SUMMARY
Marijuana has been used for thousands of years as a treatment for medical conditions. However, untoward side effects limit its medical value. Here we show that synaptic and cognitive impairments following repeated exposure to Δ9-tetrahydrocannabinol (Δ9-THC) are associated with the induction of cyclooxygenase-2 (COX-2), an inducible enzyme that converts arachidonic acid to prostanoids, in the brain. COX-2 induction by Δ9-THC is mediated via CB1 receptor-coupled G-protein βγ subunits. Pharmacological or genetic inhibition of COX-2 blocks down-regulation and internalization of glutamate receptor subunits and alterations of the dendritic spine density of hippocampal neurons induced by repeated Δ9-THC exposures. Ablation of COX-2 also eliminates Δ9-THC-impaired hippocampal long-term synaptic plasticity, spatial, and fear memories. Importantly, the beneficial effects of decreasing β-amyloid plaques and neurodegeneration by Δ9-THC in Alzheimer’s disease animals are retained in the presence of COX-2 inhibition. These results suggest that the applicability of medical marijuana would be broadened by concurrent inhibition of COX-2.
INTRODUCTION
Marijuana has been used for thousands of years to treat chronic pain, multiple sclerosis, cancer, seizure disorders, nausea, anorexia, inflammatory and neurodegenerative diseases (Robson et al, 2001; Russo, 2007). However, the undesirable neuropsychological and cognitive side effects greatly limit the medical use of marijuana (Carlini, 2004). The major intoxicating effects of cannabis are the impairments in synaptic and cognitive function (Pope et al., 2001; Solowij et al., 2002; Messinis et al., 2006). These untoward effects are also the primary consequences of cannabis abuse. However, there are no currently FDA-approved effective medications for prevention and treatment of these cannabis-related disorders.
As it is clear now, Δ9-tetrahydrocannabinol (Δ9-THC) is the major psychoactive ingredient of marijuana (Gaoni and Mechoulam, 1964) and its effects are largely mediated through cannabinoid receptors (CB1R or CB2R), which are pertussis toxin (PTX) sensitive G protein-coupled receptors (Howlett, 1998; Pertwee et al., 2010). Previous studies demonstrate that deficits in long-term synaptic plasticity, learning and memory by Δ9-THC exposure are primarily mediated through CB1R expressed in the brain (Lichtman and Martin, 1996; Hoffman et al., 2007; Puighermanal et al., 2009; Fan et al., 2010; Han et al., 2012). However, the molecular mechanisms underlying the synaptic and cognitive deficits elicited by repeated Δ9-THC exposure are largely unknown.
In the present study, we unexpectedly observed that Δ9-THC increases expression and activity of cyclooxygenase-2 (COX-2), an inducible enzyme that converts arachidonic acid to prostanoids, both in vitro and in vivo via a CB1R-dependent mechanism. This action is opposite to the observations where the endogenous cannabinoid 2-arachidonylglycerol (2-AG) induces a CB1R-dependent suppression of COX-2 activity and expression in response to proinflammatory and excitotoxic insults (Zhang and Chen, 2008). The differential modulation of COX-2 by the exogenous cannabinoid Δ9-THC and endogenous cannabinoid 2-AG appears to result from intrinsic properties of the CB1R-coupled G-protein. The COX-2 induction by Δ9-THC is mediated via Gβγ subunits, while COX-2 suppression by 2-AG is mediated through the Gαi subunit. Interestingly, the impairments in hippocampal long-term synaptic plasticity, spatial, and fear memories induced by repeated Δ9-THC exposure can be occluded or attenuated by pharmacological or genetic inhibition of COX-2. Finally, the beneficial effects of reducing Aβ and neurodegeneration by Δ9-THC are retained in the presence of COX-2 inhibition. Our results reveal a previously unknown signaling pathway that is linked to synaptic and cognitive deficits induced by Δ9-THC exposure, suggesting that Δ9-THC would display its beneficial properties with fewer undesirable side effects when its COX-2 induction effect is inhibited, which may form a novel therapeutic intervention for medical treatments.
RESULTS
Δ9-THC induces dose- and time-dependent increase in COX-2 expression
Identification of CBRs led to discovery of several endogenous cannabinoids, including anandamide (AEA) and 2-arachidonylglycerol (2-AG), which are the most studied endocannabinoids involved in a variety of physiological, pharmacological, and pathological processes (Kano et al., 2009; Pertwee et al., 2010). 2-AG, the most abundant endocannabinoid, plays significant roles in synaptic modification, resolution of neuroinflammation, and neuronal survival (Alger, 2009; Chevaleyre et al., 2006; Lovinger, 2008; Panikashvili, et al., 2001; Zhang and Chen, 2008). In particular, its anti-inflammatory and neuroprotective effects in response to proinflammatory and neurotoxic insults appear to be through limiting COX-2 signaling (Chen et al., 2011, Du et al., 2011; Zhang and Chen, 2008). Since acute inhibition of COX-2 by selective COX-2 inhibitors has been shown to decrease hippocampal long-term potentiation (LTP) and impairs memory consolidation (Chen et al., 2002; Teather et al., 2002; Cowley et al., 2008). We thus wondered whether impairments of synaptic plasticity and memory by marijuana result from a COX-2 suppressive effect. To assess this, we first analyzed hippocampal expression and activity of COX-2 in mice that received Δ9-THC. Unexpectedly, in vivo exposure to Δ9-THC produced a dose- and time-dependent induction of COX-2 in the brain, rather than suppression (Fig.1A&B), while expression of COX-1 was unaffected by Δ9-THC (supplementary Fig. S1A). The increase in COX-2 expression induced by Δ9-THC was accompanied by elevated production of prostaglandin E2 (PGE2), which could be inhibited by the selective COX-2 inhibitor Celebrex or genetic inhibition of COX-2 (Fig.1C, Fig. S1B). To confirm the ability of exogenous cannabinoids to induce COX-2, we assessed COX-2 expression and PGE2 production in animals injected with the synthetic cannabinoid CP55,940 (CP). As expected, CP produced more pronounced effects on COX-2 expression and PGE2 synthesis (Fig.S1C-E). The increase in PGE2 could be blocked by NS398, another selective COX-2 inhibitor. In addition, we observed that COX-2 expression was steadily elevated in animals injected with Δ9-THC once daily for 7 consecutive days although the magnitude of increase in COX-2 was not as intensified as that of a single injection (Fig. 1D). This indicates that expression of COX-2 is persistently elevated upon repeated exposure to Δ9-THC Fig.S7).
Figure 1.
