Abstract
Neonatal ethanol exposure in the rat is known to partially damage the hippocampus, but such exposure causes only modest or inconsistent deficits on hippocampus-dependent behavioral tasks. This may reflect variable sensitivity of these tasks or residual function following partial hippocampal injury. The context preexposure facilitation effect (CPFE) is a variant of context conditioning in which context exposure and immediate shock occur on successive occasions. During testing, preexposed rats freeze more than non-preexposed controls. The CPFE is more sensitive to anterograde hippocampal injury than standard contextual fear conditioning (e.g., Rudy & O’Reilly, 2001, Cogn Affect Behav Neurosci, 1, 66–82). We report that rats exposed to a high binge dose of ethanol (5.25 g/kg/day) over Postnatal Days [PD] 4–9 failed to demonstrate the CPFE when preexposed to the conditioning context on PD31, relative to sham-intubated and undisturbed controls (Exp. 1). Neonatal alcohol disrupted conditioned freezing to a much lesser extent relative to controls when context preexposure was followed by a standard context conditioning trial rather than immediate shock (Exp. 2). Fear conditioning to a discrete auditory cue (tone) was unaffected by neonatal alcohol exposure ruling out possible performance effects (Exp. 3). These findings suggest that the CPFE is an especially sensitive task for detecting hippocampal injury produced by neonatal alcohol. Mixed results with other tasks may reflect residual hippocampal function and/or the use of alternate neurobehavioral systems or “strategies” following alcohol-induced brain damage.
1. Introduction
Fetal Alcohol Spectrum Disorders (FASD) represent a range of adverse neurocognitive, neurobehavioral, and structural effects caused by developmental alcohol exposure [69,41]. Children with FASD demonstrate lower IQs [45], impairments on executive function tasks (e.g., problem solving, abstract thinking, cognitive flexibility), and significant learning deficits [46]. These deficits may reflect the consequences of neuroanatomical abnormalities commonly seen in human cases of FASD, including aberrations of the corpus callosum, the basal ganglia, the cerebellum, and the hippocampus [60,61,70,81], which are largely a product of the timing, pattern, and dosage of ethanol consumption during pregnancy [39,40]. Maternal and genetic factors also contribute to the effects of alcohol on the developing fetus [61]. FASD is considered a major public health problem [61] and constitutes one of the leading preventable causes of mental retardation [1]. Although public awareness programs have attempted to educate the public about the dangers of consuming alcohol during pregnancy, FASD persist [19], with an estimated prevalence of around 10 per 1,000 births in the United States [49].
The use of animal models of FASD has contributed greatly to our understanding of the effects of developmental alcohol exposure. For example, the craniofacial abnormalities seen in Fetal Alcohol Syndrome are the result of alcohol exposure during the 1st trimester of development [61,71,72]. In contrast, the cerebellum, prefrontal cortex, and hippocampus appear to be most sensitive to the damaging effects of alcohol during the brain growth spurt [27,80,38], which occurs during the 3rd trimester in humans, and during the first post-natal week in the rodent [17]. The effect of alcohol on the developing hippocampus has been of particular interest because of the known role of this structure in many forms of learning and memory. Alcohol exposure limited to the 3rd trimester equivalent in the rodent causes reductions in hippocampal pyramidal cell number, specifically within the CA1 subregion [8,9,38,43,77]. Disruptions in hippocampal physiology and adult neurogenesis in the dentate gyrus (DG) are also affected by developmental alcohol exposure [7,34,35,58,73,].
Rats exposed to high binge-like doses of alcohol during the brain growth spurt exhibit deficits on tasks that are impaired by hippocampal damage. For example, neonatal ethanol exposure resulting in a high blood alcohol concentration (BAC; >300 mg/dl) produces deficits during spatial conditional alteration and reversal learning in the T-maze [76,54], as well as spatial navigation impairments in the water maze [24,23,21,31,43]. Generally, the water maze impairments induced by neonatal ethanol affects the rate of acquisition, with some studies showing no differences during “free swim” probe sessions [43,75], the most stringent measure of spatial cognition tested after asymptotic learning. Water maze acquisition is prevented by hippocampal lesions [52], and the milder spatial learning deficits in this task observed in alcohol-exposed rats may or may not indicate hippocampal dysfunction [55,75]. Importantly, spatial learning in the water maze is also impaired by lesions of the cerebral cortex, striatum, basal forebrain, and cerebellum [16], and the effects of alcohol on these structures cannot be completely discounted. For example, rats administered 5.25 g/kg of alcohol over PD7-9 showed CA1 pyramidal cell reductions and water maze impairments [43]. Vitamin E co-administered with alcohol over PD7-9 prevented CA1 pyramidal cell loss, but did not rescue the water maze deficits [43], suggesting that water maze deficits in these rats was caused by damage to other structures or by an action on hippocampus (e.g., plasticity) that is not related substantially to CA1 cell counts.
Characterizing the impact of neonatal alcohol exposure on the hippocampus may benefit from other, more sensitive tests. For example, contextual fear conditioning has been widely used as a test of hippocampal function [56,33,28]. A variant of standard contextual fear conditioning is the context preexposure facilitation effect (CPFE), where a rat preexposed to the testing context 24h prior to an immediate shock shows enhanced contextual conditioning relative to rats not preexposed to the context [18,66,64]. In a series of experiments, Rudy & colleagues have demonstrated the enhanced sensitivity of the CPFE to hippocampal insult [63,4,47,65, see 62,64 for review]. The CPFE as a test of hippocampal dysfunction in ethanol-exposed rats has a number of advantages. First, robust learning can occur with a single context-shock association. Second, learning about the context and associating the context with the shock occur on separate occasions. And finally, learning about the context during preexposure and retrieval of the context memory during training and during testing requires an intact hippocampus (ibid), and therefore slight disruptions of hippocampal function may disrupt the CPFE.
In the current set of experiments we tested rats exposed to a high binge dose of ethanol (5.25 g/kg/day) over PD4-9 on two variants of contextual fear conditioning. In Experiment 1, ethanol-exposed and control rats were trained on the CPFE paradigm starting at PD31, an age where the CPFE is robust in developing rats [68]. We hypothesized that if ethanol-exposure over this window disrupts hippocampal function, then ethanol-exposed rats should fail to show the CPFE. In Experiment 2, the findings of Experiment 1 were replicated and extended with the inclusion of a standard contextual fear conditioning (sCFC) paradigm, where rats received an unsignaled shock 2 minutes after placement in the conditioning chamber. The CPFE and sCFC are differentially affected by pretraining lesions of the hippocampus [64]. If PD4-9 ethanol produces hippocampal dysfunction, we hypothesized that ethanol-exposed rats would show greater deficits in the CPFE than during sCFC. Finally, in Experiment 3 ethanol-exposed rats and controls were trained on PD32 in a tone fear conditioning task that is unaffected by hippocampal lesions [56]. We hypothesized that ethanol-exposed and control rats would not differ in their ability to condition to the tone CS. Portions of this paper have been presented in abstract form [53].
2. EXPERIMENT 1: Neonatal alcohol and the context preexposure facilitation effect
Experiment 1 examined the effects of neonatal binge ethanol exposure on the context preexposure facilitation effect (CPFE). The CPFE is sensitive to hippocampal insult [63,4,47,65]. Similar ethanol dosing methods utilized for this experiment produce reductions in CA1 pyramidal cells [77,43]. We therefore predicted that juvenile rats exposed to a binge dose of ethanol over PD4-9 would show deficits in the CPFE compared to control rats.
2.1. Materials and methods
Subjects
The subjects were 70 Long Evans rats (33 female, 37 male) derived from 14 litters. Time-mated females were housed with breeder males overnight at the animal housing colony of the Office of Laboratory Animal Medicine at the University of Delaware. If an ejaculatory plug was found the following morning, that day was designated Gestational day (GD) 0. Pregnant females were housed in clear polypropylene cages (45 × 24 × 21 cm) with standard bedding and ad lib access to water and rat chow. The animal housing facility was maintained on a 12:12 hour light/dark cycle with lights on at 7:00 am. The date of birth was determined by checking for birth during the light cycle; if litters were found, that day was designated as Postnatal day (PD) 0 (all births occurred on GD22). On PD3, litters were culled to 8 pups (4 males and 4 females whenever possible) and received subcutaneous injections of non-toxic ink into one or more paws for identification purposes. On PD21, pups were weaned from their mother and housed with same-sex litter mates in 45 × 24 × 17 cm cages with ad lib access to water and rat chow. Starting two days prior to behavioral testing (PD29) and throughout the remainder of the study, rats were individually housed in small white polypropylene cages (24 × 18 × 13 cm) with ad lib access to water and rat chow. All subjects were treated in accordance with protocol approved by the Institutional Animal Care and Use Committee at the University of Delaware.
Alcohol Dosing
Neonatal ethanol dosing via intragrastric intubation occurred over PD4 through PD9 following similar procedures reported from this laboratory [10,11,12] and others [23,75]. Pups in 7 litters were randomly assigned to receive either ethanol (Group EtOH) or sham (Group SI) intubations, with an equal number of males and females in each group whenever possible. Pups in 7 other litters remained undisturbed (Group UD) during the dosing period (PD4-9), except that their body weights were taken on PD4 and PD9. Same-sex littermates assigned to the same dosing condition (EtOH, SI, or UD) were assigned to different behavioral groups so that no more than one same-sex littermate was assigned to a particular experimental condition (dosing condition X behavioral group).
