Abstract
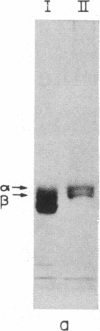
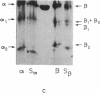
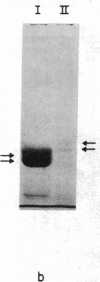
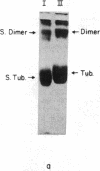
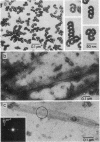
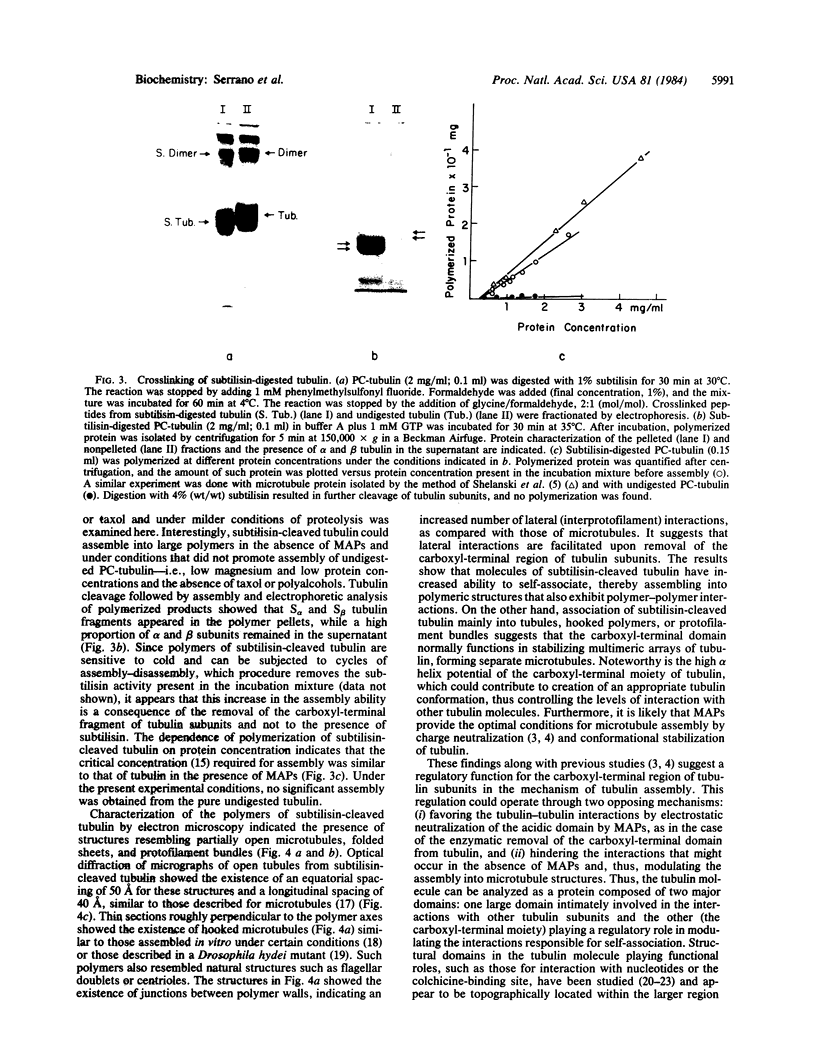
Limited proteolysis of phosphocellulose-purified tubulin with subtilisin resulted in cleavage of both alpha and beta tubulin subunits, with the formation of two major fragments (S alpha, and S beta, 48 kDa) and a small peptide (4 kDa) containing the carboxyl-terminal region of tubulin. Interestingly, tubulin cleaved under the present conditions showed an increased ability to assemble into large polymers in the absence of MAPs and under conditions that do not promote assembly of undigested tubulin--i.e., low magnesium concentrations and the absence of taxol and polyalcohols. The critical concentrations for the subtilisin-cleaved tubulin assembly was similar to that of MAPs-promoted tubulin assembly. Assembly product from subtilisin-cleaved tubulin consisted mainly of protofilament bundles, hooked polymer, and open tubules, structures showing equatorial and longitudinal spacings of 50 and 40 A, respectively. The existence of junctions between polymer walls indicates that the carboxyl-terminal removal facilitates polymer-polymer interactions. These results, together with previous studies on the involvement of the carboxyl-terminal domain of tubulin in its interaction with MAP-2, suggest a regulatory role for this domain in tubulin assembly. Thus, in general terms the tubulin molecule can be analyzed as a protein containing two essential domains with functional significance, one domain playing a major role in self-association and the other (the carboxyl-terminal moiety) playing a regulatory role in modulating the interactions responsible for self-association.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gaskin F., Cantor C. R., Shelanski M. L. Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol. 1974 Nov 15;89(4):737–755. doi: 10.1016/0022-2836(74)90048-5. [DOI] [PubMed] [Google Scholar]
- Heidemann S. R., McIntosh J. R. Visualization of the structural polarity of microtubules. Nature. 1980 Jul 31;286(5772):517–519. doi: 10.1038/286517a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maccioni R. B., Seeds N. W. Affinity labeling of tubulin's exchangeable guanosine 5'-triphosphate binding site. Biochemistry. 1983 Mar 29;22(7):1572–1579. doi: 10.1021/bi00276a008. [DOI] [PubMed] [Google Scholar]
