Abstract
YWHAH is a positional and functional candidate gene for both schizophrenia and bipolar disorder (BP). This gene has been previously shown to be associated with both disorders, and the chromosome location (22q12.3) has been repeatedly implicated in linkage studies for these disorders. It codes for the η subtype of the 14-3-3 protein family, is expressed mainly in brain, and is involved in HPA axis regulation. We investigated the association of YWHAH with BP in a large sample, consisting of 1211 subjects from 318 nuclear families including 554 affected offspring. We tested for association with the standard BP phenotype as well as subtypes defined by psychotic and mood-incongruent features. We genotyped five tag SNPs and the (GCCTGCA)n polymorphic locus present in this gene. Using a family-based association test, we found that rs2246704 was associated with BP (OR 1.31, P = 0.03) and psychotic BP (OR = 1.66, P = 0.002). The polymorphic repeat and two other SNPs were also modestly associated with psychotic BP. We have provided additional evidence for association of variants in YWHAH with major mental illness. Additional association analyses of larger sample sets will be required to clarify the role of YWHAH in schizophrenia and BP. The use of clinical sub-phenotypes such as psychotic features or other potential schizophrenia/BP overlap variables including cognitive abnormalities and poor functioning might shed further light on the potential subtypes of illness most closely associated with genetic variation in YWHAH.
Introduction
The 14-3-3 protein family is highly conserved and consists of seven subtypes (α, β, γ, δ, ε, ζ, and η) encoded by five separate genes [Ichimura et al., 1988]. Although genes belonging to this family are mainly expressed in the brain, low expression levels are also detected in other tissues [Boston et al., 1982]. These enzymes, which activate tyrosine and tryptophan hydroxylases, catalyze the initial and rate limiting step in the biosynthesis of catecholamines and serotonin. The η (or “eta”) subtype of this protein family is encoded by the YWHAH gene, also known as tyrosine 3/tryptophan 5-monooxygenase [Ichimura et al., 1988]. The gene is ~13 kb long with only two exons separated by a single intron. The protein product, 14-3-3 eta, has a number of regulatory functions including acting as a positive regulator in the glucocorticoid signal pathway by blocking the degradation of the glucocorticoid receptor, thus inducing its elevation and enhancing its transcriptional activity [Kim et al., 2005].
YWHAH is located on chromosome 22q12.3, a region which has been implicated by linkage studies in both BP and schizophrenia. In BP, Kelsoe et al. 2001 found a genome-wide significant signal, while investigators using samples from the National Institute of Mental Health (NIMH) Genetics Initiative, and the Clinical Neurogenetics pedigrees reported suggestive evidence of linkage [Edenberg et al., 1997; Detera-Wadleigh et al., 1999]. We found that BP families in which multiple members had psychotic features showed suggestive evidence of linkage to 22q12.3 [Potash et al., 2003]. The region was first implicated in major mental illness susceptibility through linkage studies of schizophrenia [Pulver et al., 1994; Gill et al., 1996]. Interestingly, a meta-analysis of 18 genome scans in schizophrenia samples along with 11 BP scans, 22q12 emerged as the strongest linkage region in the combined data [Badner and Gershon, 2002]. Subsequent meta-analyses, however, have differed in their findings [Lewis et al., 2003; Segurado et al., 2003; McQueen et al., 2005].
Several variants in YWHAH have been implicated in major mental illness susceptibility. A 7-bp repeat polymorphism (GGCTGCA) in the 5′-untranslated region (UTR) of this gene was associated with schizophrenia in a Japanese population [Toyooka et al., 1999]. However, a subsequent study using a European population failed to replicate the finding, though nominal association was reported in a 5′ UTR SNP rs11539379 [Bell et al., 2000]. Wong et al. reported nominally significant association with schizophrenia for the 7-bp repeat and a 3′ UTR SNP, rs1049583 [Wong et al., 2003]. This SNP was negative in a schizophrenia association study in a Chinese population [Duan et al., 2005]. YWHAH also showed moderately suggestive association with BP in a family-based association study done using an Ashkenazi Jewish population [Fallin et al., 2005]. Out of the eight SNPs genotyped in that study three—rs2858750, rs2853884, and rs2853887—showed evidence of association (Ann Pulver, personal communication).