On PD4, pups from to-be-dosed litters were separated from their dams and placed as a litter in one of two clear lexan containers set over a heating pad (GD model %E12107) set to the lowest temperature to provide warmth during separation. Each pup was weighed prior to the first intubation (usually around 10:00 am). Intragastric intubations involved passing PE10 tubing lubricated with corn oil down the esophagus and into the stomach of the rat pup. Group EtOH received an infusion of ethanol mixed with a custom milk formula at a v/v of 23.95% in a single binge dose of 5.25 g/kg/day [43]. The formula was delivered in a volume of 0.02778 ml/g body weight. Group SI received an intubation without an infusion of formula. After dosing/intubation was completed (< 20 minutes per litter), pups were returned as a litter to their dams. Approximately two hours (± 5 min) after the ethanol dose, pups were again briefly separated from their dams. Pups from both dosing conditions received a small tail-clip and a 20 μl blood sample was collected using a heparinized capillary tube. Blood samples from Group SI were discarded; those from Group EtOH were saved from further blood alcohol analysis (see below). The second dosing on PD4 involved an infusion of milk formula (without ethanol; Group EtOH) or a sham intubation (Group SI). A third formula-only or sham intubation was administered two hours (± 5 min) after the second dosing. Dosing occurred in a similar fashion on PD5-9, except that no blood samples were taken and pups received only one additional formula/sham intubation after the first intubation instead of two. The formula-only dosing was performed to enhance weight gain in ethanol-exposed pups [43].
Blood Alcohol Concentration Analysis
Blood samples collected from Group EtOH were centrifuged and plasma was collected and stored at −20 °C. Blood alcohol concentrations (BACs) were determined using an Analox GL5 Analyzer (Analox Instruments, Luneburg, MA) as previously described [12]. Briefly, the rate of oxidation of alcohol in each plasma sample was measured. BACs (expressed in mg/dl) were calculated based on comparisons to known values of an alcohol standard solution.
Apparatus and Stimuli
Contextual fear conditioning was based on our previously reported method [13] and occurred in three phases: preexposure; training; and testing (see below). Two separate contexts (Context A and Context B) were used for preexposure. Context A was one of four clear Plexiglas chambers (16.5 × 21.1 × 21.6 cm) with stainless steel bar floors (11.5 cm from top of chamber) consisting of 9 grid bars (0.5 cm in diameter and placed 1.25 cm apart). The grid floor was connected to a shock scrambler (Med Associates, Georgia, VT ENV-414S) and delivered a 1.5 mA, 2s footshock US. The four chambers (of Context A) were positioned on a Plexiglas stand and arranged within a fume hood that provided ambient light and background noise. The side of the chambers facing one another was made opaque. All behavior was recorded by a video camera that captured activity in all four chambers during the trial. The camera fed into a Dell computer that ran FreezeFrame software (Actimetrics, Wilmette IL). Briefly, movement was determined by measuring changes in pixel luminance. Freezing was defined as a bout of 0.75s or longer without changes in pixel luminance. Context B consisted of separate chambers (22 × 22 × 26 cm) with wire mesh cages enclosed in larger sound-attenuated chambers (BRS/LVE, Laurel, MD) lined with sound-absorbing foam. Context B chambers were located in a separate room from those of Context A.
Design and Procedures
Preexposure
Subjects from each dosing condition were preexposed to either Context A or Context B. On PD31, subjects were removed from their home cages, weighed, and placed into a distinctive transport cage. The transport cages measured 11 × 11 × 18 cm and were made of clear lexan; orange construction paper surrounded four sides. Subjects preexposed to Context A (Group Pre) were transported four at a time to a room adjacent to the preexposure room and remained there for 5 minutes while the experimenter wiped down the preexposure cages with a 5% ammonia solution. Subjects were brought into the preexposure room, placed into one of four Context A chambers, and were allowed to explore the context for a 5 minute acclimation session. Following preexposure, subjects were returned to their home cages via the transport cages. Total time from weighing to returning to their home cage was around 15 minutes. Subjects that were preexposed to Context B (Group No Pre) were treated in a similar manner except they were transported four at a time to a separate room, left in the transport cage for 5 minutes (the cages of Context B were not wiped down with any solution), and were allowed to explore the context for 5 minutes before being returned to their home cages. Handling of subjects, time away from home cages, and time within the transport cage were equated between the two preexposure groups.
Training
Twenty four hours after preexposure (on PD32), all subjects were transported four at a time to the room adjacent to the training chambers. Training for all subjects occurred in Context A; those preexposed to Context A were trained in the specific chamber where preexposure occurred 24h previously whereas those preexposed to Context B were randomly assigned to be trained in one of the four Context A chambers. After the chambers had been wiped down with ammonium solution, each subject was brought one at a time into the training room, placed into the training context, and given an immediate shock (<8 seconds upon placement into the chamber; [18]). Subjects were immediately removed from the chamber after the trial, placed back into the transport cage, and returned to their home cage.
Testing
Twenty-four hours after training (on PD33), all subjects were returned to the chamber where they had previously received an immediate shock during training. The transfer procedure was identical to that used on the preceding training day except that no shock was delivered and their activity in Context A was recorded over a 5 minute period.
Data Analysis
The data were analyzed using FreezeFrame software (Actimetrics, Wilmette IL). The bout length was set at 0.75 s and the freezing threshold (change in pixels/frame) was initially set as described in the instructions. A human observer verified the setting by watching the session and adjusting the threshold if necessary to ensure that small movements were not recorded as freezing. Freezing behavior was scored as a percent time spent freezing during the 5 minute testing session.
The data were imported into Statistica 7 data analysis software. Body weight analysis utilized a repeated measures ANOVA with between-subjects factors of sex and dosing condition and the within-subjects factor of age. Freezing behavior was analyzed with a 2 (sex) × 3 (dosing condition) × 2 (preexposure group) factorial ANOVA. No significant main effects or interactions concerning the factor of sex appeared (F<0.20) and so the remaining statistics utilized a 3 × 2 (dosing condition X preexposure) factorial design. Post hoc analyses (Newman-Keuls) were used to characterize treatment and interaction effects, when statistically significant (alpha set at p≤.05, two-tailed). Nine subjects were excluded from the final analysis: two subjects were lost due to equipment failure; and seven subjects were removed as statistical outliers (outliers were defined a priori as scores being ± 2 standard deviations from the % freezing mean of the other subjects in a particular group; EtOH Pre=1; EtOH No Pre=1; SI Pre=1; SI No Pre=1; UD Pre=1; and UD No Pre=2). Statistics were run on the remaining 61 subjects (EtOH Pre=10; EtOH No Pre=10; SI Pre=13; SI No Pre=9; UD Pre=10; UD No Pre=9).
2.2. Results
Body Weight and BACs
Body weight averages for all three dosing groups at three different ages appear in Table 1. All groups gained substantial weight during the dosing period (PD4-9) up until the age of testing (PD31). A 3 (dosing condition) × 2 (sex) × 2 (days) ANOVA on PD4 and PD9 weights revealed significant main effects of days [F(1, 55)=2046.7, p<0.001), and of dosing condition [F(2, 55)=23.6, p< 0.001], as well as interactions of Days X Dosing Condition [F(2, 55)=130.4, p<0.001)]. Newman-Keuls tests revealed group weights did not differ on PD4 (ps>0.6), but on PD9, Group EtOH mean body weight (15.1 ± 0.4 g) was significantly less (ps<0.01) than both Group UD and SI (20.6 ± 0.4 and 19.7 ± 0.4, respectively), which did not differ. Growth retardation in ethanol treated animals over the dosing period (PD4-9) has been reported previously [11,12]. A 3 (dosing condition) × 2 (sex) factorial ANOVA performed on PD31 weights revealed significant main effects of dosing condition [F(2,55)=6.6, p<0.01] and sex [F(1,55)=26.2, p<0.01]. Group EtOH weighed less than Group UD & SI (95.9±1.7g vs. 104.5±1.7g & 102.2±1.6g, respectively) and females showed reduced body weights compared to males (females=95.9±1.4g; males=105.9±1.4g). Table 1 breaks down PD31 body weights by dosing condition and sex, although this interaction did not reach significance (p>0.4).
Table 1.
Body weights and BACs for Experiment 1, 2, and 3. Average body weights (in grams; ± SE) are given from three dosing conditions (UD=undisturbed; SI=sham intubated; EtOH=5.25g/kg/day) at the first and last day of the dosing period (PD4 and PD9, respectively) and the first day of behavioral training (PD31 for Exps. 1 & 2; PD32 for Exp. 3). BACs were taken from blood samples collected on PD4 from Group EtOH (reported in mg/dl).
| Experiment | Dose | Body Weight
|
BACs (mg/dl) PD4 | |||
|---|---|---|---|---|---|---|
| PD4 | PD9 | PD31-2 (males) | PD31-2 (females) | |||
| Exp 1 | ||||||
| UD | 11.0 ± 0.2 | 20.6 ± 0.4 | 111.3 ± 2.5 | 97.2 ± 2.4 | N/A | |
| SI | 11.2 ± 0.2 | 19.7 ± 0.4 | 106.3 ± 2.5 | 98.1 ± 2.1 | N/A | |
| EtOH | 11.5 ± 0.2 | 15.1 ± 0.4* | 100.0 ± 2.3* | 91.9 ± 2.5* | 407.7 ± 42.2 | |
| Exp 2 | ||||||
| UD | 9.5 ± 0.3 | 17.3 ± 0.7 | 98.3 ± 2.3 | 91.1 ± 2.4 | N/A | |
| SI | 10.4 ± 0.3 | 18.6 ± 0.6 | 101.1 ± 2.1 | 92.5 ± 2.3 | N/A | |
| EtOH | 10.9 ± 0.3 | 15.0 ± 0.7* | 93.6 ± 2.3* | 87.9 ± 2.4* | 397.9 ± 50.5 | |
| Exp 3 | ||||||
| UD | 11.3 ± 0.4 | 18.9 ± 0.8 | 101.7 ± 6.6 | 96.8 ± 6.7 | N/A | |
| SI | 10.3 ± 0.3 | 18.9 ± 0.6 | 97.4 ± 5.1 | 95.4 ± 5.4 | N/A | |
| EtOH | 10.2 ± 0.3 | 13.6 ± 0.7* | 89.8 ± 5.1* | 79.4 ± 6.1* | 425.5 ± 91.9 | |
“*” indicates significant differences between EtOH vs. UD and SI groups.