Δ9-THC in vivo exposure induces CB1R-dependent activation and elevation of COX-2 expression in the hippocampus. A-B. Δ9-THC induces a dose- and time-dependent increase in hippocampal COX-2 expression (n=5) C. Δ9-THC increases synthesis of PGE2 and the increase is blocked by Celebrex (Celeb) or genetic inhibition of COX-2 (COX-2 knockout). PGE2 was detected 4 hrs after Δ9-THC injection (10 mg/kg). Celebrex (10 mg/kg) was injected 30 min prior to Δ9-THC injection (n=10/group). D. COX-2 is persistently elevated in animals that received repeated injections of Δ9-THC (10 mg/kg, i.p.) once a day for 7 consecutive days. COX-2 was analyzed 24 hrs after secession of the last injection (n=3). E. COX-2 induction by Δ9-THC (10 mg/kg) is blocked by Rimonabant (RIM, 5 mg/kg). Hippocampal COX-2 was detected 4 hr after Δ9-THC injection (n=3). RIM was injected 30 min prior to Δ9-THC injection. F. Δ9-THC fails to increase COX-2 in CB1R knockout mice (n=3). G. Δ9-THC increases COX-2 both in neurons and astroglial cells in culture and the increase is blocked by RIM. COX-2 was assayed 12 hr after treatments (n=6). All the data are presented as mean ± SEM, *P<0.05, **P<0.01 compared with the vehicle controls, #P<0.05, ##P<0.01 compared with Δ9-THC (one-way ANOVA, Fisher’s PLSD). (See also Figures S1,S7.)
COX-2 induction by Δ9-THC is CB1R-dependent
Since undesirable side effects elicited by cannabinoids are primarily mediated by CB1R (Lichtman and Martin, 1996; Hoffman et al., 2007; Han et al., 2012), we wondered whether COX-2 induction by Δ9-THC is mediated via CB1R. As shown in Fig.1E&F, Δ9-THC-induced increase in COX-2 in the hippocampus was blocked either by Rimonabant (RIM), a selective CB1R antagonist, or by genetic deletion of CB1R. To determine whether the increase in COX-2 by Δ9-THC occurs in neurons or astroglial cells, we made different conditions in cultures as described previously (Zhang and Chen, 2008). We found that while Δ9-THC induced a CB1R-dependent increase in COX-2 expression both in neuronal and astroglial cell-enriched cultures, the increased was more pronounced in astroglial cell-enriched cultures than in neuronal culture (Fig. 1G). Our data provide convincing evidence that COX-2 induction by Δ9-THC both in vivo and in vitro is mediated via CB1R.
COX-2 induction by Δ9-THC is via CB1R-coupled G protein βγ subunits
Since the suppression of COX-2 by 2-AG in response to proinflammatory stimuli occurs via a CB1R-dependent mechanism (Zhang and Chen, 2008), we questioned why the exogenous cannabinoid Δ9-THC increases COX-2 and the endogenous cannabinoid 2-AG suppresses COX-2 acting through the same CB1R-dependent mechanism, and speculated that CB1R may not be the key molecule responsible for differential regulation of COX-2 expression upon exposure to cannabinoids. CB1R is coupled to a PTX-sensitive Gi/o protein, and activation of CB1R releases Gβγ subunits from the GTP-bound Gαi subunit (Howlett, 1998; Pertwee et al., 2010). Earlier studies show that activation of CB1R is capable of inducing Gβγ-mediated response (Guo and Ikeda, 2004; Wilson et al., 2001; Yao et al., 2003). We hypothesized that Gβγ and Gαi may differentially mediate COX-2 induction or suppression by exogenous Δ9-THC or endogenous 2-AG. To test this prediction, we first over-expressed Gβγ subunits by transfection with plasmids carrying β1 and γ2 subunits in NG108-15 cells, which express native CB1R (Fig. S2A&B). While Δ9-THC still increased expression of COX-2 mRNA in culture transfected with the control vector, it did not increase COX-2 in culture overexpressing β1 and γ2 subunits (Fig. 2A1). In subsequent experiments, β1 and γ2 subunits were silenced by shRNA. Knockdown of β1γ2 by shRNA suppressing endogenous β1γ2 also blocked COX-2 induction by Δ9-THC in NG108-15 cells, and the blockade was rescued by concurrently expressing shRNA-resistant β1γ2 (Fig. 2A2, Fig. S2E). This indicates that COX-2 induction by Δ9-THC is likely mediated through Gβγ. To further confirm that Gβγ mediate COX-2 induction by Δ9-THC, we treated mixed culture of hippocampal neurons and astroglial cells (~5-10%) with a membrane-permeable Gβγ-binding peptide mSIRK to disrupt the function of Gβγ (Delaney et al., 2007; Goubaeva et al., 2003). As a negative control, we used a variant mSIRK with a point mutation of Leu9 to Ala (L9A-mSIRK). As shown in Fig 2B, disruption of Gβγ activity by mSIRK also blocked COX-2 induction by Δ9-THC, while it failed to block the suppression of COX-2 by 2-AG in response to LPS, a commonly used COX-2 inducer (Zhang and Chen, 2008). PTX treatment also blocked Δ9-THC-induced increase in COX-2. Interestingly, application of 2-AG failed to suppress Δ9-THC-induced increase in COX-2 (Fig. 2B, Fig. S2I). To test the prediction that Gαi mediates COX-2 suppressive effect by 2-AG, we silenced Gαi using a lentiviral vector in mixed culture of neurons and astroglial cells (Fig. S2C). As illustrated in Fig.2C and Fig. S2D, silencing Gαi1, but not Gαi2 or Gαi3, blocked the suppression of COX-2 by 2-AG in response to the LPS stimulus, and this blocking effect was rescued by concurrently expressing shRNA-resistant Gαi1 (Fig.2C, Fig. S2E). Knockdown of Gαi1, Gαi2 or Gαi3 did not block COX-2 induction by Δ9-THC (Fig. 2C and Fig. S2D). These results indicate that COX-2 induction by Δ9-THC is likely mediated via Gβγ, while COX-2 suppression by 2-AG is likely mediated through Gαi1 (Fig.S7).
Figure 2.
Gβγ subunits mediate Δ9-THC-elevated COX-2 expression. A. Overexpression or knockdown of β1 and γ2 subunits eliminates Δ9-THC-increased COX-2 mRNA detected by qPCR in NG108-15 cells. Error bars represent ±SEM, **P<0.01 compared with the vehicle control (ANOVA, Fisher’s PLSD, n=6). NG108-15 cells were transfected with pcDNA3.1 plasmids encoding Gβ1 and Gγ2 subunits, or the pLL3.7 vector expressing Gβ1 and Gγ2 shRNA, or the vector expressing shRNA-resistant Gβ1γ2 in the absence and presence of Δ9-THC. B. Disruption of Gβγ subunits blocks Δ9-THC-elevated COX-2, but does not prevent suppression of COX-2 by 2-AG in response to LPS stimulus in mixed culture of hippocampal neurons and astroglial cells (~ 10%). The culture was treated with a membrane permeable Gβγ-binding peptide mSIRK or a single point mutated (Leu 9 to Ala) Gβγ-binding peptide mSIRK (L9A-mSIRK) in the absence and presence of Δ9-THC, LPS, PTX, 2-AG. C, Silencing the Gαi1 subunit blocks 2-AG-suppressed COX-2, but does not affect the elevation of COX-2 by Δ9-THC in mixed culture of neurons and astroglial cells treated with the lentiviral vector expressing Gαi1 shRNA or shRNA-resistant Gαi1. D. Δ9-THC induces phosphorylation of Akt, ERK and p38MAPK and the phosphorylation is inhibited by knockdown of Gβγ2 and the inhibition is rescued by expressing shRNA-resistant Gβ1γ2. E1. Δ9-THC induces phosphorylation of NF-κB and the effect is blocked by Gβ1γ2 shRNA in NG108-15 cells. E2. Binding of NF-κB p65 in the promoter region of the COX-2 gene (ptgs2) by chromatin immunoprecipitation (ChIP) analysis. E3. Δ9-THC-induced NF-κB phosphorylation and COX-2 expression are blocked by IKKβ inhibition in mixed culture of neurons and astroglial cells. (See also Figures S2, S7.)