We have designed a family-based association study to further test whether variation in the YWHAH gene confers susceptibility to BP generally or to subtypes of illness defined by psychotic features or mood-incongruent psychotic features. We genotyped five SNPs and the previously studied 7-bp polymorphic repeat.
Materials and Methods
We selected 1,211 subjects from 318 nuclear families for genotyping. They constituted all available independent trios and quads from subjects originally ascertained in three BP family studies: the Chicago, Hopkins, NIMH Intramural Program (CHIP) study [McInnis et al., 2003]; the Clinical Neurogenetics (CNG) study [Detera-Wadleigh et al., 1999]; and the NIMH Genetics Initiative BP Collaborative (NIMH) study [1997]. Description of the ascertainment and case assessment can be found in the initial study reports. All subjects signed IRB approved informed consent forms prior to enrolling in the studies.
In the current study, cases were diagnosed with either bipolar I disorder (BPI) (N = 491), schizoaffective disorder bipolar type (SABP) (N = 24), or bipolar II disorder (BPII) (N = 39). The 318 pedigrees yielded 554 affected offspring, consisting of 257 quads and 61 trios. Our standard BP phenotype sample had 80% power to detect evidence of association for a locus of moderate effect (OR = 1.4) over a wide range of disease allele frequencies (0.2–0.6), an additive model, a disease prevalence of 1%, and α = 0.05. We had >80% power to detect larger effects of ~1.5 in the psychotic subset, and ~2.0 in the mood-incongruent psychotic subset under comparable assumptions.
Both the CHIP (71 families) and the CNG (11 families) studies were ascertained for multiplex families and primarily used the Schedule for Affective Disorders and Schizophrenia-Lifetime Version (SADS) (Endicott and Spitzer, 1978) and Research Diagnostic Criteria (RDC) [Spitzer et al., 1978] for assessment and diagnosis. The NIMH studies (236 families) used the Diagnostic Interview for Genetic Studies (DIGS) [Nurnberger et al., 1994] and Diagnostic and Statistical Manual (DSM) III-R or IV diagnostic criteria. Phenotypic analyses of the NIMH and CHIP samples were facilitated by the recently compiled BP Phenome Database [Potash et al., 2007].
Association was tested in the subset of cases with psychotic symptoms and in the smaller subset of cases with mood-incongruent psychotic symptoms. We designated a case as psychotic if they had a lifetime occurrence of auditory or visual hallucinations and/or delusions. Mood-incongruence was defined as the presence of hallucinations or delusions which are inconsistent with depressive or manic themes [Goes et al., 2007]. For the very small CNG sample, psychosis information was not available on a subset of subjects, while mood-incongruent psychosis data was not available at all. In total, of the 554 affected offspring, 341 (61.5%) were classified as psychotic, and of these, 104 (30.4%) had mood-incongruent psychotic features.
Tag SNPs spanning the YWHAH gene were selected using the QuickSNP web server utility available at http://bioinformoodics.jhmi.edu/quickSNP.pl [Grover et al., 2007]. With the tagging conditions chosen to allow dense coverage (r2 = 0.9, minor allele frequency = 0.05), six tag SNPs were selected from 12 YWHAH SNPs present in the HapMap phase II dataset. These six SNPs (rs2853884, rs2246704, rs7291050, rs933226, rs3747158, rs4820059) were genotyped using an ABI 7900HT and TaqMan assays, with call rates exceeding 99%. We checked duplicates on each genotyping plate as quality controls, and no discrepancies were found between them.
Mendelian errors were identified and removed by the FBAT software program [Horvath et al., 2001]. A total of 15 of them were found in 12 pedigrees, and genotypes in each pedigree were set to zero for analysis. After cleaning the data, FBAT was used to test for association against the null hypothesis of no association and no linkage. We tested single marker association under additive and dominant models. Haplotypes were tested with 3-, 4-, and 5-marker sliding windows under additive and dominant models, dropping individual haplotypes with a frequency of <5%. Association was tested with the psychotic and mood-incongruent psychotic phenotypes by limiting the affection status to only those subjects with the phenotype of interest. Covariate analysis was also performed using the Transmission Disequilibrium Test (TDT) as implemented in STATA 8.0 using an adaptation of the gtrr function made available by David Clayton, which was modified to include covariates (http://www.gene.cimr.cam.ac.uk/clayton/software/stata, modified by M. Daniele Fallin and K. Lasseter). We formally tested for differences across sub-groups by fitting to the entire sample a conditional logistic regression model that included a term for the covariate by genotype interaction. Likelihood ratio tests (LRTs) were then used to test whether the model including the interaction term provided a better fit to the data than a model without the interaction term, suggesting heterogeneity in the observed association. This same program was also used to determine allelic odds ratios.