BACs taken from blood samples of Group EtOH pups on PD4 are also shown in Table 1. All 20 samples taken from Group EtOH showed an average BAC of 407.72 ± 42.2 mg/dl. The BACs obtained by our dosing method (5.25g/kg/day in a single binge dose) are comparable to those obtained using a similar dosing procedure [43]. Sex did not influence BAC (p>0.61).
Behavioral Measures
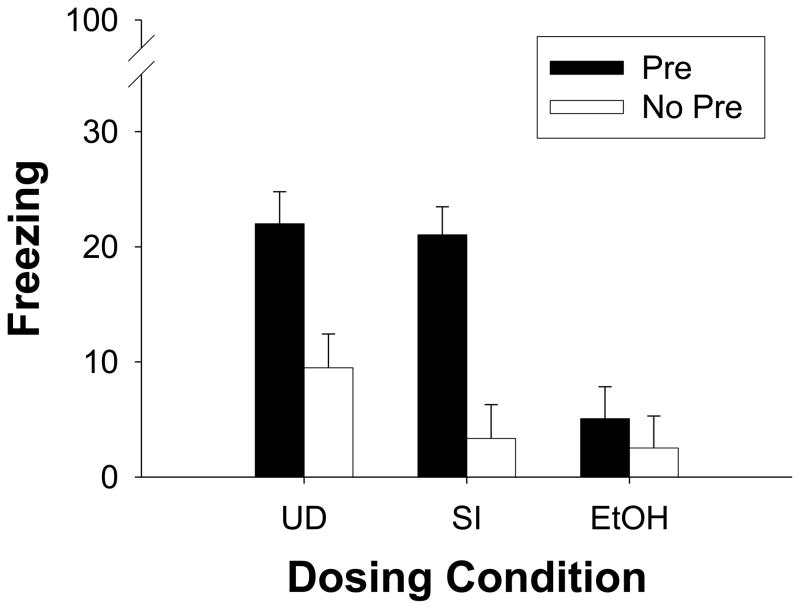
Percent freezing on the test day is shown as a function of dosing and preexposure group in Fig. 1. In UD and SI conditions, groups preexposed to Context A (Pre) froze much more during testing than their counterparts preexposed to Context B (No Pre). Group EtOH exhibited little contextual freezing regardless of preexposure condition. A 3 (dosing condition) × 2 (preexposure) factorial ANOVA revealed a significant main effect of dosing condition [F(2, 55)=9.54, p<0.001], a significant main effect of preexposure [F(1, 55)=23.01, p<0.001], and a significant interaction of Dosing Condition X Preexposure [F(2, 55)=3.90, p<0.001]. Newman-Keuls post-hoc analysis revealed that control groups (UD and SI) that were preexposed to Context A did not significantly differ from one another on percent freezing during testing (p>0.81). Control subjects preexposed to Context B also did not differ significantly from one another during testing (p>0.27). Control groups preexposed to Context A froze significantly more during testing compared to Control groups preexposed to Context B (ps<0.007). Group EtOH preexposed to Context A did not differ significantly from its non-preexposed counterpart (p>0.79). Percent freezing in Group EtOH preexposed to Context A was significantly lower than both Group UD and Group SI preexposed to Context A (ps<0.001). None of the dosing groups preexposed to Context B (No Pre) differed in their total amount of freezing during testing (ps>0.27).
Fig. 1.
Mean (± SE) percent freezing during the 5 minute test of fear conditioning in Experiment 1. Dosing conditions include Group UD (undisturbed), Group SI (sham-intubated), and Group EtOH (5.25 g/kg/day). Rats from each group were preexposed to Context A (Pre, filled bars) or Context B (No Pre, clear bars). All rats were given an immediate shock 24h later in Context A, followed 24h after that with a test of freezing in Context A. Preexposure to Context A facilitated freezing during the test in Groups UD and SI. Neonatal ethanol treatment (PD4-9) disrupted this context preexposure facilitation effect. Group EtOH showed low levels of freezing in Context A regardless of preexposure condition (Pre vs. No Pre).
Summary of Experiment 1
During behavioral testing, control groups (UD and SI) showed the CPFE whereas the EtOH group did not, with low levels of freezing regardless of context preexposure condition Thus the CPFE appears to be a variant of context conditioning that is eliminated by neonatal exposure to a high dose of alcohol.
3. EXPERIMENT 2: The effects of neonatal alcohol exposure on two variants of contextual fear conditioning
Experiment 2 examined the effects of neonatal alcohol exposure on two variants of contextual fear conditioning: the CPFE and standard contextual fear conditioning. In standard contextual fear conditioning (sCFC), the subject receives a footshock some time (e.g., 2 min) after placement into the context. Although many studies show disruptions in sCFC after posttraining hippocampal lesions [56,32], under certain conditions, rats with pretraining lesions of the hippocampus show normal context conditioning [42,20,15,59]. To account for these discrepancies, Rudy and colleagues [66,64] suggest that there are two systems capable of mediating context conditioning, one hippocampal and the other extrahippocampal in nature. If the ethanol-induced deficits in the CPFE were related to hippocampal dysfunction in Experiment 1, we were interested to learn if neonatal ethanol exposure additionally disrupted sCFC. We predicted that if an extrahippocampal system is capable of mediating contextual learning when the hippocampus is disrupted, then rats exposed to neonatal alcohol may show less or no impairment in sCFC.
3.1 Materials and methods
Subjects
A total of 144 rats (76 males & 68 females) derived from 33 litters were run in Experiment 2. The housing, animal care and maintenance, alcohol dosing, BAC analysis, apparatus, stimuli, design, and procedures were the same as those of Experiment 1, except where noted (see below).
Apparatus
As in Experiment 1, contextual fear conditioning occurred in three phases: preexposure; training; and testing. Two separate contexts (Context A and Context B) were used for preexposure. Context A was the same as in Experiment 1. Context B involved modifications to the testing chambers used for Context A. In Context B, a wire-mesh floor and back wall, which protruded into the chamber altering the spatial extent of the chamber, was inserted into the testing chamber. Three of the four clear walls of the chamber were covered with a paper drape, leaving only the wall facing the camera unobstructed. These alterations to Context B allowed for video recording of both preexposure groups during preexposure.
Design and Procedures
Preexposure
On PD31, subjects were preexposed to either Context A or Context B. The transport of animals to and from the testing room was similar to that in Experiment 1. Rats were brought over to the preexposure chambers in squads of four and exposed to either Context A or to Context B. Prior to exposure to either context, a 5% ammonium solution was used to clean out the chamber prior to the animal being placed into it. During exposure to Context B, additional room lighting was left on. Animals were preexposed to the context for 5 minutes, as in Experiment 1, and immediately returned to their home cage.
Training
Twenty four hours after preexposure (on PD32), all subjects were transported four at a time to the room adjacent to the training chambers. Training for all subjects occurred in Context A. Again, those animals preexposed to Context A were trained in the exact chamber where preexposure had occurred. Those exposed to Context B, however, were trained in the chamber placed opposite to the one they had been preexposed in; that is, if an animal was preexposed to Context B on the top left chamber, it was trained in Context A on the bottom right chamber. This was done in an effort to change the external spatial dimensions of the room the rats exposed to Context B may have encountered through the one clear chamber wall. The design differed from Experiment 1, in that we tested two time-to-shock intervals for Experiment 2. One group of animals was trained to immediate shock as in Experiment 1 (Group Imm). Rats were placed into the context, given an immediate 1.5 mA, 2s shock (<8s), and immediately removed and returned to their home cage. A second group of animals was placed into the context and given a 1.5 mA, 2s delayed shock following 120s of context exploration (Group 120s). Rats in Group 120s were removed immediately after receiving a shock and returned to their home cage. Again, we predicted that ethanol-exposed rats might perform differently during the CPFE (Group Imm) and during sCFC (Group 120s), because rats with hippocampal damage can use nonhippocampal “strategies” to perform sCFC but not the CPFE [see 64].
Testing
Twenty-four hours after training (on PD33), all subjects were returned to the chamber where they had previously received either an immediate or delayed shock during training, and their activity was recorded over a 5 minute period as in Experiment 1.