Akt, ERK, p38MAPK and NF-κB are downstream signaling of Gβγ
To determine downstream signaling pathways of Gβγ, we detected phosphorylation of Akt, ERK, and p38MAPK by overexpression or knockdown of Gβγ in the presence and absence of Δ9-THC. As shown in Fig. 2D and Fig.S2F, Δ9-THC induced phosphorylation of these signaling molecules and the phosphorylation was inhibited by knockdown or over-expression of Gβ1γ2. Inhibition of phosphorylation of these mediators by shRNA was rescued by concurrently expressing shRNA-resistant Gβ1γ2 (Fig. 2D). These data indicate that COX-2 induction by Δ9-THC is likely through signaling of these downstream molecules of Gβγ. To further characterize this signaling pathway that regulates COX-2 expression by Δ9-THC, we targeted NF-κB, which is a transcription factor regulating expression of genes including the COX-2 gene (ptgs2). We observed that Δ9-THC induced NF-κB phosphorylation in NG-108-15 cells and this phosphorylation was inhibited by overexpression or knockdown of Gβγ, and rescued by concurrently expressing shRNA-resistant G β1γ2 (Fig. 2E1, Fig. S2G). To determine regulation of COX-2 transcription by NF-κB, we performed a chromatin immunoprecipitation (CHIP) analysis in mixed culture of neurons and astroglial cells. As shown in Fig. 2E2, a binding activity of NF-κB p65 was detected in the promoter positions (−419 to −428 bp) of ptgs2, and this interaction was enhanced by Δ9-THC and inhibited by SC-514, a specific IKKβ inhibitor that inhibits p65-associated transcriptional activation of the NF-κB pathway. To further confirm the involvement of NF-κB in Δ9-THC-induced increase in COX-2, COX-2 expression and NF-κB phosphorylation by Δ9-THC were determined in the absence and presence of SC-514. Inhibition of IKKβ blocked Δ9-THC-induced COX-2 and NF-κB phosphorylation (Fig. 2E3). Phosphorylation of Akt, ERK, p38MAPK and NF-κB was confirmed in the hippocampus of animals that received Δ9-THC (Fig. S2H).
Inhibition of COX-2 eliminates impairments in hippocampal long-term synaptic plasticity
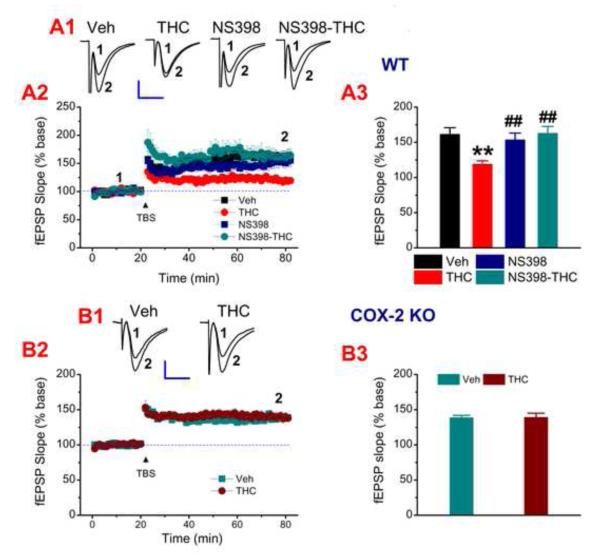
If sustained elevation of COX-2 expression and activity following repeated Δ9-THC exposure contribute to impairments in long-term synaptic plasticity and cognitive function, then inhibition of COX-2 should be able to eliminate or attenuate the impairments. To test this hypothesis, we recorded hippocampal LTP in mice receiving daily injections of Δ9-THC (10 mg/kg, the dosage used by other studies, Fan et al., 2010; Hoffman et al., 2007; Puighermanal et al., 2009; Tonini et al., 2006), NS398, Δ9-THC+NS398 or vehicle for 7 consecutive days. We found that COX inhibition by NS398 rescued decreased hippocampal LTP induced by repeated in vivo exposure to Δ9-THC for 7 days both at CA3-CA1 synapses (Fig. 3A) and perforant path synapses in the dentate gyrus (Fig. S3A). Similarly, genetic inhibition of COX-2 also prevented LTP deterioration induced by Δ9-THC at both CA3-CA1 synapses (Fig. 3B) and the perforant path (Fig. S3B). To verify whether persistent over-expression of COX-2 impairs LTP, we recorded LTP in animals repeatedly treated with LPS, which increases COX-2. As we expected, repeated injection of LPS significantly reduced LTP, and this decrease was prevented by inhibition of COX-2 (Fig. S3C). These data suggest that persistent elevation of COX-2 in the brain will be detrimental to integrity of synaptic structure and plasticity. Since a single dose of Δ9-THC produced an increase in COX-2 expression, we wondered whether this increase alters synaptic function. To this end, we recorded long-term depression (LTD) induced by low-frequency stimulation (LFS) at hippocampal CA3-CA1 synapses, and found that LTD is impaired by a single Δ9-THC exposure. However, LTD is normal in COX-2 knockout animals that received a single injection of Δ9-THC (Fig. S4). This information suggests that a single Δ9-THC exposure induces a COX-2-associated impairment in LTD (Mato et al., 2004; 2005).
Figure 3.
Inhibition of COX-2 eliminates deficits in long-term potentiation (LTP) by repeated Δ9-THC exposure. A1. Representative fEPSPs recorded at hippocampal CA3-CA1 synapses from WT animals repeatedly injected with vehicle, Δ9-THC (10 mg/kg), NS398 (10 mg/kg), or Δ9-THC+NS398 once daily for 7 consecutive days. LTP was measured 24 hr after cessation of the last injection. A2. Time courses of changes in fEPSP slope under different treatment. A3. Mean values of the potentiation of fEPSPs averaged from 56 to 60 min following TBS (n=6 to 8 slices/5~6 animals). B1. Representative fEPSPs recorded from COX-2 knockout (KO) mice injected with vehicle, or Δ9-THC (10 mg/kg) once daily for 7 consecutive days. B2. Time courses of changes in fEPSP slope induced by Δ9-THC. B3. Mean values of the potentiation of fEPSPs averaged from 56 to 60 min following TBS (n=8~12 slices/6~8 animals). Error bars represent ±SEM, **P<0.01 compared with vehicle controls; ##P<0.01 compared with Δ9-THC (ANOVA with Bonferronni post-hoc test). Scale bars in A1 and B1: 0.3 mV/10 msec. (See also Figures S3, S4.)
