optimal method to assess the vagal tone. The contribution of vagal nerve in hypotension requires further study.
Therapeutic implications
The prevention of syncope trial (POST)22 showed metoprolol (a selective β1 blocker) is not an effective therapy for vasovagal syncope in humans. In comparison, our data show that selective β2 blocker may prevent the hypotensive episodes. In Group 1 dogs, β2 blocker may selectively prevent vasodilation effects of sympathetic activation during the large SGNA. When SGNA abruptly terminates, the residual cardiac stimulation effects of β1 activation may prevent precipitous drop of BP. In Group 2 dogs, β2 blocker may directly inhibit the vasodilation during large sympathetic surge. We used ICI, a specific β2 adrenoceptor blocker,10, 11 to test this hypothesis. The results showed that ICI lead to an overall increase in BP and an elimination of the hypotensive episodes in ambulatory dogs. Our results are consistent with a previous study in a rat model of orthostatic hypotension, which showed that β1-adrenoceptors played a role in compensatory tachycardia reflex and β2-adrenoceptors in blood pressure reflex.23 The latter conclusion is supported in part by the finding that ICI had a tendency to improve orthostatic symptoms in that rat model. Our findings may also help explain the mechanisms by which non-selective β blockers (propranolol and nadolol) improve the symptoms of vasovagal syncope.24
In addition to implications for drug therapy, the present study is also relevant to nonpharmacological therapies of vasovagal syncope. A recent study showed that endocardial ablation of the intrinsic cardiac nerves is effective in the treatment of vasovagal syncope in human patients.25 That procedure benefited not only cardioinhibitory type but also vasodepressor and the mixed types of vasovagal syncope. Because of these observations, the authors suggested that endocardial ablation procedure might have affected the nerve structures outside of the heart to achieve its therapeutic benefits. Other studies show that renal sympathetic denervation is effective in treating drug refractory hypertension.26 These findings suggest that the autonomic nerve structures are interconnected both functionally and anatomically to achieve BP control. Better understanding of ANS control of BP may help further development of the neuromodulation procedures for clinical application.
Limitation of the study
Because humans are biped and dogs (as well as rats and many other laboratory animals) are quadruped, it is difficult to develop an optimal canine model of human orthostatic intolerance. Therefore, whether or not our observations are applicable to humans remain unclear. Unlike humans, the symptoms associated with orthostasis are difficult to ascertain. Due to the limitation of recording devices, we were only able to measure the autonomic nerve activities on the left side of the body. While the autonomic nerves of the right and left side of body often activate together,4, 27 the absence of right sided recording is a limitation. The absolute values of integrated nerve activity may be affected by technical factors such as the amount of scarring, the size of the stellate ganglion and the electrode orientation to the axis of electrical activity. However, the moment to moment correlation of sympathetic and vagal nerve activities is likely to be independent of these technical factors. The duration of the recording is not long enough to determine if there are seasonal variations of the iSGNA-iVNA correlations. A previous clinical study showed that ICI (in doses up to 80 mg orally) has no side-effects to humans.11 However, all drugs have potential side effects. The absence of apparent side effects in dogs does not rule out the potential side effects for humans, especially on long term use.
Acknowledgement
We thank Lei Lin, Nicole Courtney, Jessica Warfel and Jian Tan for their assistance.
Funding Source
This study was supported by the National Heart, Lung and Blood Institute of the National Institutes of Health under award number P01HL78931, R01HL71140, R21HL106554, a Piansky Endowment (M.C.F.), a Medtronic-Zipes Endowment (P.-S.C.) and the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ANS
autonomic nervous system
- BP
blood pressure
- BPM
beats per minute
- HR
heart rate
- iSGNA
integrated stellate ganglion nerve activity
- iVNA
integrated vagal nerve activity
- MSNA
muscle sympathetic nerve activity
- SAP
systolic arterial pressure
- SGNA
stellate ganglion nerve activity
- VNA
vagal nerve activity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Our laboratory received equipment donations from Medtronic Inc., Cyberonics Inc., St Jude Inc. and Cryocath Inc.
