Summary
N1731 is a recombinant activated factor VII (rFVIIa) analogue with increased intrinsic activity. This also applies to its reactivity towards antithrombin (AT), the role of which was investigated in a pharmacokinetic (PK) study. NN1731 or rFVIIa was administered to normal and haemophilia A dogs and elimination was measured by FVIIa clot activity, FVIIa- and FVIIa-AT antigen. In vitro AT complex formation was studied in canine plasma spiked with NN1731 or rFVIIa. Based on FVIIa antigen concentrations, PK profiles in normal and haemophilia A dogs were similar for NN1731 and rFVIIa with antigen half lives, t½ ≈ 1·8 h. In contrast, PK profiles based on activity measurements were distinctly different. NN1731 induced a strong, short lasting (t½ ≈ 0·5 h) pro-coagulant response, whereas rFVIIa induced a lower, longer lasting (t½ ≈ 1·1 h) response. Western Blot and FVIIa-AT antigen analysis demonstrated in vivo AT complex formation that accounted for these divergences. AT complex formation with FVIIa or NN1731 in vitro in canine plasma was considerably slower than the in vivo reaction. The results suggest that in vivo inhibition by AT contributes significantly to define drug duration in haemophilia treatment with rFVIIa and in particular with the NN1731 analogue.
Keywords: factor VIIa, antithrombin
Recombinant activated factor VII (rFVIIa, NovoSeven®; Novo Nordisk A/S, Bagsværd, Denmark) has a proven record as a safe and efficient drug in the treatment of bleeding episodes in haemophilia patients with inhibitory antibodies against FVIII or FIX. At pharmacological doses it directly activates FX on the surface of activated platelets in a tissue-factor (TF)-independent manner whereby the normal pathway via FVIII and FIX is by-passed. Clinical practice has revealed that some patients with haemophilia vary widely in their response to rFVIIa with an occasional low and inadequate response (Hayashi et al, 2004; Berntorp, 2009). The reason for this remains unclear, although a substantial individual difference in the capacity of platelets to generate thrombin is likely to contribute to this variability (Hedner & Brun, 2007; Sørensen & Ingerslev, 2004).
By substitution of three amino acids (V158D/E296V/M298Q) it has been possible to design a rFVIIa analogue, NN1731, with an increased intrinsic activity (Persson et al, 2001). The high intrinsic activity provided an analogue with increased pro-coagulant activity on the surface of activated platelets, as indicated by in vitro studies applying a cell-based model of haemophilia (Allen et al, 2007) and by spiking experiments in whole blood from haemophilia patients (Sørensen et al, 2007). Furthermore, in vivo studies in a mouse haemophilia A model showed that NN1731 efficiently shortened the bleeding time and decreased the blood loss (Holmberg et al, 2009; Tranholm et al, 2003). NN1731 is currently in clinical development and the PK profile was recently studied in male human volunteers (Moss et al, 2009). The data confirmed that the pro-coagulant activity of NN1731 compared to rFVIIa was markedly enhanced. However, the results also indicated that the NN1731 activity was eliminated relatively fast, and that NN1731 compared to rFVIIa was cleared with a shorter functional half-life. This contrasts PK studies in mice based on antigen measurements, which suggested that the clearance half-life was similar for rFVIIa and NN1731 (Tranholm et al, 2003). These apparently conflicting data might be resolved by assuming that inactivation of NN1731 by AT runs in parallel with the clearance of these proteases and their AT complexes from the circulation. Data showing that FVIIa is remarkably inert to inhibition when added to plasma in vitro (Kondo & Kisiel, 1987) opposes this interpretation and indicate that AT in the circulation is unlikely to appreciably inhibit rFVIIa within the first few hours following its administration. It should be noted, though, that this applies to the un-stimulated reaction between FVIIa and AT, and that it is unknown to what extent this reaction is stimulated in vivo by known stimulators, such as glucoseaminoglycans and TF. Of further note is the fact that the increased activity of NN1731 is paralleled by an enhanced reactivity towards AT (Persson et al, 2001) indicating that the functional half-life of NN1731, more than that of rFVIIa, is likely to be affected by AT inhibition. We have recently shown that FVIIa-AT complexes accumulated in the circulation upon i.v. administration of human rFVIIa to mice (Petersen et al, 2009). Complex formation was extensive and suggested that AT inhibition might play a more important role than hereto anticipated. The aim of the present work was to study the PK profiles of NN1731 and rFVIIa and to analyse the data with special emphasis on the role of AT. Haemophilia A and normal dogs were used for this purpose because canine models, particularly haemophilia A and B dogs, are considered excellent PK models for human coagulation factors including FVIIa (Brinkhous et al, 1989).
Material and methods
Reagents
Human rFVIIa (Thim et al, 1988), NN1731 (FVIIa(V158D,E296V,M298Q)) (Persson et al, 2001), active site-inactivated rFVIIa (FFR-rFVIIa) (Sorensen et al, 1997), soluble TF (TF1–209), monoclonal mouse anti-FVII antibodies, αF1A2, αFVII-4F7 and αFVII-4F9 were from Novo Nordisk A/S. Unfractionated heparin (UFH) was from Leo Pharma (Ballerup, Denmark). Protamine-free base (P4005) was from Sigma Aldrich (St. Louis, MO, USA).
Pharmacokinetics of rFVIIa and NN1731 FVIIa in dogs
Two haemophilia A dogs (J69, E06) from the Francis Owen Blood Research Laboratory (University of North Carolina at Chapel Hill, NC, USA) were included in the PK study with NN1731 and rFVIIa. The experiments were approved by the Institutional Animal Care and use Committee at the University of North Carolina at Chapel Hill. Each dog was dosed with both compounds at a dose of 0·28 mg/kg, at an interval of 2 d (day 1: rFVIIa and day 3: NN1731). Blood was sampled at various time points after dosing of test compounds. Stabilyte® tubes (Stabilyte Biopool Trinity Biotech Plc. Bray, Co. Wicklow, Ireland) were used for blood collection after NN1731 dosing while 0·12 mol/l sodium citrate (10:1) was used after dosing with rFVIIa.