Data Analysis
Data from each time-to-shock group (Imm & 120s) were analyzed separately using a 2 × 3 (preexposure X dosing conditioning) factorial ANOVA followed by Newman-Keuls tests as in Experiment 1. A larger ANOVA involving time-to-shock as a factor was not performed because the greater range of freezing scores in the 120s-shock condition produced an error variance that was five-fold higher than in the Imm-shock condition, thereby violating the heterogeneity-of-variance assumption of ANOVA. Comparison of Imm- vs. 120s-shock conditions involving a proportion-of-control-freezing measure that overcomes this limitation was also performed (see Results). Fourteen subjects were excluded from the final analysis as statistical outliers (defined as in Experiment 1). Animals excluded as outliers included: EtOH Pre Imm=1; EtOH No Pre Imm=1; SI Pre Imm=1; SI No Pre Imm=1; UD Pre Imm=1; UD No Pre Imm=1; EtOH Pre 120s= 1; EtOH No Pre 120s=1; SI Pre 120s=2; SI No Pre 120s=2; UD Pre 120s=1; and UD No Pre 120s=1. Statistics were run on the remaining 130 subjects. Animals conditioned to an immediate shock included: EtOH Pre=11; EtOH No Pre=10; SI Pre=13; SI No Pre=13; UD Pre=10; and UD No Pre=13. Animals conditioned to a 120s shock included: EtOH Pre=10; EtOH No Pre=9; SI Pre=11; SI No Pre=12; UD Pre=9; and UD No Pre=9.
3.2. Results
Body Weight and BACs
Body weight averages for all groups in Experiment 2 are also found in Table 1. All groups gained substantial weight during the dosing period (PD4-9). A 3 (dosing condition) × 2 (sex) × 2 (days) ANOVA of PD4 and PD9 weights revealed significant main effects of days [F(1, 124)=1622.7, p<0.001] and of dosing condition [F(2, 124)=14.1, p< 0.001], with a significant interaction of Days X Dosing Condition [F(2, 124)=81.1, p<0.01]. Newman-Keuls tests showed no group differences on PD4 (p>0.1), followed by an alcohol effect on PD9 (p<0.01, Table 1). A 2 (dosing condition) × 2 (sex) factorial ANOVA run on PD31 weights revealed a main effect of sex [F(1, 124)=14.7, p<0.01], with females weighing less than males (90.5±1.4g vs. 97.7±1.3g, respectively), and a main effect of dosing condition [F(2, 124)=3.6, p<0.04], with Group EtOH weighing less than Groups UD & SI, (90.8 ± 1.7g vs. 94.7 ± 1.7g vs. 96.8 ± 1.5g, respectively).
BACs taken from blood samples of Group EtOH pups on PD4 are shown in Table 1. Of the 39 ethanol-treated rats in Experiment 2, 6 blood samples were dropped due to equipment failure and an addition 3 samples were dropped as statistical outliers. The remaining 30 samples taken from Group EtOH on PD4 show an average BAC of 397.9 ± 50.5 mg/dl. The BACs obtained in Experiment 2 (5.25g/kg/day in a single binge dose) are comparable to those obtained during Experiment 1. As previously found, sex did not influence BAC (p>0.53).
Behavioral Measures
Preexposure
In Experiment 2 we were able to collect data from all groups (Pre and No Pre) during the preexposure period. A 3 (dosing condition) × 2 (preexposure) factorial ANOVA revealed a main effect of preexposure (p<0.01). Rats preexposed to Context B showed slightly more freezing during preexposure than rats preexposed to Context A (7.29% ± 0.9 vs. 4.14% ± 0.9), possibly due to the more confined internal space within Context B compared to Context A. There were no main or interactive effects of dosing condition on preexposure freezing (ps>0.30).
Immediate shock Training Groups (CPFE)
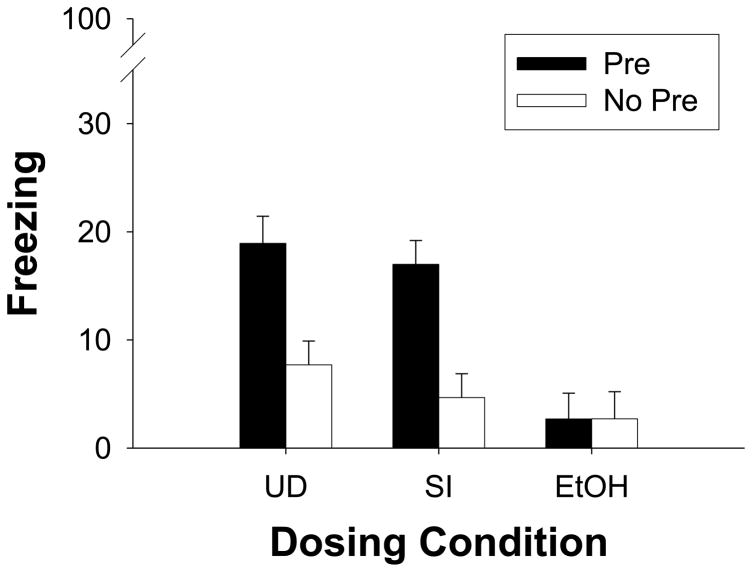
Test performance in the groups receiving immediate shock on the training day is shown in Fig. 2. A 3 (dosing condition) × 2 (preexposure) factorial ANOVA revealed a significant main effect of dosing condition [F(2, 64)=10.63, p<0.001], a significant main effect of preexposure [F(1, 64)=16.89, p<0.001], and a significant interaction of Dosing Condition X Preexposure [F(2, 64)=4.10, p<0.03]. Newman-Keuls post-hoc analysis revealed that control groups (UD and SI) did not significantly differ from one another on percent freezing during testing regardless of preexposure condition, Pre (p>0.56), No Pre (p>0.36). Control groups in the Pre condition (preexposed to Context A) froze significantly more during testing than their No Pre counterparts (preexposed to Context B, all ps<0.007). In contrast, there was no preexposure effect in Group EtOH (p>0.99), with EtOH-Pre rats freezing significantly less than their counterparts in both Group UD and Group SI (ps<0.001). No Pre groups in all three dosing conditions failed to differ in their total amount of freezing during testing (ps>0.36).
Fig. 2.
Mean (± SE) percent freezing during the 5 minute test of fear conditioning that occurred 24h after Immediate-shock training in Experiment 2. Group and Treatment designations were as described in the Figure 1 caption. The context preexposure facilitation effect appeared in Groups UD and SI but not in Group EtOH.
120s Training groups (cCFC)
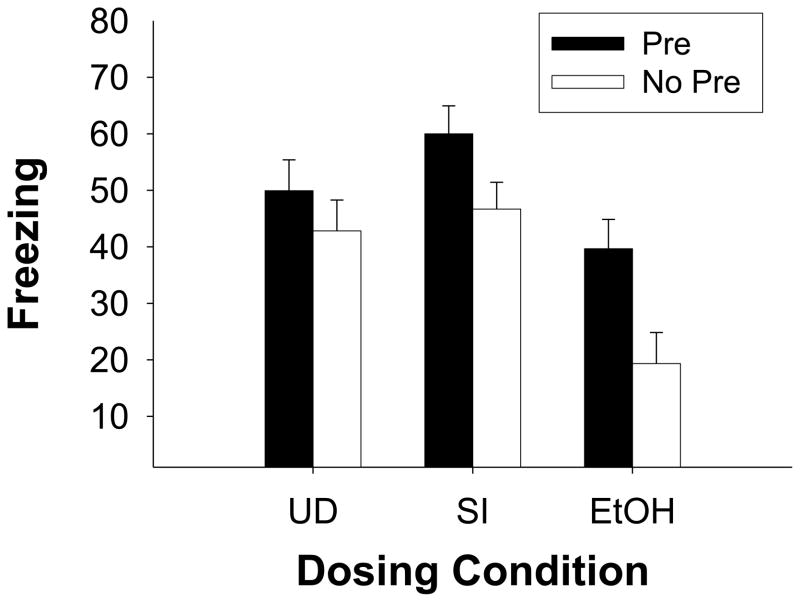
Data from groups receiving delayed shock on the training day are shown in Fig. 3. All groups exposed to the testing context 2 minutes prior to receiving a shock showed substantial freezing to context as measured during testing 24hs after training. A repeated measures ANOVA comparing preexposure freezing vs. testing freezing showed that all groups froze significantly more during testing compared to preexposure [F(1,54)=246.15, p<0.001] (data not shown). A 3 (dosing condition) × 2 (preexposure) factorial ANOVA on rats conditioned to a 120s shock (Group 120s) revealed significant main effects of dosing condition [F(2, 54)=11.32, p<0.001], and of preexposure [F(1, 54)=10.17, p<0.003], but no significant interaction of Dosing Condition X Preexposure (F<0.80). Newman-Keuls tests of the main effect of Dosing condition revealed that Group EtOH differed significantly from both Group SI and Group UD (ps<0.003), which did not differ from one another (p>0.20). Preexposure groups also differed significantly from one another (p<0.004), with groups preexposed to the training context freezing more than those preexposed to the alternate context.
Fig. 3.
Mean (± SE) percent freezing during the 5 minute test of fear conditioning that occurred 24h after 120s-shock training in Experiment 2.. Dosing conditions were Group UD (undisturbed), Group SI (sham-intubated), and Group EtOH (5.25 g/kg/day). Rats from each group were preexposed to Context A (Pre, filled bars) or Context B (No Pre, clear bars). Twenty-four hours later, all rats were given shock 120s after placement into Context A, followed 24h after that with a 5-min test of freezing in Context A. Main effects of dosing condition and preexposure indicated that Group EtOH showed reduced levels of freezing relative to the other groups and preexposure facilitated conditioning in all groups.