- Maccioni R. B., Seeds N. W. Limited proteolysis of tubulin: nucleotide stabilizes an active conformation. Biochemistry. 1983 Mar 29;22(7):1567–1572. doi: 10.1021/bi00276a007. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. M., Mandelkow E. Junctions between microtubule walls. J Mol Biol. 1979 Mar 25;129(1):135–148. doi: 10.1016/0022-2836(79)90064-0. [DOI] [PubMed] [Google Scholar]
- McEwen B. F., Ceska T. A., Crepeau R. H., Edelstein S. J. Structural changes in tubulin sheets upon removal of microtubule-associated proteins. J Mol Biol. 1983 May 15;166(2):119–140. doi: 10.1016/s0022-2836(83)80002-3. [DOI] [PubMed] [Google Scholar]
- Ponstingl H., Krauhs E., Little M., Kempf T., Hofer-Warbinek R., Ade W. Amino acid sequence of alpha- and beta-tubulins from pig brain: heterogeneity and regional similarity to muscle proteins. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):191–197. doi: 10.1101/sqb.1982.046.01.022. [DOI] [PubMed] [Google Scholar]
- Ponstingl H., Krauhs E., Little M. Tubulin amino acid sequence and consequences. J Submicrosc Cytol. 1983 Jan;15(1):359–362. [PubMed] [Google Scholar]
- Rao A. G., Hare D. L., Cann J. R. A circular dichroism study of the interaction of chlorpromazine with mouse brain tubulin. Biochemistry. 1978 Oct 31;17(22):4735–4739. doi: 10.1021/bi00615a020. [DOI] [PubMed] [Google Scholar]
- Rungger-Brändle V. E. Abnormal microtubules in testes of the mutant l(3)pl (lethal-polyploid) of Drosophila hydei, cultured in vivo. Exp Cell Res. 1977 Jul;107(2):313–324. doi: 10.1016/0014-4827(77)90354-8. [DOI] [PubMed] [Google Scholar]
- Serrano L., Avila J., Maccioni R. B. Limited proteolysis of tubulin and the localization of the binding site for colchicine. J Biol Chem. 1984 May 25;259(10):6607–6611. [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger P., Jaussi R., Gehring H., Brunschweiler K., Christen P. Peptide mapping of protein bands from polyacrylamide gel electrophoresis by chemical cleavage in gel pieces and re-electrophoresis. Anal Biochem. 1982 May 15;122(2):298–301. doi: 10.1016/0003-2697(82)90285-8. [DOI] [PubMed] [Google Scholar]
- Vallee R. Structure and phosphorylation of microtubule-associated protein 2 (MAP 2). Proc Natl Acad Sci U S A. 1980 Jun;77(6):3206–3210. doi: 10.1073/pnas.77.6.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J., Villasante A., Corral J., Avila J. Factors implicated in determining the structure of zinc tubulin-sheets: lateral tubulin-tubulin interaction is promoted by the presence of zinc. J Supramol Struct Cell Biochem. 1981;17(2):183–196. doi: 10.1002/jsscb.380170208. [DOI] [PubMed] [Google Scholar]