Studies in normal dogs were performed in accordance with the guidelines from the Danish Animal Experiments Council, The Danish Ministry of Justice. Five male beagle dogs, 5–6 months of age weighing 10·6–11·5 kg from Harlan, Germany, were included. The dogs were dosed 0·27 mg/kg rFVIIa via a Venflon in a cephalic or saphenous vein. The dose was given over a maximum of 5 s followed by 2 ml 0·9% NaCl. Dogs assigned for pre-treatment with heparin were dosed i.v. with 200 iu/kg heparin 10 min prior to treatment with rFVIIa or NN1731. Blood samples (3·0 ml) were taken by puncture of a jugular vein by use of a 3·0 ml Vacutainer containing 0·3 ml 0·13 mol/l sodium citrate obtained from Biopool® Stabylite™ tubes and 0·01 ml protamine solution (15 mg/ml). Protamine was included to avoid in vitro AT complex formation in samples from dogs pre-treated with heparin. Samples were taken at various time points and inverted gently 10 times and stored on ice for a maximum of 10 min before centrifugation at 4000 g for 5 min at 5°C. Within 5 min after centrifugation, plasma was divided in aliquots and kept frozen until analysis.
In vitro assays of plasma samples
FVII antigen concentration was measured by FVII- enzyme immune assay (EIA) (DakoCytomatic, Dako, Ejby, Denmark) as described previously (Petersen et al, 2009). Measurement FVIIa-AT antigen concentration was by FVIIa/AT-EIA based on a combination of the monoclonal αFVIIa antibody F9A4 from the FVII-EIA for capture and a polyclonal horseradish peroxidase-labelled αAT antibody from the AT-EIA kit (Fitzgerald, RDI, Concord, MA, USA) for detection. A pre-formed complex between human rFVIIa and human AT was used as a standard for calibration. FVIIa and NN1731 clotting activity in plasma samples was measured using a modified version of the soluble TF-based FVIIa-specific coagulation assay (Morrissey et al, 1993; Wildgoose et al, 1992) as described (Johansen et al, 2010). The assay was performed in FVII-deficient human plasma (Helena Biosciences, Sunderland, UK) using rabbit brain cephalin (Heptest Laboratories Inc, St. Louis, MO, USA; St. Louis, MO, USA) as lipid source. A FVIIa substandard calibrated against the international FVIIa reference (National Institute for Biological Standards and Control, World Health Organization) was used for assaying both rFVIIa and NN1731.
For thrombelastography analysis blood was drawn at selected time points and analysis was initiated within 2 min after collection using the Haemoscope TEG 5000 Model 5000 Thrombelastograph Haemostasis Analyser (TEG) according to the manufacturer’s instruction. The first 3 ml blood were discarded, and 1 ml blood was premixed with TF solution (Innovin®; Dade-Behring, Marburg, Germany) to a final dilution of 1:200 000, and 360 µl of the premix was transferred to a cup for testing. TEG recordings were allowed to proceed for 90 min. The following variables were recorded: R time (reaction clotting time, i.e. the time from initiation of coagulation until an amplitude of 2 mm was obtained), K time (the time from the end of R time until the clot reaches 20 mm and this represents the speed of clot formation), α-angle (clot development measured as the angle between the R value and the inflection point of the TEG trace), MA (maximal amplitude of the TEG trace reflecting the maximal mechanical strength of the clot).
Spiking of canine plasma with human rFVIIa
Canine plasma (Novo Nordisk A/S) stabilized with 200 nmol/l tick anticoagulant protein (TAP) (Novo Nordisk A/S) and 1·0 µmol/l hirudin (Enzyme Research Laboratories, Swansea, UK) was spiked with 200 nmol/l human rFVIIa and kept at room temperature for various time intervals in the absence and presence of 2 u/ml UFH and subjected to non-reduced sodium dodecyl sulphate polyacrylamide gel electrophoresis and FVII Western blot (WB) analysis. Alternatively, samples were analysed for FVIIa clot activity, FVIIa antigen concentration and FVIIa-AT antigen concentration as described (Petersen et al, 2009).
Pharmacokinetic analysis
The pharmacokinetic parameters for the individual dogs were accessed by use of non-compartmental methods (NCA) using the PC-based software WinNonlin (Version 5.2; Pharsight, St. Louis, MO, USA). Briefly, the areas under the plasma FVIIa concentration versus time curves were calculated according to the trapezoidal rule. The infinite part of the curve was determined as Clast/λ with Clast the last concentration and λ the slope for the last phase. The peak concentration (Cmax) and time to reach the peak concentration (Tmax) were read from the individual plasma concentrations versus time curves. Half-lives were estimated for both the initial/distribution phase (t½α) and for the terminal part of the curve (t½β). Half-lives described in the text exclusively refer to estimates of t½α values for the initial 0–4 h post administration phase of the curve.
Results
PK profiles of NN1731 in haemophilia A dogs
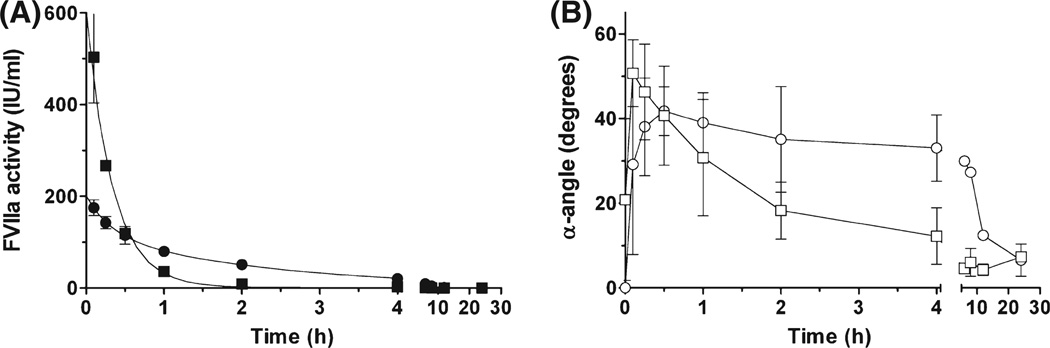
The pharmacokinetic properties of NN1731 was analysed in two haemophilia A dogs and compared to rFVIIa in a crossover experiment in the same dogs. FVIIa clot activity in plasma samples was measured relative to a rFVIIa standard, and TEG analysis was performed in whole blood triggered with Innovin® (1/200 000). The FVIIa plasma concentration profile (FVIIa activity) and the TEG parameter (α angle) over time are shown in Fig 1. The PK profile obtained after i.v. administration of 0·28 mg/kg NN1731 was distinctly different from that obtained after the same dose of rFVIIa. As expected, the Cmax and the corresponding TEG value (α angle) were significantly increased after administration of NN1731. However, as shown in Fig 1 and also illustrated by the functional half-lives, t½ = 0·2 h for NN1731 and t½ = 0·7 h for rFVIIa, the activity of NN1731 decreased more rapidly than the activity of rFVIIa. In essence, NN1731 worked as a potent and short lasting pro-coagulant agent compared to rFVIIa, which was more moderate and longer lasting. This profile was substantiated by TEG analysis (α-angle measurements) shown in Fig 1. The α-angle reflects progression in fibrin clot formation, and the data in Fig 1 suggested that administration of NN1731 initially increased clot propagation markedly, more so than did rFVIIa. In agreement with measurements of clot activity, the effect of rFVIIa on α-angle was, however, more long lived and surpassed the effect of NN1731 at later time points. Similar patterns were evident from measurements of other TEG parameters, such as R-time and MA, and by activated partial thromboplastin time measurements (data not shown).