To assess the size of the polymorphic repeat in the 5′-UTR region of YWHAH, Genescan analysis was performed using the Applied Biosystems 3100 Genetic Analyzer. PCR primers were designed to amplify a 205 bp region containing the polymorphic locus (forward primer: TCTCCTCCCTCGGCGTTGTCCG and reverse primer: GCCTCACACCGCTGGCTCGCTAG). The forward primer was fluorescently labeled with FAM fluorochrome at its 5′ end, ROX500 was used as a size marker, and the results were analyzed using GeneScan and Genotyper software version 3.7. We also resequenced this region on an Applied Biosystems 3100 Genetic Analyzer. DNA sequences were aligned with NCBI database reference sequences (Build 36.1) using the Vector NTI Advance™ multiple alignment tool.
Results
We successfully genotyped five out of the six tag SNPs selected for the gene (Fig. 1). The SNP rs7291050, for which the genotyping experiment did not work, is in high LD with one of the successfully genotyped SNPs, rs2853884 (r2 = 0.84). The allelic transmissions for the five SNPs, using both additive and dominant genetic models, were analyzed with FBAT. Results are shown in Table I.
FIG. 1.
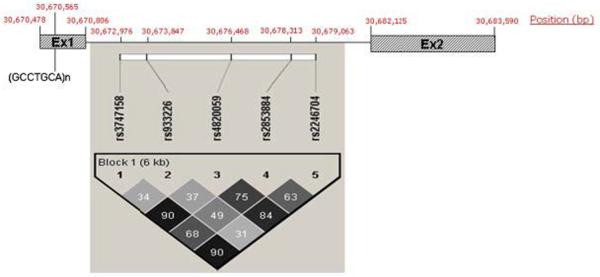
Graphical representation of the YWHAH gene with physical location of exons, intron, five genotyped SNPs, and a seven base pair (bp) repeat indicated in bp from the p-telomere. In addition, an LD plot generated by HAPLOVIEW of the tag SNPs is shown with r2 values given for each pair-wise calculation.
TABLE I.
Allelic Association Analysis for 5 Tag SNPs in the YWHAH Gene
| Standard BP |
Psychotic BP |
Mood-incongruent psychotic BP |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Major allele | Minor allele | Major allele | Minor allele | Major allele | Minor allele | |||||||
|
|
|
|
||||||||||
| Marker | Additive | Dominant | Additive | Dominant | Additive | Dominant | Additive | Dominant | Additive | Dominant | Additive | Dominant |
| rs2853884 | ||||||||||||
| S:E(S) | 406:413 | 101:100 | 278:271 | 179:171 | 251:261.5 | 60:61.5 | 179:168.5 | 117:108 | 83:79.5 | 20:18 | 47:50.5 | 31:32.5 |
| P-value | 0.51 | 0.86 | 0.51 | 0.33 | 0.21 | 0.73 | 0.21 | 0.17 | 0.44 | 0.40 | 0.44 | 0.68 |
| rs2246704 | ||||||||||||
| S:E(S) | 396:408 | 151:147 | 370:357 | 189:172 | 243:261 | 93:91.7 | 239:221 | 131:111.7 | 83:82.5 | 31:28.5 | 71:71.5 | 36:34 |
| P-value | 0.27 | 0.57 | 0.27 | 0.03 | 0.04 | 0.82 | 0.04 | 0.002 | 0.92 | 0.42 | 0.92 | 0.57 |
| rs933226 | ||||||||||||
| S:E(S) | 362:358 | 51:13.5 | 166:170 | 45:60.5 | 224:225 | 31:33 | 131:111.7 | 108:107 | 75:72.5 | 9:9.5 | 31:33.5 | 26:29 |
| P-value | 0.66 | 0.79 | 0.66 | 0.52 | 0.89 | 0.50 | 0.89 | 0.87 | 0.54 | 0.75 | 0.54 | 0.39 |
| rs3747158 | ||||||||||||
| S:E(S) | 386:391.5 | 142:140.2 | 346:340.5 | 173:165.7 | 229:241.5 | 89:88.5 | 219:206.5 | 119:106 | 75:76 | 28:27.5 | 67:66 | 34:32.7 |
| P-value | 0.62 | 0.80 | 0.62 | 0.35 | 0.16 | 0.93 | 0.16 | 0.03 | 0.84 | 0.93 | 0.84 | 0.71 |
| rs4820059 | ||||||||||||
| S:E(S) | 401:409 | 143:137.7 | 337:329 | 191:177.7 | 238:254 | 88:87.2 | 220:204 | 129:112.2 | 76:76.5 | 28:26.5 | 64:63.5 | 35:33.5 |
| P-value | 0.47 | 0.43 | 0.47 | 0.10 | 0.07 | 0.89 | 0.07 | 0.008 | 0.91 | 0.67 | 0.91 | 0.61 |