Impairments in spatial and fear memories by Δ9-THC is occluded by COX-2 inhibition
Administration of marijuana or Δ9-THC impairs learning and memory. If this impairment is associated with COX-2 induction, then inhibition of COX-2 would prevent or attenuate the deficits. To test this prediction, we determined the effect of COX-2 inhibition on spatial learning and memory using the Morris water maze test in mice that received repeated Δ9-THC exposure in WT and COX-2 KO mice. As shown in Fig.4B&C, pharmacological or genetic inhibition of COX-2 prevented Δ9-THC-impaired spatial memory. To further determine the role of COX-2 in Δ9-THC-impaired memory, hippocampus-dependent contextual memory was determined using the fear conditioning protocol (Chen et al., 2006). As seen in Fig. 4A, repeated Δ9-THC exposure impaired fear memory, and this impairment was attenuated by COX-2 inhibition. These results suggest that COX-2 plays a critical role in synaptic and cognitive function deterioration consequent to repeated in vivo Δ9-THC exposure (Fig.S7).
Figure 4.
Impaired spatial and fear memories by repeated Δ9-THC exposure are occluded by COX-2 inhibition. A. Impaired fear memory is attenuated by COX-2 inhibition. 24 hrs after a footshock coniditioning, animals were administered with Δ9-THC (10 mg/kg) or NS398 (10 mg/kg) once a day for 7 days. Freezing behavior was recorded 24 hrs after the cessation of the last injections. B. COX-2 KO and WT mice received training in the Morris water maze for 5 days without any treatments (naïve). Starting at day 6, WT animals received vehicle, Δ9-THC (10 mg/kg), NS398 (10 mg/kg), Δ9-THC+NS398, once a day for 7 days. COX-2 KO mice received vehicle or Δ9-THC (10 mg/kg) for 7 days. Tests were performed 30 min following the injections. C1-3, Probe trial test was conducted 24 hrs after the cessation of the last Δ9-THC injection. The number of times crossed the target zone, the amount of time stayed in the target quadrant, and swim speed in different treatments in probe trial tests were detected. Error bars represent ±SEM, **P<0.01 compared with the vehicle control (n=9~12 animals/group, two-way ANOVA, Bonferronni post-hoc test). (See also Figures S5, S7.)
Cataleptic effect and hypomotility are behavioral response upon administering Δ9-THC (Burstein et al., 1989; Long et al., 2009). We observed that the cataleptic and locomotor depressive effects of Δ9-THC were attenuated or prevented by pharmacological or genetic inhibition of COX-2 (Fig. S5). This means that cannabis-elicited catalepsy and locomotor depression are associated with the COX-2 induction.
Functional synaptic integrity in Δ9-THC -treated animals is maintained by COX-2 inhibition
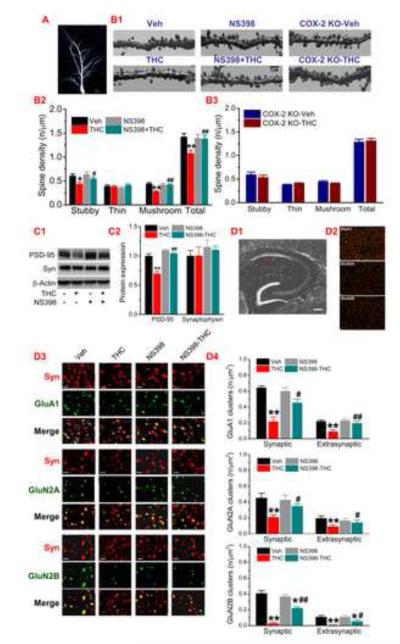
Impaired long-term synaptic plasticity and memory induced by Δ9-THC are largely associated with altered expression and function of glutamate receptors (Fan et al., 2010; Han et al., 2012). Recent evidence shows that adolescent chronic treatment with Δ9-THC results in reduced density of dendritic spines and lowered length and number of dendrites in the hippocampus (Rubino et al., 2009). We used Thy1-GFP expressing transgenic mice to detect morphology of dendritic spines (Chen et al., 2012). As seen in Fig. 5A&B, repeated Δ9-THC exposure significantly reduced density of dendritic spines of CA1 pyramidal neurons, especially mushroom spines where AMPA and NMDA receptors are expressed. We found that the reduction in spines was prevented by pharmacological or genetic inhibition of COX-2. (We should mention it here that the comparatively low number of mushroom-type spines in Fig. 5A&B may be due to the scoring criteria). Meanwhile, Δ9-THC-reduced expression of PSD-95, an important postsynaptic marker, was rescued by COX-2 inhibition (Fig. 5C). However, Δ9-THC did not alter expression of synaptophysin (Syn), a presynaptic marker. This information indicates that increased COX-2 by repeated Δ9-THC exposure decreases dendritic spines and postsynaptic density. We show previously that repeated Δ9-THC exposure for 7 days induces CB1R-dependent decreases in functional and surface expression of AMPA and NMDA receptor subunits (Fan et al., 2010). We speculated that reduced expression of glutamate receptor subunits in the hippocampus of animals that received repeated in vivo Δ9-THC exposure are likely regulated by a homeostatic mechanism. Δ9-THC increased synthesis of COX-2 and its reaction product PGE2, which stimulates glutamate released from presynaptic nerve terminals and astroglial cells, resulting in an extracellular accumulation of glutamate (Fig. S6A). The increased extracellular glutamate may also result from the reduced uptake of glutamate by glutamate transporters since expression of these transporters was down-regulated by repeated exposure to Δ9-THC (Fig. S6B). To this end, we used immunostaining to determine expressions of synaptic and extrasynaptic GluA1, GluN2A, GluN2B in the hippocampal CA1 area. As shown in Fig. 5D, hippocampal expressions of both synaptic and extrasynaptic GluA1, GluN2A, GluN2B were significantly reduced by repeated Δ9-THC exposure and the reduction was attenuated or prevented by COX-2 inhibition. This was consistent with the observations where total and surface expressions of GluA1, GluN2A, GluN2B detected by immunoblot in WT mice were significantly decreased following exposure to Δ9-THC for 7 days, but the decreases were not seen in COX-2 knockout mice (Fig. 6). These results indicate that reduced expression of glutamate receptor subunits and density of dendritic spines are associated with the COX-2 induction effect of Δ9-THC (Fig.S7).
Figure 5.
Decreases in dendritic spine density and glutamate receptor expression by Δ9-THC are prevented by inhibition of COX-2. A-B, Two-photon imaging of dendritic spines in CA1 hippocampal pyramidal neurons expressing GFP of transgenic mice (n=5 animals/group). Scale bars in A and B: 20 and 3 μm. C, Expression of PSD-95 and synaptophysin (Syn) in animals treated with Δ9-THC or NS398 for 7 days (n=3 animals). D1, Schematic of a hippocampal section. The red dash-line box marks the sampling field of immunostaining analysis. Scale bar: 200 μm. D2, Representative GluA1, GluN2A, GluN2B, and Syn immunoreactivities (Scale bar: 5 μm). D3, Enlarged immunosignals of GluA1, GluN2A, GluN2B, Syn, and their overlay. Scale bars: 1.5 μm. D4. Quantification of synaptic (colocalized with Syn) and extrasynaptic (non-colocalized) GluA1, GluN2A, and GluN2B (n=5 animals/group). Error bars represent ±SEM, **P<0.01 compared with the vehicle control; #P<0.05, ##P<0.01compared with Δ9-THC (ANOVA with Fisher’s PLSD or Bonferronni post-hoc tests).