Fig 1.
FVIIa clot activity and clot formation during in vivo elimination of NN1731 or rFVIIa in haemophilia A dogs. A bolus of 0·28 mg/kg NN1731 (squares) or rFVIIa (circles) was administered i.v. to haemophilia A dogs. (A) FVIIa clotting activities (closed symbols) measured according to standard procedures using a rFVIIa calibrator for analysis of both NN1731 and rFVIIa samples. (B) Clot propagation (α-angle) (open symbols) measured by thrombelastographic profiling. Data are means ± SD (n = 2).
PK profiles and formation of AT complexes in vivo during clearance of NN1731 and rFVIIa in normal dogs
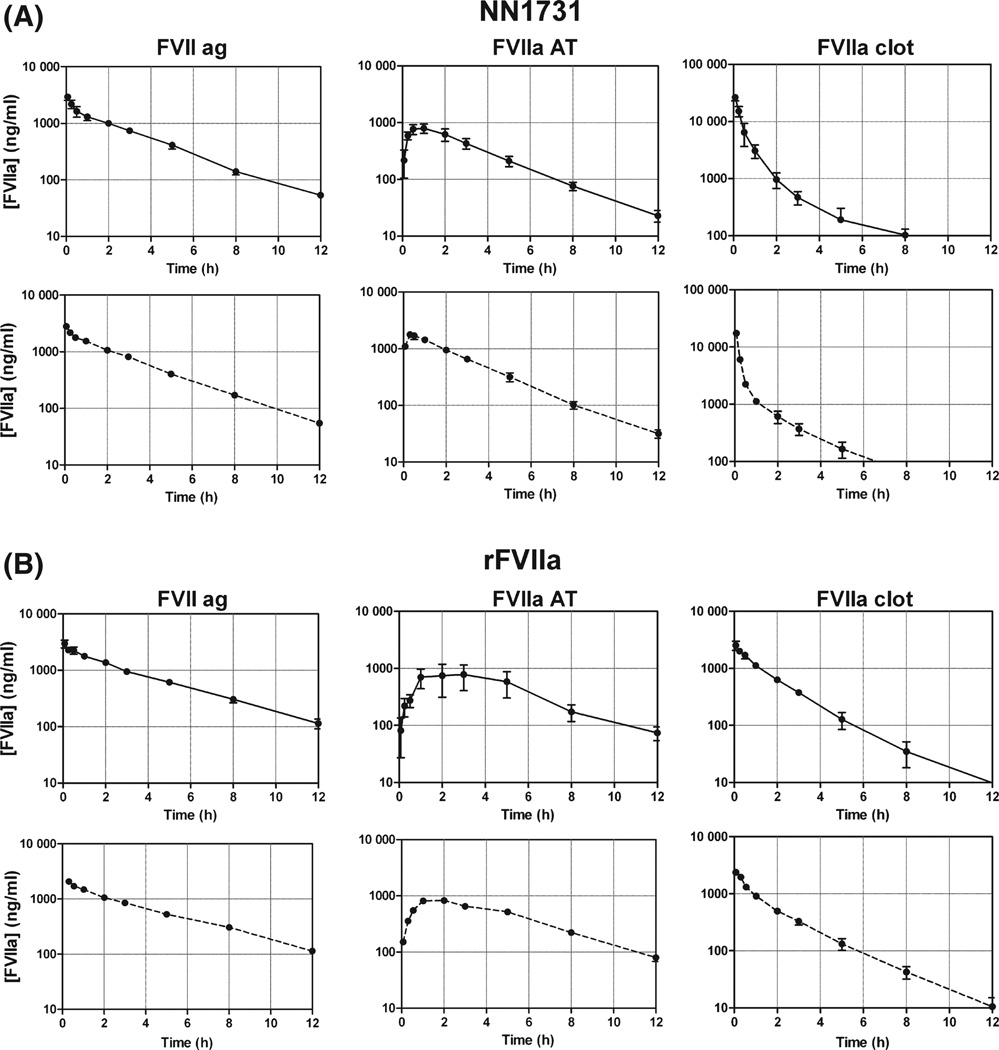
We wanted to explore whether inhibition by AT could explain the difference in functional half lives between rFVIIa and NN1731. To examine this we designed a PK study with NN1731 and rFVIIa in normal dogs where, in addition to FVIIa activity and FVII antigen, we measured AT complex formation by EIA and subjected selected samples to WB analysis. The experiments were performed in the absence and presence of a pre-dose of heparin. The PK profiles are shown in Fig 2 and estimates of PK data for FVII antigen, FVIIa activity and FVIIa-AT antigen are listed in Table I. WB analysis is shown in Fig 3. Consistent with our observations in haemophilia A dogs (Fig 1) the PK profiles in normal dogs based on activity (Fig 2, Table I) resulted in a functional half-life determined within the first 4 h for NN1731 (t½ = 0·5 ± 0·1 h) that was significant shorter than that of rFVIIa (t½ = 1·1 ± 0·1 h). In contrast FVII antigen measurements resulted in similar PK profiles for NN1731 and rFVIIa with considerably longer half-lives of about 1·8 h, just as other PK parameters for rFVIIa and NN1731 were comparable and individually not significantly different. Figure 2 shows that considerable amounts of the respective AT complexes with NN1731 and rFVIIa were formed. The FVIIa antigen and FVIIa-AT antigen curves in the logarithmic presentation enter into terminal linear declines of similar slopes. This applied to both rFVIIa and NN1731 profiles and indicates that NN1731-and rFVIIa proteins, and also the respective AT complexes, are all cleared with similar rates. This result agrees with our previous findings in mice (Petersen et al, 2009) showing that the AT complex is cleared at the same rate as free FVIIa. Pre-treatment with 200 iu/kg heparin initially accelerated complex formation with AT as seen in Fig 2 and illustrated by the Tmax parameter for NN1731-AT and rFVIIa-AT in Table I, and also by the initial decline in FVIIa clot activity of both NN1731 and rFVIIa (Fig 2). The effect of heparin on AT complex formation was also apparent from the area under the curve (AUC) values for NN1731-AT, which doubled in the presence of heparin (Table I). In contrast, the heparin dose was presumably too low to significantly affect the AUC values for the rFVIIa-AT complex.