Using the standard BP phenotype, only rs2246704 showed nominal significance (OR = 1.31, P = 0.03) under the dominant model. Using the psychotic BP phenotype, three SNPs were nominally significant under the dominant model; rs2246704 (OR = 1.66, P = 0.002), rs3747158 (OR = 1.46, P = 0.03) and rs4820059 (OR = 1.52, P = 0.008). Using the additive model and psychotic BP phenotype, a P-value of 0.04 (OR = 1.25) was obtained for rs2246704. For all significant transmissions, over-transmission of the minor allele was observed. None of the SNPs showed any difference between observed and expected allelic transmissions when the mood-incongruent psychotic BP phenotype was used. The results for genotypic transmission were similar to those for allelic transmission; for rs2246704, P = 0.03 (standard BP), P = 0.002 (psychotic BP); for rs3747158 P = 0.03 (psychotic BP) and for rs4820059, P = 0.008 (psychotic BP). No haplotype association result was as strong as the individual SNP results (data not shown).
Genescan analysis revealed three alleles of the GCCTGCA polymorphic repeat in our sample set, which we refer to here as alleles 1, 2, and 3, corresponding to two, three and four copies of this repeat sequence, respectively. The prevalence of these alleles was 39.9% (allele 1), 59.9% (allele 2), and ~0.2% (allele 3). The allele frequencies observed here were similar to those observed by Toyooka et al., but different from those of Bell et al. who found allele 1 as the rare allele rather than allele 3. To address this discrepancy, we confirmed our results by DNA sequencing. We performed a family-based association test to find differences between observed and expected allelic transmission for this locus using standard, psychotic and mood-incongruent BP phenotypes (Table II). We found moderate over-transmission of allele 1 under both additive (P = 0.04) and dominant (P = 0.01) genetic models, but only under the psychotic BP phenotype.
TABLE II.
Allelic Association Analysis for the (GCCTGCA)n Polymorphic Repeat in the 5′ UTR of YWHAH
| Standard BP |
Psychotic BP |
Mood-incongruent psychotic BP |
||||
|---|---|---|---|---|---|---|
| Additive | Dominant | Additive | Dominant | Additive | Dominant | |
| Allele 1a | ||||||
| S:E(S) | 333:321 | 181:169 | 214:197 | 121:106 | 63:62 | 33:32 |
| P-value | 0.29 | 0.14 | 0.04 | 0.01 | 0.83 | 0.77 |
| Allele 2a | ||||||
| S:E(S) | 383:394 | 135:135 | 220:237 | 86:88 | 73:73 | 27:26.5 |
| P-value | 0.30 | 1.00 | 0.05 | 0.70 | 0.92 | 0.93 |
Another allele with three copies of the 7-bp repeat was also observed, but FBAT analysis could not be performed since the number harboring that allele was insufficient.
To formally test the impact of the psychotic features and mood-incongruent psychotic features variables, a genotypic TDT was calculated for each of these five SNPs, using psychosis and mood-incongruence as covariates. Results are shown in Table III. Three SNPs had evidence of association with BP that was impacted by the psychotic features covariate, namely rs3747158 (P = 0.01), rs4820059 (P = 0.03), and rs2246704 (P = 0.02). In addition, the 7-bp repeat was also nominally positive while using psychotic features as an interaction term (P = 0.03). However, neither the repeat nor any of the SNPs were impacted by the mood-incongruence covariate.