Summary of Experiment 2
Findings from the immediate-shock condition replicate the findings of Experiment 1. The variation in apparatus (altered context B) across these experiments did not change the outcome. Both control groups (UD and SI) preexposed to the testing context (Context A) showed elevated freezing to an immediate shock, compared to those animals that were preexposed to an alternate context (Context B). Preexposure to Context A did not facilitate conditioning to an immediate shock for Group EtOH. All groups trained to a 120s shock showed elevated levels of freezing during testing when contrasted to freezing in their counterparts that received immediate shock following preexposure to Contex B (No Pre Imm condition). However, Group EtOH froze significantly less than control groups (Group SI and Group UD).
Comparison of contextual freezing deficits in Group EtOH across tasks
To better examine the relative size of the ethanol deficit across experiments, the data from ethanol groups were analyzed as a proportion of control freezing. Group SI and Group UD did not differ significantly on any measure and their data were therefore combined to form a single control (CTL) group. During the CPFE, the average freezing of Group No Pre from each dosing condition (Group EtOH & Group CTL) represents a non-associative freezing baseline. To determine the proportion of CTL freezing exhibited by Group EtOH during each experiment (CPFE & sCFC), the average score of Group EtOH No Pre (CPFE) was subtracted from an individual ethanol rat’s score. This score was then divided by the difference of Group CTL Pre (CPFE) and Group CTL No Pre (CPFE) and multiplied by 100 to form a percentage. Data for Group EtOH run in sCFC was transformed in a similar manner, with the exception that for EtOH Pre (sCFC) the divisor was the difference between Group CTL Pre (sCFC) and Group CTL No Pre (CPFE) and that for Group No Pre (sCFC) the divisor was the difference between Group CTL No Pre (sCFC) and Group CTL No Pre (CPFE). For the transformation, a score of zero represents the level of freezing in non-associative controls (No Pre Imm condition) whereas a score of 100% represents the level of freezing in the (treatment) control groups for each of the three training conditions. The transformed scores for the EtOH groups were as follows: CPFE Pre = −0.15 ± 8.5; sCFC No Pre=43.16 ± 9.44; and sCFC Pre=75.75 ± 8.95. These scores were analyzed via one-way ANOVA which revealed a significant main effect of Group, F(2,27)=19.07, p<0.001. Newman-Keuls tests revealed that all three ethanol groups differed significantly from one another (ps<.003). These results indicate that the ethanol deficit in contextual conditioning was most apparent during CPFE, where no learning occurred. The ethanol deficit was less robust during sCFC, and was further reduced by preexposure to the testing context.
4. Experiment 3: Neonatal Alcohol Exposure and Discrete CS (Tone) Fear Conditioning
Experiment 3 examined the effects of developmental alcohol exposure on fear conditioning to a discrete CS in juvenile rats. The previous experiments demonstrated deficits in contextual fear conditioning in rodents neonatally exposed to alcohol. These deficits are consistent with hippocampal injury produced by developmental exposure to alcohol. However, they could also reflect more general “performance deficits” such as the inability to freeze because of hyperactivity or to learn fear conditioning in general because of amygdala damage or alterations in sensitivity to foot shock. To exclude these possibilities, rats exposed to alcohol over PD4-9 and controls were trained as juveniles in a tone fear conditioning paradigm that makes similar sensory, motor, and motivational demands but does not depend on contextual learning or the integrity of the hippocampus. Rats received either one or two tone-shock pairings during the training session. Number of pairings was added as a factor to determine whether strength of conditioning would alter the effect of alcohol on tone fear conditioning. The design of this experiment (see below) also enabled us to compare groups for levels of post-shock freezing, a measure of short-term memory of conditioned fear.
4.1 MATERIALS AND METHODS
Subjects
The subjects were 48 juvenile Long Evans rats (22 female, 26 male) derived from 8 litters (3 undisturbed litters, 5 dosed litters). The same procedures as Exp. 1 & 2 were used in the current experiment for housing, animal care and maintenance, developmental alcohol exposure, and BAC analysis. As before, subjects were individually housed on PD29. However, behavioral testing occurred on PD32 and PD33 (see below).
Apparatus
Behavioral training and context testing (see below) occurred in the same context (Context A) as in Exp. 1 & 2. Testing to the tone CS was conducted in a distinct context (Context B), measuring 50cm × 22cm × 33cm. Context B consisted of four clear Plexiglas walls and ceiling and a grid floor consisting of 38 evenly spaced bars that measured 4mm in diameter (not attached to the shock generator). Context B was positioned within a fume hood, in the same room as Context A, which provided ambient light and background noise. The auditory CS (1600 Hz, 80 dB tone) was delivered via two adjacent speakers connected to a Dell computer running FreezeFrame software (Actimetrics, Wilmette IL). Behavior was captured by a video camera, as described for the previous experiments.
Design and Procedures
Training
On PD32, rats were weighed and transported to and from the testing room in distinct transport cages (see Exp. 1). Rats were brought over to the training room in squads of four and placed into one of four training chambers (Context A). As in the context-conditioning experiments, a 5% ammonium solution was used to clean out the chamber prior to the animal being placed into it. Subjects were randomly assigned to one of two training procedures, and received either 1 or 2 tone-shock pairings. Rats in the 2 tone-shock pairings group received the 1st tone-shock pairing at 120s upon placement into the context, with a 10s, 1200-Hz tone terminating with the onset of a 2s, 1.5 mA footshock. The 2nd tone-shock pairing occurred 60s later (192s into the trial). Rats were left in the chamber for an additional 60s after the 2nd shock offset and returned to their home cage following the trial. For the 1 tone-shock pairing group, a 10s, 1200 Hz tone terminating with the onset of a 2s, 1.5 mA footshock occurred at 192s upon placement into the chamber (the same procedure as the 2 tone-shock group but omitting the 1st tone-shock pairing). Rats remained in the chamber for an additional 60s after US termination and were then returned to their home cage
Testing
Testing for tone and context conditioning occurred on PD33, 24h after training. For tone testing, rats were individually transported to the tone testing chamber (Context B); they were transported in their home cage with a piece of white paper covering the top to obscure any visual cues. Prior to placement, Context B was wiped down with a 5% isopropyl alcohol solution. The rat was then placed into Context B where it received a 120s baseline period where no CS was presented followed by 5-tone-alone presentations, spaced 60s apart. Testing for contextual conditioning occurred as before; rats were transported to the testing room in transport cages (see above); they were then placed into the training context (Context A) and freezing was measured over a 5 min period. Order of testing was counterbalanced, such that half of the subjects were tested for tone conditioning first, while the other half were tested for context conditioning first.
Data Analysis
In all analyses, the percentage of time freezing was the dependant measure. Analyses involved baseline freezing (freezing prior to CS-US pairing during training in Context A and freezing prior to CS onset during testing in Context B), post-shock freezing (freezing during the 60s interval following US termination), tone freezing (freezing to tone presentations over a 5 minute testing interval), and context freezing (freezing within the training context over a 5 minute testing interval). For post-shock freezing, a 3 (dosing condition) × 2 (sex) × 2 (tone-shock pairings) factorial ANOVA was performed. Tone and context conditioning were analyzed separately. A 3 (dosing condition) × 2 (sex) × 2 (tone-shock pairings) × 2 (order of testing) factorial ANOVA was performed.
4.2 Results
Body Weight and BACs
The body weights on PD4, PD9, and PD32 for the three dosing conditions (UD, SI, and EtOH) and the BACs for group EtOH are summarized in Table 1. A 3 (dosing condition) × 2 (sex) × 2 (days) repeated measures ANOVA on PD4 and PD9 weights again revealed a significant main effect of days [F(1, 42)=809.5, p<0.01], dosing condition [F(2, 42)=12.4, p<0.01], and a significant Days X Dosing Condition interaction [F(2, 42)=53.3, p<0.01]. Again, Newman-Keuls revealed that group weights did not differ on PD4 (ps>0.29) but, on PD9, Group EtOH weighed significantly less than Groups SI & UD (ps<0.01), which did not differ from one another (p>0.9). A 3 (dosing condition) × 2 (sex) factorial ANOVA performed on PD32 weights reveal a main effect of dosing condition [F(2,42)=3.58, p<0.04], with Group EtOH weighing less than Group SI & UD (p<.05), which did not differ. Analysis of blood samples taken on PD4 from the 17 pups in Group EtOH showed an average BAC of 425.5 mg/dl, comparable to the other reported BACs in Exps 1 & 2.
Behavioral Measures
Preexposure Freezing
Freezing was measured during the first two minutes prior to stimulus onset in both the training context (Context A) and the tone testing context (Context B). A 3 (dosing condition) × 2 (sex) × 2 (context) factorial ANOVA revealed a significant main effect of context [F(1, 42)=9.00, p<0.01]. Subjects spent less time freezing in Context A (0.76% ± 0.2%) than in Context B (6.47% ± 1.9%). Baseline freezing in either context, however, was significantly less than freezing during the test (see below). Importantly, treatment groups did not differ in baseline freezing (F<0.1). A subsequent analysis of difference scores (subtracting test minus baseline freezing) did not result in significantly different results as reported below, with the exception that sex was no longer a significant factor during tone testing (p>0.08; data not shown).