Fig 2.
Pharmacokinetics of in vivo elimination of NN1731 and rFVIIa. Dogs received 0·27 mg/kg bw NN1731 (A) or rFVIIa (B) in absence (full lines) and presence of 200 iu/kg of un-fractionated heparin pre-dosed i.v. 10 min before treatment with NN1731 or rFVIIa (dotted lines). Plasma was sampled at various time points and FVIIa antigen (ag) concentration, FVIIaAT antigen concentration and FVIIa clot activity were measured as indicated. Note that the activity of NN1731 was calibrated against a rFVIIa standard and given as rFVIIa equivalents in ng/ml. Data are means ± SD (n = 2–4).
Table I.
Pharmacokinetic parameters after i.v. administration of NN1731 or rFVIIa to normal dogs.
| Treatment | Assay | Tmax (h) | AUC (h µg/ml) | Cmax (µg/ml) | t½* (h) | Cl (ml/h per kg) | Vss (ml/kg) |
|---|---|---|---|---|---|---|---|
| rFVIIa | FVII EIA | 9·4 ± 0·9 | 3·0 ± 0·5 | 1·9 ± 0·2 | 29 ± 3 | 110 ± 12 | |
| rFVIIa + heparin | FVII EIA | 8·1 ± 0· 5 | 2·3 ± 0·1 | 1·7 ± 0·7 | 34 ± 2 | 140 ± 2 | |
| NN1731 | FVII EIA | 6·5 ± 0· 7 | 2·9 ± 0·4 | 1·6 ± 0·2 | 42 ± 5 | 127 ± 17 | |
| NN1731 + heparin | FVII EIA | 6·5 ± 0· 7 | 2·7 ± 0·2 | 1·8 ± 0·1 | 42 ± 5 | 131 ± 20 | |
| rFVIIa | FVIIa-AT EIA | 2·3 ± 1·0 | 5·1 ± 2· 0 | 0·8 ± 0·4 | 2·1 ± 0·04 | – | – |
| rFVIIa + heparin | FVIIa-AT EIA | 1·5 ± 0·7 | 5·3 ± 0· 7 | 0·8 ± 0·1 | 2·0 ± 0·1 | – | – |
| NN1731 | FVIIa-AT EIA | 0·8 ± 0·2 | 3·2 ± 0· 6 | 0·8 ± 0·1 | 2·7 ± 0·6 | – | – |
| NN1731 + heparin | FVIIa-AT EIA | 0·4 ± 0·2 | 6·3 ± 0·3 | 2·0 ± 0·1 | 2·9 ± 0·1 | – | – |
| rFVIIa | FVIIa clot | 4·1 ± 0· 5 | 2·5 ± 0·5 | 1·1 ± 0·1 | 67 ± 8 | 120 ± 14 | |
| rFVIIa + heparin | FVIIa clot | 3·5 ± 0· 2 | 2·4 ± 0·3 | 0·8 ± 0·3 | 76 ± 4 | 150 ± 20 | |
| NN1731 | FVIIa clot | 16 ± 2·7 | 26 ± 3·6 | 0·5 ± 0·1 | 201 ± 37 | 205 ± 27 | |
| NN1731 + heparin | FVIIa clot | 9·2 ± 0· 1 | 17 ± 0·2 | 0·6 ± 0·1 | 345 ± 3 | 540 ± 64 |
Tmax, time to reach peak concentration; AUC, area under the curve; Cmax, peak concentration; t½, half-life; Cl, clearance; Vss, steady-state volume of distribution.
Determined from data in the interval 0–4 h.
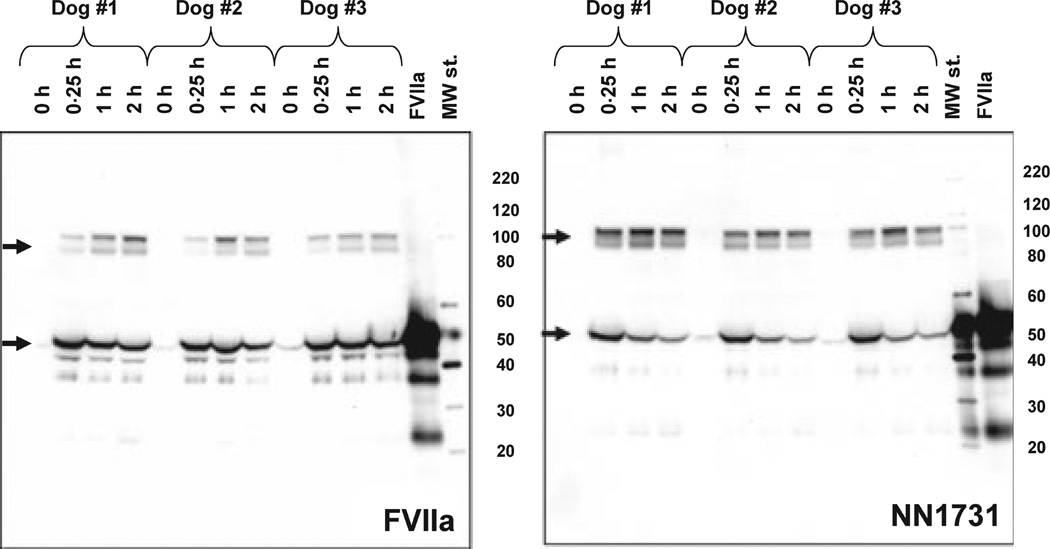
Fig 3.