TABLE III.
Impact of Psychotic Features and Mood-Incongruent Psychotic Features on Genotypic Association
| LRT P-valuea |
||
|---|---|---|
| Polymorphism | Psychotic features | Mood-incongruent psychotic features |
| (GCCTGCA)n | 0.03 | 0.68 |
| rs3747158 | 0.01 | 0.82 |
| rs93322G | 0.44 | 0.82 |
| rs4820059 | 0.03 | 0.76 |
| rs2853884 | 0.43 | 0.38 |
| rs2246704 | 0.02 | 0.30 |
The LRT P-value reflects the comparison between the evidence for association for each SNP and bipolar disorder with and without the presence of the covariate. Analyses were done under a dominant model.
Discussion
We genotyped five tag SNPs from the YWHAH gene in a sample set consisting of 1,211 subjects from 318 nuclear families. We found that rs2246704 was associated with BP under the standard phenotype, while under the psychotic phenotype this SNP showed stronger nominal association, and two additional SNPs, rs3747158 and rs4820059, also showed evidence of association. We further validated the significance of the psychotic subtype by performing covariate analysis using psychosis as the interaction term; all three positive SNPs were shown to be impacted by this covariate with moderate significance. We also found a 7-bp polymorphic repeat in the 5′-UTR region to be moderately associated with the psychotic BP phenotype (P = 0.01).
This study is consistent with that of Fallin et al., who also found a moderate level of association for this gene with BP using 323 Ashkenazi Jewish families. The three associated SNPs in that study were in perfect LD with each other and also in substantial LD (r2 = 0.56) with our best SNP, rs2246704. Additionally, our associated SNP rs4820059 is in LD (r2 = 0.66) with the Wong et al. schizophrenia-associated SNP, rs1049583 [Wong et al., 2003]. Modest association of YWHAH with BP was also seen in the genome-wide association study conducted by Baum et al. 2008. The finding was observed in the “NIMH sample” consisting of 461 unrelated bipolar I probands as cases and 563 unrelated controls. The associated SNP in this case–control GWA study was the SNP rs2246704 (P = 0.015), which is also the best finding in our family-based study. Further, in both studies, over-transmission of the minor allele to cases was observed. We note that there was substantial overlap between that sample and ours, with about half of our probands being cases in that study. Neither the Baum et al. German sample, the Wellcome Trust Case Control Consortium sample 2007, nor the STEP-BD sample [Sklar et al., 2008] showed evidence of association between YWHAH and BP. Coverage of the gene was limited, however, with only 33% on the Illumina HumanHap550 chip and 50% on the Affymetrix 5.0. Even the Affymetrix 6.0 chip (containing ~900,000 SNPs), which is being used in several genome-wide association studies, captures only 60% of the common variation in this gene.
Our study adds to the evidence that YWHAH is implicated in both BP and schizophrenia. This is the first study to find association of the (GCCTGCA)n repeat with the psychotic BP phenotype, which was previously found to be associated with schizophrenia [Toyooka et al., 1999]. Our results are consistent with this finding as we also observed over-transmission of allele 1 to cases.
The Stanley Medical Research Institute online genomics database (SMRIDB) shows significant under-expression of YWHAH in BP postmortem brain samples (P = 0.018) based on data from 12 controlled studies across six different microarray platforms [Higgs et al., 2006]. Several reports have also shown significant downregulation of YWHAH mRNA in schizophrenia brains [Vawter et al., 2001; Middleton et al., 2005], though others have failed to replicate this [Wong et al., 2005]. While speculative, it is intriguing to note the finding that the protein encoded by another 14-3-3 gene, YWHAE, binds to the strong schizophrenia candidate gene DISC1 in a complex that influences axon elongation, suggesting a potential mechanism through which variation in YWHAH might also act [Taya et al., 2007].
The advantages of this study are, firstly, the thorough coverage of the gene with selection of tag SNPs using the HapMap II database. Secondly, because we used a family-based study design, our results are immune to the issues of population stratification.