Figure 6.
Reduced expression of glutamate receptor subunits and phosphorylation of CREB by Δ9-THC is rescued by COX-2 inhibition. A. Immunoblot analysis of hippocampal expression of GluR1, NR2A and NR2B subunits in WT and COX-2 KO mice treated with vehicle or Δ9-THC for 7 days (n=3). B. Surface expression of GluR1, NR2A, and NR2B in WT and COX-2 KO mice treated with vehicle or Δ9-THC for 7 days (n=4). C. Phosphorylation of hippocampal CREB in WT and KO mice treated with vehicle or Δ9-THC for 7 days (n=3). Error bars represent ±SEM, *P<0.05, **P<0.01 compared with the vehicle control (ANOVA with Fisher’s PLSD). (See also Figures S6, S7.)
The beneficial effects of decreasing Aβ and neurodegeneration by Δ9-THC are preserved in the presence of COX-2 inhibition
A critical issue is whether COX-2 inhibition would eliminate the beneficial effects of marijuana. To answer this question, we used 5XFAD APP transgenic mice, an animal model of Alzheimer’s disease (AD) as described previously (Chen et al., 2012), to determine whether Δ9-THC is capable of reducing Aβ and neurodegeneration and whether these effects are retained when COX-2 is inhibited. As shown Fig. 7A&B, treatment of Δ9-THC once daily for four weeks significantly reduced the numbers of Aβ plaques and degenerated neurons in the absence and presence of Celebrex in AD animals. This information indicates that the beneficial effects of Δ9-THC are preserved while COX-2 is inhibited. Meanwhile, we revealed that the reduction of Aβ by Δ9-THC is not through inhibiting expression of β-site amyloid precursor protein cleaving enzyme 1 (BACE1), an enzyme responsible for synthesis of Aβ, but likely through elevating neprilysin, an important endopeptidase that degrades Aβ (Fig. 7C).
Figure 7.
The beneficial effects of reducing Aβ and neurodegeneration by Δ9-THC are preserved in the presence of COX-2 inhibition. A. Δ9-THC significantly reduces Aβ plaques detected using anti-4G8 antibody in 4-month-old 5XFAD APP transgenic (TG) mice in the absence and presence of COX-2 inhibition. TG mice received Δ9-THC (3 mg/kg) or Celebrex (1 mg/kg) once daily for 4 weeks starting at 3 months of age. B. Δ9-THC significantly reduces degenerated neurons detected by Fluoro-Jade C (FJC) staining in 6-month-old TG mice treated with/out Celebrex. TG mice received Δ9-THC (3 mg/kg) or Celebrex (1 mg/kg) once daily for 4 weeks starting at 5 months of age. C. Δ9-THC increases expression of neprilysin (NEP), but not β-site amyloid precursor protein cleaving enzyme 1 (BACE1) in TG mice. Error bars represent ± SEM, **P<0.01 compared with the vehicle control (n=3 to 5 animals/group; One-way ANOVA, Bonferronni post-hoc tests). Scale bars in A and B: 400 μm.
DISCUSSION
The results presented here demonstrate that impaired synaptic and cognitive function induced by repeated Δ9-THC exposure is associated with a previously unrevealed CB1R-Gβγ-Akt-ERK/MAPK-NF-κB-COX-2 signaling pathway. It has been long known that use of marijuana induces neuropsychiatric and cognitive deficits, which greatly limit medical use of marijuana. Synaptic and memory impairments are also the consequence of cannabis abuse. However, the molecular mechanisms underlying undesirable effects by cannabis are largely unknown. We discovered in this study that pharmacological or genetic inhibition of COX-2 eliminates or attenuates synaptic and memory impairments elicited by repeated Δ9-THC exposure, suggesting that these major adverse effects of cannabis on synaptic and cognitive function can be eliminated by COX-2 inhibition, which would broaden the use of medical marijuana.
CB1R is the primary target of cannabinoid exposures causing synaptic and memory impairments (Lichtman and Martin, 1996; Hoffman et al., 2007; Puighermanal et al., 2009; Fan et al., 2010; Han et al., 2012). Previous studies show that the endocannabinoid 2-AG suppresses COX-2 via a CB1R-depedent mechanism in response to proinflammatory and excitotoxic insults (Zhang and Chen, 2008). Surprisingly, we found in the present study that the exogenous cannabinoid Δ9-THC increases COX-2 activity and expression, which are also mediated via CB1R. We demonstrate that COX-2 induction by Δ9-THC is mediated via Gβγ subunits, while COX-2 suppression by 2-AG is mediated via the Gαi1 subunit, suggesting that activation of the same CB1 receptor may induce opposite biological effects. Indeed, previous studies showed that endogenous cannabinoids and exogenous Δ9-THC exhibit different behavioral responses via CB1R (Long et al., 2009). However, it is still not clear how activation of CB1R and its coupled Gi/o by the endogenous cannabinoid 2-AG results in Gαi-mediated suppression of COX-2 in response to proinflammatory insults but by the exogenous cannabinoid Δ9-THC leads to Gβγ-mediated induction of COX-2. Activation of CB1R/Gi/o either by 2-AG or Δ9-THC should induce both Gαi- and Gβγ-mediated effector responses through different downstream signaling events. For example, inhibition of N-type calcium channel currents by 2-AG appears to be mediated via Gβγ (Guo and Ikeda, 2004), suggesting that 2-AG is also capable of triggering Gβγ-mediated responses in addition to Gαi-mediated responses. In the case of COX-2 induction, the Gβγ-mediated COX-2 induction by Δ9-THC may be predominant, which may mask Gαi-mediated COX-2 suppression. In addition, our results showing that the beneficial effects of Δ9-THC are retained in the presence of COX-2 inhibition further suggest that activation of CB1R by Δ9-THC may have both Gαi- and Gβγ-mediated effector responses. It is likely that COX-2 induction by Δ9-THC may be just one of several Gβγ-mediated effects, and we cannot exclude the possibility that other biological effects are mediated via Gβγ. The divergent roles of G-protein subunits in mediating endogenous and exogenous cannabinoids may be a consequence the intrinsic mechanisms of CB1R/G-protein coupling, such as the agonist binding sites in the receptor, the efficacy of binding, or different conformational changes in the receptor/G-protein upon binding with different agonists.
Synaptic and cognitive impairments by Δ9-THC are apparently associated with alterations in glutamatergic synaptic transmission and functional expression of glutamate receptor subunits (Fan et al., 2010; Han et al., 2012; Monory et al., 2007; Tonini et al., 2006). It has been demonstrated that cannabinoid exposure leads to down-regulation, internalization, and endocytosis of glutamate receptor subunits (Fan et al., 2010; Han et al., 2012; Suárez et al., 2003). In this study, we also demonstrate that density of dendritic spines in hippocampal neurons is reduced in animals that received Δ9-THC for seven days. The reduced expressions of synaptic and extrasynaptic of glutamate receptor subunits as well as PSD-95 by Δ9-THC are likely associated with elevated extracellular glutamate levels. Indeed, it has been shown that cannabinoids elevate extracellular glutamate levels, which may result from increased synaptic and astrocytic release of glutamate or reduced uptake of glutamate by glutamate transporters (Fan et al., 2010; Ferraro et al., 2001; Han et al., 2012; Navarrete et al., 2008; Tomasini et al., 2002; Suárez et al., 2004; Tonini et al., 2006). We detected that expression of glutamate transporters is significantly decreased in Δ9-THC exposed animals, and this decrease is attenuated by COX-2 inhibition (Fig. S6). These previous studies together with our results suggest that accumulation of glutamate in the extracellular apartment by repeated Δ9-THC exposure contributes to reductions in total and surface expression of the glutamate receptors and the density of dendritic spines.