NN1731/rFVIIa-AT complex formation during in vivo elimination of NN1731 and rFVIIa. NN1731 or rFVIIa (0·27 mg/kg) was administered i.v. to dogs, and plasma was sampled at various time points as indicated. Plasma was subjected to non-reduced sodium dodecyl sulphate polyacrylamide gel electrophoresis and FVIIa Western Blot analysis. Arrows indicate free NN1731/rFVIIa and complexes with AT with molecular masses of c. 50 and 100 kDa, respectively.
WB analysis of selected plasma samples with an antibody against FVII (Fig 3) further documented that clearance of rFVIIa, particularly NN1731, was indeed accompanied by a marked accumulation of AT complexes during the first few hours following i.v. administration of rFVIIa or NN1731. WB of plasma samples showed two FVII-containing bands as expected for complex formation with AT; one band with an apparent mass of c. 100 kDa, representing AT complex with rFVIIa or NN1731, and another band with a mass of c. 50 kDa, representing free enzymes. The complex formation with AT was further advanced with heparin pre-treatment for both FVIIa variants (data not shown).
Active site blockage prevents AT complex formation, and to further evaluate AT complex formation as a crucial step in the elimination of FVIIa and NN1731 protein from the circulation we performed a cross-over PK study based on FVII antigen measurements where the free enzyme (NN1731 or rFVIIa) and the active site blocked form (FFR-NN1731 or FFR-rFVIIa) were administered in sequential order to normal dogs. The PK data obtained (results not shown) suggested that primarily the functional half lives were affected by AT inhibition of rFVIIa or NN1731, whereas the FVII antigens of the active proteases and active site-blocked derivatives were cleared at approximately the same rate, indicating that complex formation with AT was without an appreciable affect on the clearance of FVII protein from the circulation or its detection by the EIA.
In vitro reaction of rFVIIa and NN1731 with inhibitors in canine plasma
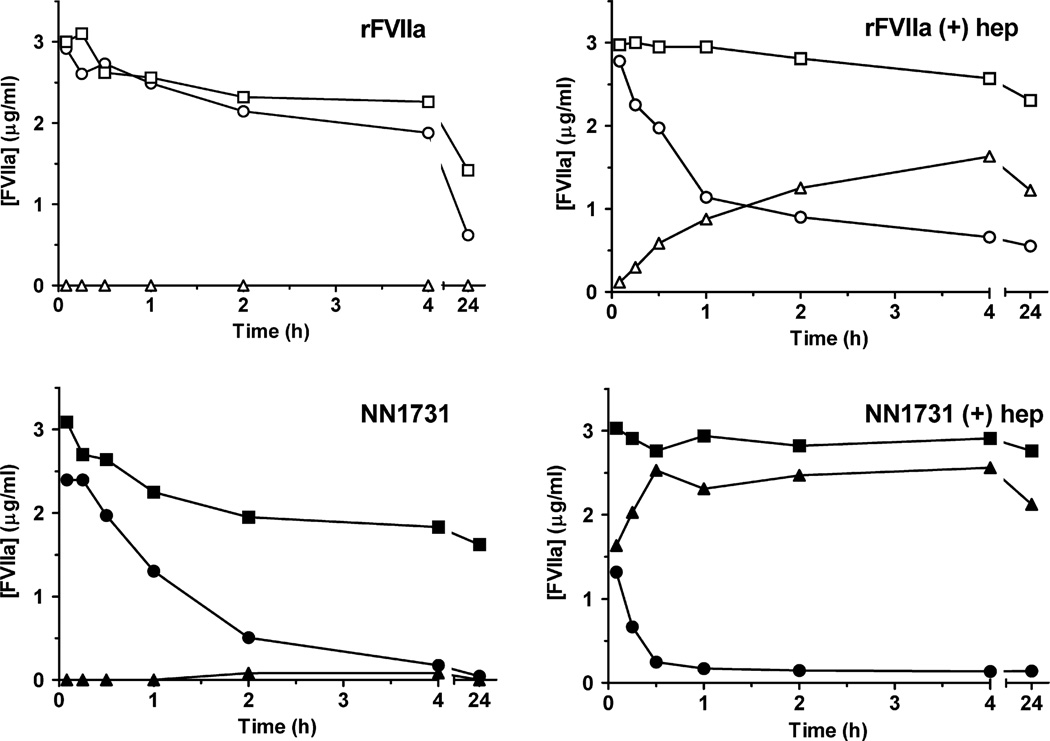
In vitro spiking experiments with rFVIIa and NN1731 in canine plasma confirmed previous studies in human plasma showing that rFVIIa did not react with plasma inhibitors at an appreciable rate under in vitro conditions. This was evident when rFVIIa inhibitor complex formation was followed over time by WB analysis of FVIIa antigen (results not shown) and by measurements of FVIIa activity, FVIIa antigen and FVIIa-AT antigen (Fig 4). This holds true also when the Ca2+ concentration and thereby the Ca2+-dependent rFVIIa activity was retained as it is in TAP/hirudin-stabilized plasma. WB data showed that spiking with rFVIIa did not result in significant complex formation with plasma inhibitors within 4 h. High molecular mass (>200 kDa and c. 100 kDa) bands containing FVIIa, suggesting complex formation with α2 macroglobulin (α2M) and AT respectively, were however observed when the reaction was allowed to proceed over night. Spiking of TAP/hirudin-stabilized canine plasma with NN1731 in the absence of heparin did, in contrast to rFVIIa, result in detectable AT complexes at c. 100 kDa on WB (not shown). Spiking experiments measuring the time courses of FVIIa activity, FVIIa antigen and AT complexes in the absence and presence of heparin are shown in Fig 4. Undetectable amounts of rFVIIa-AT complexes were formed in the absence of heparin within a 4-h observation period, whereas spiking with NN1731 produced measurable amounts of the NN1731-AT complex. As expected, the presence of 2 iu/ml heparin markedly enhanced complex formation with both rFVIIa and NN1731 (Fig 4).