References
- 1.Liard JF, Tarazi RC, Ferrario CM, Manger WM. Hemodynamic and humoral characteristics of hypertension induced by prolonged stellate ganglion stimulation in conscious dogs. Circ Res Mar. 1975;36:455–464. doi: 10.1161/01.res.36.3.455. [DOI] [PubMed] [Google Scholar]
- 2.Westcott EB, Segal SS. Perivascular innervation: A multiplicity of roles in vasomotor control and myoendothelial signaling. Microcirculation. 2013;20:217–238. doi: 10.1111/micc.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toda N. Vasodilating beta-adrenoceptor blockers as cardiovascular therapeutics. Pharmacol Ther. 2003;100:215–234. doi: 10.1016/j.pharmthera.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Jung BC, Dave AS, Tan AY, et al. Circadian variations of stellate ganglion nerve activity in ambulatory dogs. Heart Rhythm. 2006;3:78–85. doi: 10.1016/j.hrthm.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa M, Zhou S, Tan AY, et al. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. Journal of the American College of Cardiology. 2007;50:335–343. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 6.Tan AY, Zhou S, Ogawa M, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–925. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen MJ, Choi EK, Tan AY, et al. Neural mechanisms of atrial arrhythmias. Nat Rev Cardiol. 2011;27:30–39. doi: 10.1038/nrcardio.2011.139. [DOI] [PubMed] [Google Scholar]
- 8.Shen MJ, Choi EK, Tan AY, et al. Patterns of baseline autonomic nerve activity and the development of pacing-induced sustained atrial fibrillation. Heart Rhythm. 2011;8:583–589. doi: 10.1016/j.hrthm.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinohara T, Shen MJ, Han S, et al. Heart Failure Decreases Nerve Activity in the Right Atrial Ganglionated Plexus. J Cardiovasc Electrophysiol. 2011;23:404–412. doi: 10.1111/j.1540-8167.2011.02204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bilski AJ, Halliday SE, Fitzgerald JD, Wale JL. The pharmacology of a beta 2-selective adrenoceptor antagonist (ICI 118,551) Journal of cardiovascular pharmacology. 1983;5:430–437. doi: 10.1097/00005344-198305000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Arnold JM, O'Connor PC, Riddell JG, Harron DW, Shanks RG, McDevitt DG. Effects of the beta 2-adrenoceptor antagonist ICI 118,551 on exercise tachycardia and isoprenaline-induced beta-adrenoceptor responses in man. British journal of clinical pharmacology. 1985;19:619–630. doi: 10.1111/j.1365-2125.1985.tb02689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen MJ, Shinohara T, Park HW, et al. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation. 2011;123:2204–2212. doi: 10.1161/CIRCULATIONAHA.111.018028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinohara T, Park HW, Han S, et al. Ca2+ clock malfunction in a canine model of pacinginduced heart failure. Am J Physiol Heart Circ Physiol. 2010;299:H1805–H1811. doi: 10.1152/ajpheart.00723.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jardine DL, Ikram H, Frampton CM, Frethey R, Bennett SI, Crozier IG. Autonomic control of vasovagal syncope. The American journal of physiology. 1998;274:H2110–H2115. doi: 10.1152/ajpheart.1998.274.6.H2110. [DOI] [PubMed] [Google Scholar]
- 15.Vaddadi G, Esler MD, Dawood T, Lambert E. Persistence of muscle sympathetic nerve activity during vasovagal syncope. Eur Heart J. 2010;31:2027–2033. doi: 10.1093/eurheartj/ehq071. [DOI] [PubMed] [Google Scholar]
- 16.Jardine DL, Krediet CT, Cortelli P, Frampton CM, Wieling W. Sympatho-vagal responses in patients with sleep and typical vasovagal syncope. Clinical science. 2009;117:345–353. doi: 10.1042/CS20080497. [DOI] [PubMed] [Google Scholar]
- 17.Mano T, Iwase S. Sympathetic nerve activity in hypotension and orthostatic intolerance. Acta physiologica Scandinavica. 2003;177:359–365. doi: 10.1046/j.1365-201X.2003.01081.x. [DOI] [PubMed] [Google Scholar]
- 18.Mosqueda-Garcia R, Furlan R, Fernandez-Violante R, et al. Sympathetic and baroreceptor reflex function in neurally mediated syncope evoked by tilt. J Clin Invest. 1997;99:2736–2744. doi: 10.1172/JCI119463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jardine DL, Melton IC, Crozier IG, et al. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. AmJPhysiol Heart CircPhysiol. 2002;282:H1804–H1809. doi: 10.1152/ajpheart.00640.2001. [DOI] [PubMed] [Google Scholar]
- 20.Fu Q, Verheyden B, Wieling W, Levine BD. Cardiac output and sympathetic vasoconstrictor responses during upright tilt to presyncope in healthy humans. J Physiol. 2012;590:1839–1848. doi: 10.1113/jphysiol.2011.224998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corcoran SJ, Lambert EA. Vasovagal syncope--the electricity, the pump or the input pressure? J Physiol. 2012;590:1775–1776. doi: 10.1113/jphysiol.2012.231282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheldon R, Connolly S, Rose S, et al. Prevention of Syncope Trial (POST): a randomized, placebo-controlled study of metoprolol in the prevention of vasovagal syncope. Circulation. 2006;113:1164–1170. doi: 10.1161/CIRCULATIONAHA.105.535161. [DOI] [PubMed] [Google Scholar]
- 23.Funakami Y, Hata T, Itoh E, Itano S. Effects of some beta-adrenoceptor antagonists on orthostatic hypotension in repeatedly cold-(SART-) stressed rats. Biological & pharmaceutical bulletin. 2007;30:303–308. doi: 10.1248/bpb.30.303. [DOI] [PubMed] [Google Scholar]
- 24.Flevari P, Livanis EG, Theodorakis GN, Zarvalis E, Mesiskli T, Kremastinos DT. Vasovagal syncope: a prospective, randomized, crossover evaluation of the effect of propranolol, nadolol and placebo on syncope recurrence and patients' well-being. J Am Coll Cardiol. 2002;40:499–504. doi: 10.1016/s0735-1097(02)01974-5. [DOI] [PubMed] [Google Scholar]
- 25.Yao Y, Shi R, Wong T, et al. Endocardial autonomic denervation of the left atrium to treat vasovagal syncope: an early experience in humans. Circ Arrhythm Electrophysiol. 2012;5:279–286. doi: 10.1161/CIRCEP.111.966465. [DOI] [PubMed] [Google Scholar]
- 26.Symplicity HTNI. Esler MD, Krum H, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 27.Park HW, Shen MJ, Han S, et al. Neural control of ventricular rate in ambulatory dogs with pacing-induced sustained atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:571–580. doi: 10.1161/CIRCEP.111.967737. [DOI] [PMC free article] [PubMed] [Google Scholar]