However, this study should also be considered in light of several limitations; for example, family-based designs may be more vulnerable to issues arising from genotyping error. However, since our study replicates previous findings, and our best finding (rs2246704) is consistent with the results of a genome-wide association analysis, it seems less likely that genotyping errors have influenced the results. The modest P-values obtained for association do withstand Bonferroni correction for the number of markers used and the multiple phenotypes used (6 × 3 = 18; P = 0.002 × 18 = 0.036). However, additional tests were also done, including additive versus dominant, allelic versus genotypic, and single-marker versus haplotypic tests. Because the SNPs are in high LD with each other, and the tests are highly correlated, the “correct” P-value adjustment is not clear. We add that since these tests were carried out to examine a prior hypothesis, the appropriate P-value threshold may be lower than it would be otherwise. An additional concern is that the potential risk conferred by rare variants within YWHAH was not tested in the present study, since the minor allele frequency cutoff used was 0.05.
Additional association results in large samples will be required to clarify the role of YWHAH in schizophrenia and BP. The use of clinical phenotypes such as psychosis or other potential overlap variables including cognitive abnormalities and poor functioning might shed further light on the potential subtypes of illness most closely associated with genetic variation in YWHAH. Functional studies could also help to elucidate the mechanisms through which YWHAH might influence vulnerability to psychiatric illness.
Acknowledgements
This work was supported by grants from the NIMH (R01 MH-042243 [JBP] and R01 MH-061613 [ESG]), the National Alliance for Research on Schizophrenia and Depression, and the Stanley Medical Research Institute, and by the NIMH Intramural Research Program (FJM). VLW and JBP were also supported by Margaret Price Investigatorships. We are grateful to Y. Huo, K. Miao, and B. Craighead for their contributions, and to Ann Pulver for making genotyping results available to us. Some DNA samples were prepared and distributed by The Rutgers University Cell and DNA Repository under a contract from the NIMH. We are also grateful to the many interviewers and diagnosticians who contributed to this project, and to the families who devoted their time and effort to the study. The BP Phenome Group consists of F.J. McMahon, J. Steele, J. Pearl, L. Kassem, V. Lopez and T. Schulze from the Genetic Basis of Mood and Anxiety Disorders Unit, Mood and Anxiety Program, National Institute of Mental Health, National Institutes of Health, Bethesda, MD; J. Potash, D. MacKinnon, E. Miller, J. Toolan from the Department of Psychiatry, Johns Hopkins School of Medicine, Baltimore, MD; P. Zandi from the Department of Mental Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD; S. Simpson from the Department of Psychiatry, University of Colorado at Denver, Denver, CO. Some of the data and biomaterials were collected in four projects that participated in the NIMH BP Genetics Initiative from 1991 to 1998. The Principal Investigators and Co-Investigators were: Indiana University, Indianapolis, IN, U01 MH46282, J. Nurnberger, M.D., Ph.D., M. Miller, M.D., and E. Bowman, M.D.; Washington University, St. Louis, MO, U01 MH46280, T. Reich, M.D., A. Goate, Ph.D., and J. Rice, Ph.D.; Johns Hopkins University, Baltimore, MD, U01 MH46274, J.R. DePaulo Jr., M.D., S. Simpson, M.D., MPH, and C. Stine, Ph.D.; NIMH Intramural Research Program, Clinical Neurogenetics Branch, Bethesda, MD, E. Gershon, M.D., D. Kazuba, B.A., and E. Maxwell, M.S.W. Other data and biomaterials were collected in ten NIMH BP Genetics Initiative projects from 1999 to 2003. The Principal Investigators and Co-Investigators were: Indiana University, Indianapolis, IN, R01 MH59545, J. Nurnberger, M.D., Ph.D., M.J. Miller, M.D., E.S. Bowman, M.D., N.L. Rau, M.D., P.R. Moe, M.D., N. Samavedy, M.D., R. El-Mallakh, M.D. (at University of Louisville), H. Manji, M.D., D.A. Glitz, M.D. (at Wayne State University), E.T. Meyer, M.S., C. Smiley, R.N., T. Foroud, Ph.D., L. Flury, M.S., D.M. Dick, Ph.D., H. Edenberg, Ph.D.; Washington University, St. Louis, MO, R01 MH059534, J. Rice, Ph.D., T. Reich, M.D., A. Goate, Ph.D., L. Bierut, M.D.; Johns Hopkins University, Baltimore, MD, R01 MH59533, M. McInnis M.D., J.R. DePaulo Jr., M.D., D.F. MacKinnon, M.D., F.M. Mondimore, M.D., J.B. Potash, M.D., P.P. Zandi, Ph.D., D. Avramopoulos, Ph.D. and J. Payne, M.D.; University of Pennsylvania, PA, R01 MH59553, W. Berrettini M.D., Ph.D.; University of California at Irvine, CA, R01 MH60068, W. Byerley M.D., and M. Vawter M.D.; University of Iowa, IA, R01 MH059548, W. Coryell M.D., and R. Crowe M.D.; University of Chicago, IL, R01 MH59535, E. Gershon, M.D., J. Badner Ph.D., F. McMahon M.D., C. Liu Ph.D., A. Sanders M.D., M. Caserta, S. Dinwiddie M.D., T. Nguyen, D. Harakal; University of California at San Diego, CA, R01 MH59567, J. Kelsoe, M.D., R. McKinney, B.A.; Rush University, IL, R01 MH059556, W. Scheftner M.D., H.M. Kravitz, D.O., M.P.H., D. Marta, B.S., A. Vaughn-Brown, MSN, RN, and L. Bederow, MA; NIMH Intramural Research Program, Bethesda, MD, 1Z01MH002810-01, F. McMahon, M.D., L. Kassem, PsyD, S. Detera-Wadleigh, Ph.D., L. Austin, Ph.D., D.L. Murphy, M.D.