Fig 4.
Time courses of FVIIa clot activity, FVIIa antigen and FVIIa-AT antigen concentrations in canine plasma spiked with NN1731 or rFVIIa in the absence and presence of heparin. Canine plasma stabilized with TAP and hirudin was spiked with 3 µg/ml NN1731 (lower panels) or 3 µg/ml rFVIIa (upper panels) at room temperature and incubated for various times in the absence (left panel) and presence of 2 u/ml heparin (right panels). FVIIa antigen concentration (closed circles), FVIIa clot activity (open circles) and FVIIa-AT antigen concentration (open squares) were measured.
Discussion
Using a canine haemophilia A model the distinct PK profiles of NN1731 and rFVIIa were illustrated by the present study (Fig 1). Following an i.v. bolus administration, the enhanced intrinsic activity of NN1731 resulted in an increased pro-coagulant activity which initially was markedly higher than that produced by an equivalent bolus of rFVIIa. In addition, it was shown in Fig 1 that the increased reactivity of NN1731 towards AT affected the subsequent clearance such that the activity of NN1731 declined with a shorter half-life than the activity of rFVIIa. This contrasted measurements of protein levels which indicated similar half lives for the clearance of NN1731 and rFVIIa.
PK studies in normal dogs confirmed these patterns of NN1731 and rFVIIa, and additional measurements of AT complex formation provided strong support for a significant effect of AT on the PK profiles of both NN1731 and rFVIIa. In vivo inhibition of rFVIIa and in particular NN1731 by AT was shown to result in a passing accumulation of AT complexes in the circulation. In vitro spiking experiments (Fig 4) confirmed previous reports (Persson et al, 2001) that NN1731 reacted more avidly with AT than rFVIIa, and the increased reactivity of NN1731 appeared therefore to explain its shorter functional half-life and the higher level of accumulated AT complexes that compared with rFVIIa characterized the clearance of NN1731 (Fig 2). Further support for a significant effect of AT on clearance comes from the experiments with pre-treatment with a low dose of heparin showing that this affected the initial decline in activity and AT complex accumulation as is expected from in vitro observations (Kondo & Kisiel, 1987; Lawson et al, 1993; Hamamoto & Kisiel, 1998). Taking together this suggest that inhibition by AT in the circulation plays an important role for the elimination and duration of the pro-coagulant effect of rFVIIa and especially NN1731.
Our spiking experiments with rFVIIa in human, murine and canine plasma (Fig 4, and (Petersen et al, 2009)) confirmed previous observations on the un-stimulated in vitro reaction (Kondo & Kisiel, 1987; Lawson et al, 1993; Hamamoto & Kisiel, 1998) and showed that hardly any detectable AT complex was formed within the first 4 h after the addition of rFVIIa. This slow reaction contrasted our observations of a significant rFVIIa-AT complex formation in vivo, which markedly affected the functional half-life of FVIIa in the circulation. As previously suggested from similar studies in mice (Petersen et al, 2009), this indicated that in vivo complex formation between rFVIIa and AT was somehow influenced by a stimulatory effect when the reaction took place within the circulatory organ. It is interesting in this context that FVIIa-AT complexes were found to be present in normal individuals at a level of 156 ± 63 pmol/l close to the level of free FVIIa as reported by a recent study in which a novel FVIIa-AT assay was applied to plasma from normal individuals and patients with arterial and venous thrombosis (Spiezia et al, 2010). The level of FVIIa-AT complexes was furthermore observed to correlate with the level of free FVIIa suggesting that the reaction with AT also, in general terms, plays a significant role for the regulation of the intrinsic FVIIa activity level.
Another significant conclusion can be drawn from the data in Fig 2 regarding the clearance of rFVIIa and NN1731 based on FVIIa antigen measurements. In apparent contrast to the data on clearance based on FVIIa clot activity, the data suggested that the clearance of rFVIIa and NN1731 antigen was similar, if not identical. This allowed us to assume that clearance of rFVIIa and NN1731 is independent of active site occupancy and complex formation with AT. Further support for this interpretation is provided by experiments showing that PK parameters for FFR-chloromethylketone-blocked rFVIIa and NN1731 were indistinguishable from the PK parameters for the uninhibited forms (data not shown), and also by our previous studies in mice (Petersen et al, 2009), which showed that a pre-formed rFVIIa-AT complex was cleared with the same rate as free rFVIIa. In this context it is also noticeable (Fig 2) that clearance of AT complexes in the late phase appeared to follow the same clearance trait as that followed by FVII antigen measurements of rFVIIa and NN1731. These observations imply that complex formation between FVIIa and AT does not induce neo-epitopes, resulting in recognition by scavenger receptors and rapid clearance as is the case for complex formation between a number of other proteases and serpins (Mast et al, 1991; Wells et al, 1999). Finally, the data indicated that antibody recognition in the assays applied to monitor FVII antigen was essentially unaffected by FVIIa-AT complex formation.
It was of interest, if possible, to obtain an estimate of the rate constant for the in vivo reaction between rFVIIa and AT in order to evaluate the magnitude of the stimulatory effect and to get an impression of a possible effect of AT on the normal endogenous plasma level of FVIIa in the circulation. A rough estimate of the in vivo rate constant, k1, for the reaction between rFVIIa and AT might be obtained by assuming that the steady state accumulation of rFVIIa-AT complex is described by rate Eq. (1):
| (1) |
We further presumed a canine AT concentration of c. 3·5 µmol/l in agreement with a recent study, which estimated that canine plasma contained 135% of the normal AT concentration in human plasma (Tarnow et al, 2007) and with our own estimate based on titration of canine plasma with rFVIIa/TF in the presence of heparin (data not shown). Using this information, we next assumed (i) that the rate of AT complex formation equals the rate of clearance, d[rFVIIa-AT]/dt = 0, at the maximal peak value for [rFVIIa-AT], (ii) that rFVIIa-AT is cleared at a rate identical to the FVIIa antigen clearance (Petersen et al, 2009), and (iii) that [rFVIIa]ss, the concentration of free rFVIIa at d[rFVIIa-AT]/dt = 0, can be determined from the clot activity curve in Fig 2. Comparing this with the clearance rate we derived at a second order constant k1 c. 29/M/s for the in vivo reaction between human rFVIIa and canine AT. The rate constant is approximately an order of magnitude faster than the un-stimulated in vitro reaction between AT and free FVIIa (kinh = 1·2 ± 0·2/M/s) (Olson et al, 2004). What actually causes the stimulation in vivo is unclear. TF and heparin/glucoseaminoglycans are known stimulators of the reaction between FVIIa and AT in vitro. The rate constant determined for the reaction stimulated by TF (kinh = 33 ± 3/M/s) is similar to that obtained for the in vivo reaction, whereas stimulation with un-fractionated heparin reaches a maximal level of kinh = 650 ± 30/M/s (Olson et al, 2004). Rather than assuming extensive binding of FVIIa to TF in the circulation, we find it more obvious to propose that in vivo enhancement of FVIIa inhibition by AT is caused by sub-maximal stimulation of the reaction by glucoseaminoglycans on the vessel walls.