Earlier studies showed that the levels of the eicosanoid PGE2 in circulation and the brain are elevated in humans and animals exposed to marijuana or Δ9-THC and the elevation could be antagonized by indomethacin, an NSAID (Burstein et al., 1989; Fairbairn and Pickens, 1979; 1980; Perez-Reyes et al., 1991). NSAIDs are non-selective inhibitors for both COX-1 and COX-2. This suggests that COX-1 and/or COX-2 may be involved in marijuana- or Δ9-THC-induced increase in PGE2. While both COX-1 and COX-2 are capable of converting arachidonic acid (AA) into five primary prostanoids and prostaglandins (PGD2, PGE2, PGF2α, PGI2, and TXA2), they exhibit preferences in synthesizing these substances. It is evident that PGE2 is primarily derived from the COX-2 pathway (Brock et al., 1999; Sang et al., 2005). Since COX-1 expression is not affected by Δ9-THC (Fig. S1) and COX-2 is expressed both in constitutive and inducible forms in the brain, it is likely that COX-2 is responsible for the marijuana- or Δ9-THC-induced elevation of PGE2. Our data showing that Δ9-THC increases PGE2 in the brain and this increase is blocked by COX-2 inhibition support this speculation. Interestingly, Δ9-THC-induced cataleptic response can be eliminated by NSAIDs and mimicked by direct administration of PGE2 (Burstein et al., 1989; Fairbairn and Pickens, 1979). We also provide convincing evidence that pharmacological or genetic inhibition of COX-2 prevents or attenuates cataleptic and locomotor depressive responses by Δ9-THC. Importantly, synaptic and cognitive deficits following repeated Δ9-THC exposure are eliminated or attenuated by COX-2 inhibition.
The elevated levels of extracellular glutamate by Δ9-THC result likely from induction of COX-2, which makes PGE2. It has been shown that PGE2 stimulates or facilitates both synaptic and astrocytic release of glutamate (Bezzi et al., 1998; Chen et al., 2002; Dave et al., 2010; Sang et al., 2005; Sanzgiri et al., 1999). In fact, COX-2 and PGE2 signaling have been shown to regulate glutamatergic synaptic transmission and plasticity via EP2 or EP3 receptors (Akaneya and Tsumoto, 2006; Chen et al., 2002; Cowley et al., 2008; Sang et al., 2005). It is possible that Δ9-THC exposure stimulates COX-2 expression and activity through CB1R-coupled Gβγ subunits and downstream Akt-ERK/MAPK-NF-κB signaling pathway, resulting in increase of COX-2 transcription, expression, and activity, which in turn enhance the release of PGE2 from neurons and astroglial cells. Our results show that Δ9-THC-induced COX-2 expression in astroglial cells is more pronounced than that in neurons. A recent study also shows that CB1R expressed in astroglial cells is responsible for LTD and working memory impairment in animals exposed to cannabinoids (Han et al., 2012). This suggests that glutamate released from astroglial cells triggered by COX-2-derived PGE2 and reduced uptake of glutamate by glutamate transporters in astrocytes resulting from repeated Δ9-THC exposure may play an important role in extracellular glutamate accumulation. Sustained elevation and accumulation of extracellular glutamate upon repeated exposure to Δ9-THC induce downregulation and internalization of glutamate receptor subunits and reduction in the density of dendritic spines in hippocampal neurons, leading to the deficits in long-term synaptic plasticity and cognitive function (Fig. S7).
It has been well recognized that cannabinoids possess antioxidant, anti-inflammatory, and neuroprotective properties (Bahr et al., 2006; Campbell and Gowran, 2007; Centonze et al., 2007; Chen et al., 2011; Du et al., 2011; Gowran et al., 2011; Marchalanta et al., 2008; Marsicano et al., 2003; Zhang and Chen, 2008). Also cannabis has been used for thousands of years as medical treatments. However, neuropsychiatric and cognitive side effects limit medical use of marijuana, especially for a long-term treatment. The results presented here suggest that the unwanted side effects of cannabis could be eliminated or reduced, while retaining its beneficial effects, by administering a COX-2 inhibitor or NSAID along with Δ9-THC for treatments of intractable medical conditions such as Alzheimer’s disease (AD). In the present study, we did observe that brain Aβ and neurodegeneration in 5XFAD transgenic mice are significantly reduced by Δ9-THC and these beneficial effects are preserved in the presence of COX-2 inhibition. We also discovered that Δ9-THC significantly elevates expression of neprilysin, an important endopeptidase for Aβ degradation. To our knowledge, this is the first demonstration that Δ9-THC is capable of reducing Aβ and neurodegeneration in an animal model of AD and that the Aβ reducing effect is likely through elevating expression of neprilysin. This suggests that Δ9-THC (brand name: Marinol) may have therapeutic potential for prevention and treatment of Alzheimer’s disease if its undesirable side effects (e.g., synaptic and cognitive impairments) can be eliminated by COX-2 inhibition. In particular, there are no effective medications currently available for preventing and treating AD or halting disease progression. Our results also suggest that selective COX-2 inhibitors or NSAIDs may be useful for treating the neuropsychological and cognitive side effects of cannabis abuse.
EXPERIMENTAL PROCEDURES
Animals
C57BL/6, CB1 knockout, Thy1-EGFP transgenic, COX-2 knockout and 5XFAD APP transgenic mice were used in the present study.
Cell culture
Relative pure hippocampal neurons (astroglial cells<2%), mixed neurons and astroglial cells (astroglial cells ~ 10%), and astroglial cell-enriched (astroglial cells>95%), and NG108-15 cell cultures were made as described previously (Sang et al, 2005; Zhang and Chen, 2008).
Electrophysiological recordings
Hippocampal LTP both at CA3-CA1 and perforant path synapses were recorded in acutely hippocampal slices and induced by a theta-burst stimulation (TBS) as described previously (Hoffman et al., 2007).
Immunoblots
Western blot assay was conducted using specific antibodies (Table S1) to determine expressions of COX-2, glutamate receptor subunits, PSD-95, G-protein subunits, phosphoproteins, BACE1 and neprilysin in hippocampal tissue and/or in cultured cells as described previously (Chen et al., 2012). Surface biotinylation assays were performed to determine surface expression of glutamate receptor subunits in hippocampal slices as described previously (Fan et al., 2010).