Post-shock Freezing
During training, juvenile rats received either 1 or 2 tone-shock pairings. We assessed post-shock freezing by analyzing the rate of freezing following the last shock during training (the last minute of training). A 3 (dosing condition) × 2 (sex) × 2 (tone-shock pairs) factorial ANOVA revealed a main effect of the number of tone-shock pairs [F(1,36)=6.19, p<0.02]; one tone-shock pairing produced 37.5% (± 5.6%) freezing compared to 57.8% (± 5.9%) freezing in the two tone-shock pairing group. No other factors were significant (Fs<1.0). The absence of a treatment effect after either one- or two pairings indicates that the amount of freezing that a given training protocol produces per se (37.5 vs. 57.8 %) is not important for revealing alcohol-related behavioral deficits. Rather it appears that the type of learning---contextual vs. cued fear---is the important factor.
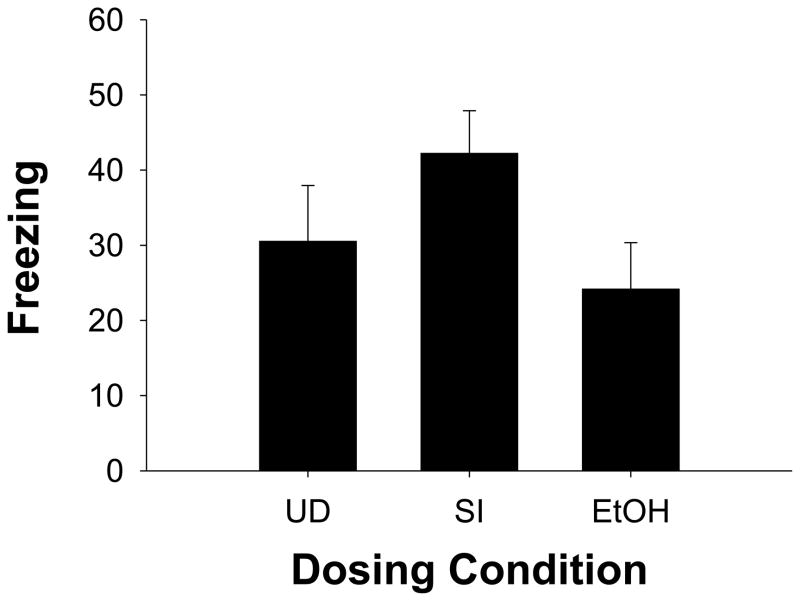
Tone Freezing
Tone freezing is summarized in Figure 4. Freezing to the discrete CS (tone) was assessed with a 3 (dosing condition) × 2 (sex) × 2 (number of tone-shock pairings) × 2 (order of testing) repeated measures ANOVA. The number of tone-shock pairings during training and the order of testing were non-significant factors (ps>0.3). There were also no differences in the amount of tone-elicited freezing across dosing conditions (F<2.3; Figure 5). A main effect of sex [F(1, 24)=6.08, p<0.02] was the only significant effect observed, with females freezing less than males (F=29.1% ± 5.5 vs. M=47.5% ±5.1).
Fig. 4.
Percent freezing (mean ± SE) during 5 minute testing period with 5 10s presentations of a discrete auditory (tone) conditioned fear stimulus. Group UD (undisturbed), Group SI (sham-intubated), and Group EtOH (5.25 g/kg/day) did not differ significantly in CS-elicited freezing.
Context Freezing
Finally, we examined contextual freezing to the training context (Context A) with a 3 (dosing condition) × 2 (sex) × 2 (tone/shock pairings) × 2 (order of testing) factorial ANOVA, which demonstrated a significant main effect of dosing condition [F(2,24)=7.70, p<0.01]. Although contextual freezing did not differ between Groups UD and EtOH (25.2 ± 5.3 vs. 18.69 ± 4.4, respectively; p>0.18), both groups differed significantly from Group SI (41.5 ± 4.0; ps<0.03). No other differences were found to be significant (Fs<3.0). This result supports the conclusion of Experiment 2 that standard contextual fear conditioning is less sensitive than the CPFE to neonatal alcohol treatment.
Summary of Findings
The preceding findings suggest that animals exposed to a high binge-dose of alcohol during early neonatal development do not differ from controls on two measures of fear conditioning: post-shock freezing or CS (tone) elicited freezing. This lack of an effect of alcohol was robust across other factors such as sex and the number of tone-shock pairings. Importantly, the failure of alcohol treatment to alter post-shock or tone-elicited freezing shows that alcohol-exposed rats can freeze as readily as controls when contextual processing is not required for learned fear. Thus, the impairment of the CPFE by alcohol is unlikely to be secondary to “performance effects” such as hyperactivity that competes with freezing behavior or alterations in shock sensitivity.
5. General discussion
The current set of experiments examined the performance of rats neonatally exposed to a high binge dose of ethanol over postnatal days (PD) 4-9 on two variants of contextual fear conditioning---the context preexposure facilitation effect (CPFE, Experiments 1 and 2) and standard context fear conditioning (sCFC) involving 120 sec of context exposure terminating with a single footshock (Experiment 2), as well as on cued (tone) fear conditioning (Experiment 3). As in previous studies of the CPFE [18,66,64], control rats (Groups SI & UD) preexposed to the testing context demonstrated the CPFE, with elevated levels of contextual freezing to an immediate shock compared to control groups preexposed to the alternate context, which displayed the immediate shock deficit (ISD;[18]). In contrast, rats neonatally exposed to ethanol (Group EtOH) demonstrated the ISD regardless of preexposure experience. This is the first report of a CPFE deficit in this rodent model of third trimester ethanol exposure. Experiment 2 replicated and extended this finding by including a standard contextual fear conditioning procedure. Preexposure to the testing context facilitated conditioning during sCFC for all groups. In addition, Group EtOH rats displayed a significant reduction in contextual freezing compared to control groups. Comparisons between the two variants of contextual fear conditioning reveal that the CPFE is more sensitive to developmental alcohol exposure than is sCFC. Additionally, Experiment 3 ruled out various performance effects that may have been caused by developmental alcohol exposure by showing intact post-shock and cued (tone) freezing in alcohol-exposed rats.
Relatively few experiments have examined fear conditioning in rodents exposed to ethanol during development. Wagner & Hunt [78] demonstrated trace fear conditioning deficits in juvenile rats exposed to 5.25 g/kg/day of ethanol over PD4-9 compared to control rats. However, ethanol-exposed rats and controls did not differ during delay fear conditioning (consistent with our results in Exp. 3). Lesions of the dorsal hippocampus (DH) have been show to disrupt trace fear conditioning, while leaving delay conditioning intact [14]. Wagner & Hunt [78] conclude that trace conditioning deficits in ethanol exposed rodents are likely to result from ethanol-induced disruptions in the hippocampus and/or basal forebrain. Two additional studies examined sCFC in adult rodents prenatally exposed to ethanol [79,2]. In these studies, conditioning involved pairing a tone CS with an aversive footshock US over a number of trials. Ethanol-exposed rodents showed reduced levels of freezing when tested in the training context 24h later compared to controls, but showed normal levels of freezing during tone presentation, a task dissociation that has been seen in rodents with hippocampal lesions [56,33]. The hippocampus has also been implicated in contextual fear conditioning (see below), and deficits in sCFC in rodents prenatally exposed to ethanol may result from hippocampal dysfunction. Our results are in general agreement with these findings. Rats exposed to ethanol over PD4-9 showed disruptions in contextual freezing in two separate variants of the task whereas these rats showed conditioned freezing to a tone CS that is comparable to control groups.
Contextual fear conditioning requires that the shock become associated with the context in which the shock occurs and is mediated by interactions between the hippocampus and the amygdala [56,33]. The standard contextual fear circuit involves reciprocal connections between the CA1/subiculum border regions and the basaolateral complex (BLA), which includes the basal and accessory basal nuclei of the amygdala [36,30,5]. Although posttraining lesions of the hippocampus have been shown to disrupt contextual fear conditioning [32,42,3,37,74], pretraining lesions of the hippocampus have inconsistently affected contextual fear conditioning [42,20,15,59], suggesting that an extrahippocampal system is capable of mediating contextual conditioning when the hippocampus is “offline” [66,64]. The extrahippocampal system is likely to involve reciprocal connections between the entorhinal cortex and the BLA [57;62 for review]. The hippocampus actively competes with and dominates the extrahippocampal system at the level of the BLA during contextual conditioning [42,5]. However, when the competition from the hippocampus is disrupted (e.g., through lesions, inactivations, etc.), the extrahippocampal system is capable of mediating contextual learning [5]. In Experiment 2, ethanol-exposed rats showed a more modest but significant impairment of standard contextual conditioning (sCFC) relative to control groups, a reduction similar to those induced by pretraining excitotoxic lesions of the dorsal hippocampus [82]. The more modest impairment of sCFC relative to the CPFE (Experiment 2) may occur because sCFC may be mediated by an extrahippocampal system following neonatal alcohol exposure.