FVIIa, the activated form of the serine protease zymogen FVII, stays in the circulation longer than other activated serine proteases in the coagulation cascade (Erhardtsen, 2000; Lindley et al, 1994), which are cleared within minutes after forming complexes with their respective plasma inhibitors (Wells et al, 1999). A marked resistance of FVIIa to inhibition by plasma inhibitors is assumed to be a major reason for the existence of measurable concentrations of activated FVIIa (c. 0·1 nmol/l) in the circulation in vivo. The steady state level of FVIIa in the circulation appears to be strictly regulated and constitutes about 1% of the FVII zymogen concentration of c. 10 nmol/l (Wildgoose et al, 1992). Regulation of the steady state level of FVIIa activity is of undisputed importance for the haemostatic balance. However, little is known about the regulation of this level in the circulation. It depends on the rate of FVII synthesis and secretion from the liver. It furthermore depends on FVII activation and FVIIa inactivation as well as on the clearance of FVII, FVIIa and FVIIa-AT. The relative rates of in vivo clearance and inactivation determined in the present study may contribute to a better understanding of the regulatory mechanisms involved.
Our data suggest that FVIIa reacts with AT in vivo at a rate that is faster than anticipated from in vitro spiking experiment. Although these observations are based on animal models, there are reasons to believe that similar conditions will exist in humans. The present study suggests that in vivo inhibition by AT contributes significantly to define the functional half-life of rFVIIa and in particular of NN1731. As expected, administration of NN1731 resulted in a functional FVIIa activity, which was initially much higher than that reached by an equivalent amount of wild-type rFVIIa. In accord with its increased reactivity towards AT (Persson et al, 2001) we also found that NN1731 reacted with AT in canine plasma at a significant rate even in the absence of a stimulator (Fig 4). Indeed, the present study suggested that AT inhibition of NN1731 played a predominant role for the fast in vivo elimination of NN1731 activity. Administration of NN1731 is expected to provide a rapid and increased haemostatic effect as compared to rFVIIa with a shorter duration of action.
Acknowledgement
Carsten L. Christoffersen, Lene Kureer, Pia Justesen and Anders A. Larsen are thanked for excellent technical assistance.
TCN acknowledge research support from Novo Nordisk A/S.
Footnotes
Authorship and disclosures
LCP, DMK, HA, MBH, HP, EP, TCN and ME designed and performed experiments and analysed data. LCP and ME wrote the manuscript. LCP, DMK, HA, MBH, HP, EP and ME are employees at Novo Nordisk A/S.
References
- Allen GA, Persson E, Campbell RA, Ezban M, Hedner U, Wolberg AS. A variant of recombinant factor VIIa with enhanced procoagulant and antifibrinolytic activities in an in vitro model of hemophilia. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:683–689. doi: 10.1161/01.ATV.0000257204.82396.2b. [DOI] [PubMed] [Google Scholar]
- Berntorp E. Differential response to bypassing agents complicates treatment in patients with haemophilia and inhibitors. Haemophilia: The Official Journal of the World Federation of Hemophilia. 2009;15:3–10. doi: 10.1111/j.1365-2516.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- Brinkhous KM, Hedner U, Garris JB, Diness V, Read MS. Effect of recombinant factor VIIa on the hemostatic defect in dogs with hemophilia A, hemophilia B, and von Willebrand disease. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:1382–1386. doi: 10.1073/pnas.86.4.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardtsen E. Pharmacokinetics of recombinant activated factor VII (rFVIIa) Seminars in Thrombosis and Hemostasis. 2000;26:385–391. doi: 10.1055/s-2000-8457. [DOI] [PubMed] [Google Scholar]
- Hamamoto T, Kisiel W. The effect of cell surface glycosaminoglycans (GAGs) on the inactivation of factor VIIa tissue factor activity by antithrombin III. International Journal of Hematology. 1998;68:67–78. doi: 10.1016/s0925-5710(98)00034-6. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Tanaka I, Shima M, Yoshida K, Fukuda K, Sakurai Y, Matsumoto T, Giddings JC, Yoshioka A. Unresponsiveness to factor VIII inhibitor bypassing agents during haemostatic treatment for life-threatening massive bleeding in a patient with haemophilia A and a high responding inhibitor. Haemophilia: The Official Journal of the World Federation of Hemophilia. 2004;10:397–400. doi: 10.1111/j.1365-2516.2004.00924.x. [DOI] [PubMed] [Google Scholar]
- Hedner U, Brun NC. Recombinant factor VIIa (rFVIIa): its potential role as a hemostatic agent. Neuroradiology. 2007;49:789–793. doi: 10.1007/s00234-007-0240-2. [DOI] [PubMed] [Google Scholar]
- Holmberg HL, Lauritzen B, Tranholm M, Ezban M. Faster onset of effect and greater efficacy of NN1731 compared with rFVIIa, aPCC and FVIII in tail bleeding in hemophilic mice. Journal of Thrombosis and Haemostasis. 2009;7:1517–1522. doi: 10.1111/j.1538-7836.2009.03532.x. [DOI] [PubMed] [Google Scholar]