Transfection of plasmid and lentiviral vectors
NG108-15 cells were used for transfection of the pcDNA3.1 plasmid encoding Gβ1 and Gγ2 subunits or the pLL3.7 vector expressing scramble, Gβ1 and Gγ2 shRNA, and shRNA-resistant Gβ1γ2. Mixed culture of neurons and astroglial cells were used for transfection of the pLL3.7 lentiviral vector expressing scramble, Gαi1 shRNA, and shRNA-resistant Gαi1.
qRT-PCR
The iScript cDNA synthesis kit (BioRad) was used for the reverse transcription reaction. Real-time RT-PCR specific primers for COX-2, β1, γ2, and GAPDH were synthesized by IDT (Coralville, IA). Samples were compared using the relative CT method as described previously (Zhang & Chen, 2008).
CHIP analysis
Chromatin Immunoprecipitation (ChIP) analysis was performed to determine the binding activity of NF-κB in the promoter of the COX-2 gene.
PGE2 assay
PGE2 in hippocampal tissue was detected using PGE2 enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) according to the procedure described by the manufacturer (Zhang and Chen, 2008).
Immunostaining and histochemistry
Aβ plaques, degenerated neurons, and glutamate receptor subunits in cryostat sectioning brain slices were performed as described previously (Chen et al., 2012; Li et al., 2011).
Two-photon imaging
Morphology of dendritic spines in hippocampal CA1 pyramidal neurons was determined in GFP-expressing transgenic mice using a two-photon laser scanning microscope as described previously (Chen et al., 2012). Shape, size, and density of spines were measured from the three-dimensional reconstructions using NeuronStudio Version 0.9.92.
Behavioral tests
The classic Morris water maze and fear conditioning tests were performed to determine spatial and fear memory as described previously (Chen et al., 2012). The ‘open field’ test was conducted to detect the locomotor activity and the bar test was used to detect catalepsy (Egashira et al., 2007).
Supplementary Material
Highlights.
Δ9-THC induces COX-2 expression and activity via CB1R-coupled Gβγ subunits.
Disruption of synaptic integrity by Δ9-THC is prevented by COX-2 inhibition.
COX-2 inhibition eliminates Δ9-THC-caused synaptic and cognitive deficits.
Δ9-THC reduces Aβ and neurodegeneration in the presence of COX-2 inhibition.
Acknowledgements
We thank Dr. Xin-Yun Huang (Weill Medical College of Cornell University) for providing us with β1 and γ2 plasmids; Dr. James Pickel (NIMH transgenic core) for providing us CB1R knockout mice; Ms. Hongmin Jin for technical support; NIDA and MIMH Drug Supply Programs for providing Δ9-THC, Rimonabant, and Celebrex. This work was supported by grants from National Institutes of Health R01NS054886 and R01NS076815 to C.C.
Footnotes
Author Contributions R.C., J.Z., N.F., Z.T., Y.W. and C.C. designed and performed the experiments and analyzed the data; H.Y., H. S. and Y.S. performed some experiments; Y.T. provided the behavioral testing setups; C.C. conceived the project and wrote the manuscript.
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alger BE. Endocannabinoid signaling in neural plasticity. Curr Top Behav Neurosci. 2009;1:141–172. doi: 10.1007/978-3-540-88955-7_6. [DOI] [PubMed] [Google Scholar]
- Akaneya Y, Tsumoto T. Bidirectional trafficking of prostaglandin E2 receptors involved in long-term potentiation in visual cortex. J. Neurosci. 2006;26:10209–10221. doi: 10.1523/JNEUROSCI.3028-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr BA, Karanian DA, Makanji SS, Makriyannis A. Targeting the endocannabinoid system in treating brain disorders. Expert Opin Investig Drugs. 2006;15:351–365. doi: 10.1517/13543784.15.4.351. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Brock TG, McNish RW, Peters-Golden M. Arachidonic acid is preferentially metabolized by cyclooxygenase-2 to prostacyclin and prostaglandin E2. J. Biol. Chem. 1999;274:11660–11666. doi: 10.1074/jbc.274.17.11660. [DOI] [PubMed] [Google Scholar]
- Burstein SH, Hull K, Hunter SA, Shilstone J. Immunization against prostaglandins reduced Δ1-tetrohydrocannabinol-induced catalepsy in mice. Mol. Pharmacol. 1989;35:6–9. [PubMed] [Google Scholar]
- Campbell VA, Gowran A. Alzheimer’s disease: taking the edge off with cannabinoids? Br. J. Pharmacol. 2007;152:655–662. doi: 10.1038/sj.bjp.0707446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlini EA. The good and the bad effects of (−) trans-delta-9-tetrahydrocannabinol (Δ9-THC) on humans. Toxicon. 2004;44:461–467. doi: 10.1016/j.toxicon.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Centonze D, Finazzi-Agro A, Bernardi G, Maccarrone M. The endocannabinoid system in targeting inflammatory neurodegenerative diseases. Trends in Pharmacol. Sci. 2007;28:180–187. doi: 10.1016/j.tips.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J. Neurophysiol. 2002;87:2851–2857. doi: 10.1152/jn.2002.87.6.2851. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhang J, Chen C. Endocannabinoid 2-arachidonoylglycerol protects neurons against β-amyloid insults. Neurosci. 2011;178:159–168. doi: 10.1016/j.neuroscience.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Zhang J, Wu Y, Wang D, Feng G, Tang YP, Teng Z, Chen C. Monoacylglycerol lipase is a therapeutic target for Alzheimer’s disease. Cell Reports. 2012;2:1329–1339. doi: 10.1016/j.celrep.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-Mediated Synaptic Plasticity in the CNS. Ann. Rev. Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Cowley TR, Fahey B, O’Mara SM. COX-2, but not COX-1, activity is necessary for the induction of perforant path long-term potentiation and spatial learning in vivo. Eur. J. Neurosci. 2008;27:2999–3008. doi: 10.1111/j.1460-9568.2008.06251.x. [DOI] [PubMed] [Google Scholar]
- Dave KA, Platel JC, Huang F, Tian D, Stamboulian-Platel S, Bordey A. Prostaglandin E2 induces glutamate release from subventricular zone astrocytes. Neuron Glia Biol. 2010;6:201–207. doi: 10.1017/S1740925X10000244. [DOI] [PubMed] [Google Scholar]
- Delaney AJ, Crane JW, Sah P. Noradrenaling modulates transmission at a central synapse by a presynaptic mechanism. Neuron. 2007;56:880–892. doi: 10.1016/j.neuron.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Du H, Chen X, Zhang J, Chen C. Inhibition of COX-2 expression by endocannabinoid 2-arachidonoylglycerol is mediated by PPAR-γ. Br. J. Pharmacol. 2011;163:1533–1549. doi: 10.1111/j.1476-5381.2011.01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egashira N, Koushi E, Mishima K, Iwasaki K, Oishi R, Fujiwara M. 2,5-Dimethoxy-4-iodoamphetamine (DOI) inhibits Delta9-tetrahydrocannabinol-induced catalepsy-like immobilization in mice. J. Pharmacol. Sci. 2007;105:361–366. doi: 10.1254/jphs.fp0071247. [DOI] [PubMed] [Google Scholar]