To better examine the role of the hippocampus during contextual fear conditioning Rudy and colleagues have developed the CPFE paradigm, where learning about the context and associating the context with the shock occur on separate occasions. This paradigm appears to be more sensitive to hippocampal insult than is sCFC [66,64]. The CPFE is negatively affected by 1) anterograde damage of the DH [63]; 2) blocking protein synthesis in the DH after context preexposure [4]; 3) muscimol infusions into the DH prior to context preexposure, prior to immediate shock, and prior to contextual fear testing [47]; 4) inactivating NMDA receptors in the DH prior to preexposure [48]; and 5) inactivation of the VH prior to preexposure or inhibiting protein synthesis in the VH after preexposure [65]. These studies demonstrate that the hippocampus makes important contributions during each of the three phases of the CPFE paradigm. The CPFE deficits observed in our ethanol-exposed rats may be related to hippocampal dysfunction during one or more of these phases.
There are a number of factors that could contribute to the deficits in contextual fear conditioning seen in rats neonatally exposed to ethanol. First, neonatal ethanol exposure that results in high BACs (>350 mg/dl) causes a significant reduction of CA1 pyramidal neurons [38,77,43,51]. Selective lesions of both dorsal and ventral CA1 disrupt sCFC [29]. Though CA1 pyramidal neurons are only reduced by 20% in most studies of neonatal ethanol exposure in rats, these small reductions in CA1 may contribute to the contextual fear deficits exhibited. Further support for the involvement of CA1 in sCFC comes from studies of the activity-regulated cytoskeletal protein (Arc), an immediate-early gene, linked to neural-plasticity mechanisms mediating learning [26,6]. Arc shows a selective increase in mRNA signal in the CA1/subiculum 30 minutes after sCFC [30]. Arc expression in the hippocampus is increased during both context exploration and during context-shock association during sCFC, but not after an immediate shock [28]. Future studies are planned to examine Arc expression during contextual fear conditioning in rats neonatally exposed to ethanol. Together, both lesion and Arc studies suggest an important role of CA1 in sCFC. Examining Arc expression during contextual fear conditioning as a function of EtOH exposure will provide more direct evidence for a link between disruptions in CA1 function and impaired contextual conditioning induced by developmental ethanol exposure.
Rudy & O’Reilly [66] suggest that during the CPFE paradigm, rats form a conjunctive representation of the context during preexposure. During training to an immediate shock, the rat samples a subset of features of the context and, through the process of pattern completion, the previously acquired conjunctive representation is retrieved and it is this retrieved representation that becomes associated with shock, producing the CPFE. Rats that are not preexposed to the context show the ISD to immediate shock because the individual features of the context sampled prior to immediate shock are insufficient to support conditioning. Area CA3 of the hippocampus has been proposed to support the process of pattern completion due to its abundance of recurrent circuits [44]. Rats with CA3 and DG lesions, but not rats with CA1 or sham lesions, were impaired during the test phase of a spatial pattern completion task [22], giving experimental support to this view. In addition, CA3 NMDA receptors are required for the rapid formation of context representation during contextual conditioning at short (between 20 and 40 seconds) placement-to-shock intervals [50]. In light of these considerations, Livy et al. [38] report reductions in CA3 density and cell number at PD10 using an artificial rearing method for developmental ethanol exposure over PD4-9, though other studies have failed to show CA3 reductions (e.g., [77]). If our dosing method produced CA3 reductions at PD31-33, the disruptions in the CPFE demonstrated by our ethanol-exposed rats may represent a disruption in the pattern completion process during the training phase of the CPFE paradigm. A deficit in pattern completion, however, is not necessary to account for deficits observed in sCFC.
An additional brain region contributing to our contextual fear conditioning deficits may be the cerebellum. Neonatal ethanol exposure has been shown to reduce the number of Purkinje cells in rodents [25] and there is some evidence that sCFC is attenuated by inactivation of the cerebellar vermis [67]. This issue has been noted in studies using the Morris water maze as a behavioral assay in neonatally exposed rodents, for mutant mice with Purkinje cell dysfunction show acquisition deficits in a spatial Morris water maze [83]. To address the issue of cerebellar effects future studies could restrict neonatal ethanol exposure to PD7-9, a period that does not result in Purkinje cell reductions [27].
The current set of experiments demonstrates reductions in contextual conditioning on two variants of contextual fear conditioning in juvenile rats neonatally exposed to a high binge dose of ethanol during early development. Further research is needed to better characterize the neural basis of these conditioning deficits, including the link between alcohol effects on hippocampus and performance on these tasks.
Acknowledgments
The authors would like to thank Jeff B. Rosen for graciously providing access to the fear conditioning equipment used in these experiments. We would also like to thank Mike A. Burman for initial training on the fear conditioning paradigm, and to Felipe L. Schifino and Henry S. Lange for technical support. This work was supported by the University of Delaware and by NIH grant RO1 AA014288-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abel EL, Sokol RJ. Fetal alcohol syndrome is now leading cause of mental retardation. Lancet. 1986;2:1222. doi: 10.1016/s0140-6736(86)92234-8. [DOI] [PubMed] [Google Scholar]
- 2.Allan AM, Chynoweth J, Tyler LA, Caldwell KK. A mouse model of prenatal ethanol exposure using a voluntary drinking paradigm. Alcoholism-Clinical and Experimental Research. 2003;27:2009–2016. doi: 10.1097/01.ALC.0000100940.95053.72. [DOI] [PubMed] [Google Scholar]
- 3.Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Barrientos RM, O’Reilly RC, Rudy JW. Memory for context is impaired by injecting anisomycin into dorsal hippocampus following context exploration. Behav Brain Res. 2002;134:299–306. doi: 10.1016/s0166-4328(02)00045-1. [DOI] [PubMed] [Google Scholar]
- 5.Biedenkapp JC, Rudy JW. Hippocampal and extrahippocampal systems compete for control of contextual fear: Role of ventral subiculum and amygdala. Learn Memory. 2009;16:38–45. doi: 10.1101/lm.1099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloomer WA, VanDongen HM, VanDongen AM. Arc/Arg3.1 translation is controlled by convergent N-methyl-D-aspartate and Gs-coupled receptor signaling pathways. J Biol Chem. 2008;283:582–592. doi: 10.1074/jbc.M702451200. [DOI] [PubMed] [Google Scholar]
- 7.Bonthius DJ, Pantazis NJ, Karacay B, Bonthius NE, Taggard Da, Lothman EW. Alcohol exposure during the brain growth spurt promotes hippocampal seizures, rapid kindling, and spreading depression. Alcohol Clin Exp Res. 2001;25:734–745. [PubMed] [Google Scholar]
- 8.Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14:107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- 9.Bonthius DJ, West JR. Permanent neuronal deficits in rats exposed to alcohol during the brain growth spurt. Teratology. 1991;44:147–163. doi: 10.1002/tera.1420440203. [DOI] [PubMed] [Google Scholar]
- 10.Brown KL, Burman MA, Duong HB, Stanton ME. Neonatal binge alcohol exposure produces dose dependent deficits in interstimulus interval discrimination eyeblink conditioning in juvenile rats. Brain Res. 2009;1248:162–175. doi: 10.1016/j.brainres.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown KL, Calizo LH, Goodlett CR, Stanton ME. Neonatal alcohol exposure impairs acquisition of eyeblink conditioned responses during discrimination learning and reversal in weanling rats. Dev Psychobiol. 2007;49:243–57. doi: 10.1002/dev.20178. [DOI] [PubMed] [Google Scholar]
- 12.Brown KL, Calizo LH, Stanton ME. Dose-dependent deficits in dual interstimulus interval classical eyeblink conditioning tasks following neonatal binge alcohol exposure in rats. Alcohol Clin Exp Res. 2008;32:277–293. doi: 10.1111/j.1530-0277.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 13.Burman MA, Murawski NJ, Schiffino FL, Rosen JB, Stanton ME. Factors governing single-trial contextual fear conditioning in the weanling rat. Behavioral Neuroscience. 123:1148–1152. doi: 10.1037/a0016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burman MA, Starr MJ, Gewirtz JC. Dissociable effects of hippocampus lesions on expression of fear and trace fear conditioning memories in rats. Hippocampus. 2006;16:103–113. doi: 10.1002/hipo.20137. [DOI] [PubMed] [Google Scholar]
- 15.Cho YH, Friedman E, Silva AJ. Ibotenate lesions of the hippocampus impair spatial learning but not contextual fear conditioning in mice. Behav Brain Res. 1999;98:77–87. doi: 10.1016/s0166-4328(98)00054-0. [DOI] [PubMed] [Google Scholar]
- 16.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 17.Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- 18.Fanselow MS. Factors Governing One-Trial Contextual Conditioning. Anim Learn Behav. 1990;18:264–270. [Google Scholar]
- 19.Fitzgerald P. FAS persists despite broad public awareness. Mich Med. 1988;87:262–264. 268. [PubMed] [Google Scholar]
- 20.Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci. 1998;112:863–874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- 21.Girard TA, Xing HC, Ward GR, Wainwright PE. Early postnatal ethanol exposure has long-term effects on the performance of male rats in a delayed matching-to-place task in the Morris water maze. Alcohol Clin Exp Res. 2000;24:300–306. [PubMed] [Google Scholar]
- 22.Gold AE, Kesner RP. The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus. 2005;15:808–814. doi: 10.1002/hipo.20103. [DOI] [PubMed] [Google Scholar]
- 23.Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19:435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- 24.Goodlett CR, Peterson SD. Sex differences in vulnerability to developmental spatial learning deficits induced by limited binge alcohol exposure in neonatal rats. Neurobiol Learn Mem. 1995;64:265–275. doi: 10.1006/nlme.1995.0009. [DOI] [PubMed] [Google Scholar]