- Johansen PB, Bjørn SE, Agersø H, Thorup I, Hermit MB, Sørensen BB, Stennicke HR, Ezban M, Tranholm M. Prolonged effect of GlycoPEGylated rFVIIa (40k-PEG-rFVIIa) in rabbits correlates to activity in plasma. Thrombosis and Haemostasis. 2010;104:157–164. doi: 10.1160/TH09-11-0797. [DOI] [PubMed] [Google Scholar]
- Kondo S, Kisiel W. Regulation of factor VIIa activity in plasma: evidence that antithrombin III is the sole plasma protease inhibitor of human factor VIIa. Thrombosis Research. 1987;46:325–335. doi: 10.1016/0049-3848(87)90294-5. [DOI] [PubMed] [Google Scholar]
- Lawson JH, Butenas S, Ribarik N, Mann KG. Complex-dependent inhibition of factor VIIa by antithrombin III and heparin. Journal of Biological Chemistry. 1993;268:767–770. [PubMed] [Google Scholar]
- Lindley CM, Sawyer WT, Macik BG, Lusher J, Harrison JF, Bairdcox K, Birch K, Glazer S, Roberts HR. Pharmacokinetics and pharmacodynamics of recombinant factor viia. Clinical Pharmacology and Therapeutics. 1994;55:638–648. doi: 10.1038/clpt.1994.80. [DOI] [PubMed] [Google Scholar]
- Mast AE, Enghild JJ, Nagase H, Suzuki K, Pizzo SV, Salvesen G. Kinetics and physiologic relevance of the inactivation of alpha 1-proteinase inhibitor, alpha 1-antichymotrypsin, and antithrombin III by matrix metalloproteinases-1 (tissue collagenase), -2 (72-kDa gelatinase/type IV collagenase), and-3 (stromelysin) Journal of Biological Chemistry. 1991;266:15810–15816. [PubMed] [Google Scholar]
- Morrissey JH, Macik BG, Neuenschwander PF, Comp PC. Quantitation of activated factor VII levels in plasma using a tissue factor mutant selectively deficient in promoting factor VII activation. Blood. 1993;81:734–744. [PubMed] [Google Scholar]
- Moss J, Scharling B, Ezban M, Sorensen TM. Evaluation of the safety and pharmacokinetics of a fast- acting recombinant FVIIa analogue, NN1731, in healthy male subjects. Journal of Thrombosis and Haemostasis. 2009;7:299–305. doi: 10.1111/j.1538-7836.2008.03253.x. [DOI] [PubMed] [Google Scholar]
- Olson ST, Swanson R, Raub-Segall E, Bedsted T, Sadri M, Petitou M, Hérault JP, Herbert JM, Björk I. Accelerating ability of synthetic oligosaccharides on antithrombin inhibition of proteinases of the clotting and fibrinolytic systems. Comparison with heparin and low-molecular-weight heparin. Thrombosis and Haemostasis. 2004;92:929–939. doi: 10.1160/TH04-06-0384. [DOI] [PubMed] [Google Scholar]
- Persson E, Kjalke M, Olsen OH. Rational design of coagulation factor VIIa variants with substantially increased intrinsic activity. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13583–13588. doi: 10.1073/pnas.241339498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen LC, Elm T, Ezban M, Krogh TN, Karpf DM, Steinø A, Olsen EH, Sørensen BB. Plasma elimination kinetics for factor VII are independent of its activation to factor VIIa and complex formation with plasma inhibitors. Thrombosis and Haemostasis. 2009;101:818–826. [PubMed] [Google Scholar]
- Sørensen B, Ingerslev J. Whole blood clot formation phenotypes in hemophilia A and rare coagulation disorders. Patterns of response to recombinant factor VIIa. Journal of Thrombosis and Haemostasis. 2004;2:102–110. doi: 10.1111/j.1538-7836.2004.00528.x. [DOI] [PubMed] [Google Scholar]
- Sorensen BB, Persson E, Freskgard PO, Kjalke M, Ezban M, Williams T, Rao LVM. Incorporation of an active-site inhibitor in factor viia alters the affinity for tissue factor. Journal of Biological Chemistry. 1997;272:11863–11868. doi: 10.1074/jbc.272.18.11863. [DOI] [PubMed] [Google Scholar]
- Sørensen B, Persson E, Ingerslev J. Factor VIIa analogue (V158D/E296V/M298Q-FVIIa) normalises clot formation in whole blood from patients with severe haemophilia A. British Journal of Haematology. 2007;137:158–165. doi: 10.1111/j.1365-2141.2007.06534.x. [DOI] [PubMed] [Google Scholar]
- Spiezia L, Rossetto V, Campello E, Gavasso S, Woodhams B, Tormene D, Simioni P. Factor VIIa-antithrombin complexes in patients with arterial and venous thrombosis. Thrombosis and Haemostasis. 2010;103:1188–1192. doi: 10.1160/TH09-08-0606. [DOI] [PubMed] [Google Scholar]
- Tarnow I, Falk T, Tidholm A, Martinussen T, Jensen AL, Olsen LH, Pedersen HD, Kristensen AT. Hemostatic biomarkers in dogs with chronic congestive heart failure. Journal of Veterinary Internal Medicine. 2007;21:451–457. doi: 10.1892/0891-6640(2007)21[451:hbidwc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Thim L, Bjoern S, Christensen M, Nicolaisen EM, Lund-Hansen T, Pedersen AH, Hedner U. Amino acid sequence and posttranslational modifications of human factor VIIa from plasma and transfected baby hamster kidney cells. Biochemistry. 1988;27:7785–7793. doi: 10.1021/bi00420a030. [DOI] [PubMed] [Google Scholar]
- Tranholm M, Kristensen K, Kristensen AT, Pyke C, Rojkjaer R, Persson E. Improved hemostasis with superactive analogs of factor VIIa in a mouse model of hemophilia A. Blood. 2003;102:3615–3620. doi: 10.1182/blood-2003-05-1369. [DOI] [PubMed] [Google Scholar]
- Wells MJ, Sheffield WP, Blajchman MA. The clearance of thrombin-antithrombin and related serpin- enzyme complexes from the circulation: role of various hepatocyte receptors. Thrombosis and Haemostasis. 1999;81:325–337. [PubMed] [Google Scholar]
- Wildgoose P, Nemerson Y, Hansen LL, Nielsen FE, Glazer S, Hedner U. Measurement of basal levels of factor VIIa in hemophilia A and B patients. Blood. 1992;80:25–28. [PubMed] [Google Scholar]