- Fairbairn JW, Pickens JT. The oral activity of Δ’-tetrahydrocannabinol and its dependence on prostaglandin E2. Br. J. Pharmacol. 1979;67:379–385. doi: 10.1111/j.1476-5381.1979.tb08691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn JW, Pickens JT. The effect of conditions influencing endogenous prostaglandins on the activity of delta’-tetrahydrocannabinol in mice. Br. J. Pharmacol. 1980;69:491–493. doi: 10.1111/j.1476-5381.1980.tb07039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan N, Yang H, Zhang J, Chen C. Reduced expression of glutamate receptors and phosphorylation of CREB are responsible for in vivo Δ9-THC exposure-impaired hippocampal synaptic plasticity. J. Neurochem. 2010;112:691–702. doi: 10.1111/j.1471-4159.2009.06489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L, Tomasini MC, Gessa GL, Bebe BW, Tanganelli S, Antonelli T. The cannabinoid receptor agonist WIN 55,212-2 regulates glutamate transmission in rat cerebral cortex: an in vivo and in vitro study. Cereb. Cortex. 2001;11:728–733. doi: 10.1093/cercor/11.8.728. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964;86:1646–1647. [Google Scholar]
- Goubaeva F, Ghosh M, Malik S, Yang J, Hinkle PM, Griendling KK, Neubig RR, Smrcka AV. Stimulation of cellular signaling and G protein subunit dissociation by G protein betagamma subunit-binding peptides. J. Biol. Chem. 2003;278:19634–19641. doi: 10.1074/jbc.M300052200. [DOI] [PubMed] [Google Scholar]
- Gowran A, Noonan J, Campbell VA. The multiplicity of action of cannabinoids: implications for treating neurodegeneration. CNS Neurosci. Ther. 2011;17:637–644. doi: 10.1111/j.1755-5949.2010.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Ikeda SR. Endocannabinoids modulate N-type calcium channels and G-protein-coupled inwardly rectifying potassium channels via CB1 cannabinoid receptors heterologously expressed in mammalian neurons. Mol Pharmacol. 2004;65:665–674. doi: 10.1124/mol.65.3.665. [DOI] [PubMed] [Google Scholar]
- Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, Koehl M, Abrous DN, Mendizabal-Zubiaga J, Grandes P, et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell. 2012;148:1039–1050. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Yang R, Lichtman AH, Lupica CR. Opposing actions of chronic Delta9-tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation. Learn. Mem. 2007;14:63–74. doi: 10.1101/lm.439007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC. The CB1 cannabinoid receptor in the brain. Neurobiol. Dis. 1998;5:405–416. doi: 10.1006/nbdi.1998.0215. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J. Neurosci. 2011;31:6627–6638. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Delta 9-tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharm. (Berl) 1996;126:125–131. doi: 10.1007/BF02246347. [DOI] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, et al. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc. Natl. Acad. Sci. USA. 2009;106:20270–20275. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. Presynaptic modulation by endocannabinoids. Handb. Exp. Pharmacol. 2008;184:435–477. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- Marchalanta Y, Brothersa HM, Wenka GL. Inflammation and aging: can endocannabinoids help? Biomed. Pharmacother. 2008;62:212–217. doi: 10.1016/j.biopha.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutiérrez SO, van der Stelt M, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Messinis L, Kyprianidou A, Malefaki S, Papathanasopoulos P. Neuropsychological deficits in long-term frequent cannabis users. Neurol. 2006;66:737–739. doi: 10.1212/01.wnl.0000201279.83203.c6. [DOI] [PubMed] [Google Scholar]
- Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schütz G, Wotjak CT, Lutz B, Marsicano G. Genetic dissection of behavioural and autonomic effects of Delta (9)-tetrahydrocannabinol in mice. PLoS Biol. 2007;5:e269. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008;57:883–893. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Burstein SH, White WR, McDonald SA, Hicks RE. Antagonism of marijuana effects by indomethacin in human. Life Sci. 1991;48:507–515. doi: 10.1016/0024-3205(91)90465-n. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, et al. International Union of Basic and Clinical Pharmacology LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol. Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch. Gen. Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat. Neurosci. 2009;12:1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- Robson P. Therapeutic aspects of cannabis and cannabinoids. Br. J. Psychiatry. 2001;178:107–115. doi: 10.1192/bjp.178.2.107. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Viganò D, Guidali C, Pinter M, Sala M, Bartesaghi R, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- Russo EB. History of Cannabis and Its Preparations in Saga, Science, and Sobriquet. Chem. Biodiv. 2007;4:1614–1648. doi: 10.1002/cbdv.200790144. [DOI] [PubMed] [Google Scholar]
- Sang N, Zhang J, Marcheselli V, Bazan NG, Chen C. Postsynaptically synthesized prostaglandin E2 modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J. Neurosci. 2005;25:9858–9870. doi: 10.1523/JNEUROSCI.2392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanzgiri RP, Araque A, Haydon PG. Prostaglandin E(2) stimulates glutamate receptor-dependent astrocyte neuromodulation in cultured hippocampal cells. J. Neurobiol. 1999;41:221–229. [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J, Marijuana Treatment Project Research Group Cognitive functioning of long-term heavy cannabis users seeking treatment. J. Am. Med. Assoc. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Suárez I, Bodega G, Fernández-Ruiz J, Ramos JA, Rubio M, Fernández B. Down-regulation of the AMPA glutamate receptor subunits GluR1 and GluR2/3 in the rat cerebellum following pre- and perinatal Δ9-tetrahydrocannabinol exposure. Cerebellum. 2003;2:66–74. doi: 10.1080/14734220310017230. [DOI] [PubMed] [Google Scholar]
- Suárez I, Bodega G, Rubio M, Fernández-Ruiz JJ, Ramos JA, Fernández B. Prenatal cannabinoid exposure down- regulates glutamate transporter expressions (GLAST and EAAC1) in the rat cerebellum. Dev. Neurosci. 2004;26:45–53. doi: 10.1159/000080711. [DOI] [PubMed] [Google Scholar]
- Teather LA, Packard MG, Bazan NG. Post-training cyclooxygenase-2 (COX-2) inhibition impairs memory consolidation. Learn Mem. 2002;9:41–47. doi: 10.1101/lm.43602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasini MC, Ferraro L, Bebe BW, Tanganelli S, Cassano T, Cuomo V, Antonelli T. Δ9-Tetrahydrocannabinol increases endogenous extracellular glutamate levels in primary cultures of rat cerebral cortex neurons: involvement of CB1 receptor. J. Neurosci. Res. 2002;68:449–453. doi: 10.1002/jnr.10242. [DOI] [PubMed] [Google Scholar]
- Tonini R, Ciardo S, Cerovic M, Rubino T, Parolaro D, Mazzanti M, Zippel R. ERK-dependent modulation of cerebellar synaptic plasticity after chronic Δ9-tetrahydrocannabinol exposure. J Neurosci. 2006;26:5810–5818. doi: 10.1523/JNEUROSCI.5469-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Yao L, Fan P, Jian Z, Mailliard WS, Gordon AS, Diamond I. Addicitng drugs untilize a synergistic molecular mechanism in common requiring adenosine and Gi-βγ dimmers. Proc. Natl. Acad. Sci. USA. 2003;100:14379–14384. doi: 10.1073/pnas.2336093100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen C. Endocannabinoid 2-arachidonoylglycerol protects neurons by limiting COX-2 elevation. J. Biol. Chem. 2008;283:22601–22611. doi: 10.1074/jbc.M800524200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.