- 25.Green JT. The effects of ethanol on the developing cerebellum and eyeblink classical conditioning. Cerebellum. 2004;3:178–187. doi: 10.1080/14734220410017338. [DOI] [PubMed] [Google Scholar]
- 26.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 27.Hamre KM, West JR. The effects of the timing of ethanol exposure during the brain growth spurt on the number of cerebellar Purkinje and granule cell nuclear profiles. Alcohol Clin Exp Res. 1993;17:610–622. doi: 10.1111/j.1530-0277.1993.tb00808.x. [DOI] [PubMed] [Google Scholar]
- 28.Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, Rudy JW. Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. J Neurosci. 2006;26:1616–1623. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunsaker MR, Kesner RP. Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiol Learn Mem. 2008;89:61–69. doi: 10.1016/j.nlm.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue K, Fukazawa Y, Ogura A, Inokuchi K. Two-dimensional neural activity mapping of the entire population of hippocampal CA1 pyramidal cells responding to fear conditioning. Neurosci Res. 2005;51:417–425. doi: 10.1016/j.neures.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Johnson TB, Goodlett CR. Selective and enduring deficits in spatial learning after limited neonatal binge alcohol exposure in male rats. Alcohol Clin Exp Res. 2002;26:83–93. [PubMed] [Google Scholar]
- 32.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 33.Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- 34.Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT. Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcohol Clin Exp Res. 2007;31:2073–2082. doi: 10.1111/j.1530-0277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 35.Krahl SE, Berman RF, Hannigan JH. Electrophysiology of hippocampal CA1 neurons after prenatal ethanol exposure. Alcohol. 1999;17:125–131. doi: 10.1016/s0741-8329(98)00043-3. [DOI] [PubMed] [Google Scholar]
- 36.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 37.Lehmann H, Lacanilao S, Sutherland RJ. Complete or partial hippocampal damage produces equivalent retrograde amnesia for remote contextual fear memories. Eur J Neurosci. 2007;25:1278–86. doi: 10.1111/j.1460-9568.2007.05374.x. [DOI] [PubMed] [Google Scholar]
- 38.Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 39.Maier SE, Chen WJ, West JR, Abel EL, editors. Fetal Alcohol Syndrome: From Mechanisms to Prevention. CRC Press; Boca Raton: 1996. The effects of timing and duration of alcohol exposure on development of the fetal brain; pp. 27–50. [Google Scholar]
- 40.Maier SE, West JR. Drinking patterns and alcohol-related birth defects. Alcohol Res Health. 2001;25:168–174. [PMC free article] [PubMed] [Google Scholar]
- 41.Manning MA, Eugene Hoyme H. Fetal alcohol spectrum disorders: a practical clinical approach to diagnosis. Neurosci Biobehav Rev. 2007;31:230–238. doi: 10.1016/j.neubiorev.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 43.Marino MD, Aksenov MY, Kelly SJ. Vitamin E protects against alcohol-induced cell loss and oxidative stress in the neonatal rat hippocampus. Int J Dev Neurosci. 2004;22:363–377. doi: 10.1016/j.ijdevneu.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 45.Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22:279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- 46.Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25:185–191. [PMC free article] [PubMed] [Google Scholar]
- 47.Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behav Neurosci. 2007;121:721–731. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- 49.May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- 50.McHugh TJ, Tonegawa S. CA3 NMDA receptors are required for the rapid formation of a salient contextual representation. Hippocampus. 2009:335–339. doi: 10.1002/hipo.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miki T, Harris SJ, Wilce PA, Takeuchi Y, Bedi KS. Effects of age and alcohol exposure during early life on pyramidal cell numbers in the CA1–CA3 region of the rat hippocampus. Hippocampus. 2004;14:124–134. doi: 10.1002/hipo.10155. [DOI] [PubMed] [Google Scholar]
- 52.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 53.Murawski NJ, Stanton ME. Fetal Alcohol Spectrum Disorder Study Group. San Diego, CA: 2009. Variants of contextual fear conditioning are differentially impaired in the juvenile rat by binge ethanol exposure on postnatal days 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Leary-Moore SK, McMechan AP, Mathison SN, Berman RF, Hannigan JH. Reversal learning after prenatal or early postnatal alcohol exposure in juvenile and adult rats. Alcohol. 2006;38:99–110. doi: 10.1016/j.alcohol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Pauli J, Wilce P, Bedi KS. Spatial learning ability of rats following acute exposure to alcohol during early postnatal life. Physiol Behav. 1995;58:1013–1020. doi: 10.1016/0031-9384(95)00120-8. [DOI] [PubMed] [Google Scholar]
- 56.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 57.Pitkanen A, Kelly JL, Amaral DG. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the entorhinal cortex in the macaque monkey. Hippocampus. 2002;12:186–205. doi: 10.1002/hipo.1099. [DOI] [PubMed] [Google Scholar]
- 58.Redila VA, Olson AK, Swann SE, Mohades G, Webber AJ, Weinberg J, Christie BR. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus. 2006;16:305–311. doi: 10.1002/hipo.20164. [DOI] [PubMed] [Google Scholar]
- 59.Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JN, Feldon J, Bannerman DM. Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behav Neurosci. 1999;113:1189–1203. doi: 10.1037/0735-7044.113.6.1189. [DOI] [PubMed] [Google Scholar]
- 60.Riikonen R, Salonen I, Partanen K, Verho S. Brain perfusion SPECT and MRI in foetal alcohol syndrome. Dev Med Child Neurol. 1999;41:652–659. doi: 10.1017/s0012162299001358. [DOI] [PubMed] [Google Scholar]
- 61.Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- 62.Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learn Men. 2009;16:573–85. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rudy JW, Barrientos RM, O’Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116:530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- 64.Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 65.Rudy JW, Matus-Amat P. The ventral hippocampus supports a memory representation of context and contextual fear conditioning: implications for a unitary function of the hippocampus. Behav Neurosci. 2005;119:154–163. doi: 10.1037/0735-7044.119.1.154. [DOI] [PubMed] [Google Scholar]
- 66.Rudy JW, O’Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn Affect Behav Neurosci. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- 67.Sacchetti B, Baldi E, Lorenzini CA, Bucherelli C. Cerebellar role in fear-conditioning consolidation. Proc Natl Acad Sci U S A. 2002;99:8406–8411. doi: 10.1073/pnas.112660399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schiffino FL, Murawski NJ, Rosen JB, Stanton ME. Ontogeny of the context preexposure facilitation effect: role of hippocampal NMDA receptors. doi: 10.1016/j.nlm.2010.11.011. (in prep) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. Jama. 2003;290:2996–2999. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- 70.Spadoni AD, McGee CL, Fryer SL, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Neurosci Biobehav Rev. 2007;31:239–245. doi: 10.1016/j.neubiorev.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sulik KK. Genesis of alcohol-induced craniofacial dysmorphism. Exp Biol Med (Maywood) 2005;230:366–375. doi: 10.1177/15353702-0323006-04. [DOI] [PubMed] [Google Scholar]
- 72.Sulik KK, Johnston MC, Webb MA. Fetal alcohol syndrome: embryogenesis in a mouse model. Science. 1981;214:936–938. doi: 10.1126/science.6795717. [DOI] [PubMed] [Google Scholar]
- 73.Sutherland RJ, McDonald RJ, Savage DD. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus. 1997;7:232–238. doi: 10.1002/(SICI)1098-1063(1997)7:2<232::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 74.Sutherland RJ, O’Brien J, Lehmann H. Absence of systems consolidation of fear memories after dorsal, ventral, or complete hippocampal damage. Hippocampus. 2008;18:0–8. doi: 10.1002/hipo.20431. [DOI] [PubMed] [Google Scholar]
- 75.Thomas JD, Sather TM, Whinery LA. Voluntary exercise influences behavioral development in rats exposed to alcohol during the neonatal brain growth spurt. Behav Neurosci. 2008;122:1264–1273. doi: 10.1037/a0013271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas JD, Wasserman EA, West JR, Goodlett CR. Behavioral deficits induced by bingelike exposure to alcohol in neonatal rats: importance of developmental timing and number of episodes. Dev Psychobiol. 1996;29:433–452. doi: 10.1002/(SICI)1098-2302(199607)29:5<433::AID-DEV3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 77.Tran TD, Kelly SJ. Critical periods for ethanol-induced cell loss in the hippocampal formation. Neurotoxicol Teratol. 2003;25:519–528. doi: 10.1016/s0892-0362(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 78.Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: Reversal by choline. Behavioral Neuroscience. 2006;120:482–487. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- 79.Weeber EJ, Savage DD, Sutherland RJ, Caldwell KK. Fear conditioning-induced alterations of phospholipase C-beta1a protein level and enzyme activity in rat hippocampal formation and medial frontal cortex. Neurobiol Learn Mem. 2001;76:151–182. doi: 10.1006/nlme.2000.3994. [DOI] [PubMed] [Google Scholar]
- 80.Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure reduces spine density without affecting dendritic morphology in rat mPFC. Synapse. 2008:566–573. doi: 10.1002/syn.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsych Soc. 2008;14:1022–1033. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]
- 82.Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26:5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Woodruff-Pak DS, Green JT, Levin SI, Meisler MH. Inactivation of sodium channel Scn8A (Na-sub(v)1.6) in Purkinje neurons impairs learning in Morris water maze and delay but not trace eyeblink classical conditioning. Behav Neurosci. 2006;120:229–240. doi: 10.1037/0735-7044.120.2.229. [DOI] [PubMed] [Google Scholar]