References
- Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry. 2002;7(4):405–411. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B, Schulze TG, Cichon S, Rietschel M, Nothen MM, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008;13(2):197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R, Munro J, Russ C, Powell JF, Bruinvels A, Kerwin RW, Collier DA. Systematic screening of the 14-3-3 eta (eta) chain gene for polymorphic variants and case-control analysis in schizophrenia. Am J Med Genet. 2000;96(6):736–743. [PubMed] [Google Scholar]
- Boston PF, Jackson P, Thompson RJ. Human 14-3-3 protein: Radioimmunoassay, tissue distribution, and cerebrospinal fluid levels in patients with neurological disorders. J Neurochem. 1982;38(5):1475–1482. doi: 10.1111/j.1471-4159.1982.tb07928.x. [DOI] [PubMed] [Google Scholar]
- Detera-Wadleigh SD, Badner JA, Berrettini WH, Yoshikawa T, Goldin LR, Turner G, Rollins DY, Moses T, Sanders AR, Karkera JD, et al. A high-density genome scan detects evidence for a bipolar-disorder susceptibility locus on 13q32 and other potential loci on 1q32 and 18p11.2. Proc Natl Acad Sci USA. 1999;96(10):5604–5609. doi: 10.1073/pnas.96.10.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Gao R, Xing Q, Du J, Liu Z, Chen Q, Wang H, Feng G, He L. A family-based association study of schizophrenia with polymorphisms at three candidate genes. Neurosci Lett. 2005;379(1):32–36. doi: 10.1016/j.neulet.2004.12.040. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Foroud T, Conneally PM, Sorbel JJ, Carr K, Crose C, Willig C, Zhao J, Miller M, Bowman E, et al. Initial genomic scan of the NIMH genetics initiative bipolar pedigrees: Chromosomes 3,5,15,16,17, and 22. Am J Med Genet. 1997;74(3):238–246. [PubMed] [Google Scholar]
- Endicott J, Spitzer RL. A diagnostic interview: The schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35(7):837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Fallin MD, Lasseter VK, Avramopoulos D, Nicodemus KK, Wolyniec PS, McGrath JA, Steel G, Nestadt G, Liang KY, Huganir RL, et al. Bipolar I disorder and schizophrenia: A 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am J Hum Genet. 2005;77(6):918–936. doi: 10.1086/497703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill M, Vallada H, Collier D, Sham P, Holmans P, Murray R, McGuffin P, Nanko S, Owen M, Antonarakis S, et al. A combined analysis of D22S278 marker alleles in affected sib-pairs: Support for a susceptibility locus for schizophrenia at chromosome 22q12. Schizophrenia Collaborative Linkage Group (Chromosome 22) Am J Med Genet. 1996;67(1):40–45. doi: 10.1002/(SICI)1096-8628(19960216)67:1<40::AID-AJMG6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Goes FS, Zandi PP, Miao K, McMahon FJ, Steele J, Willour VL, Mackinnon DF, Mondimore FM, Schweizer B, Nurnberger JI, Jr, et al. Mood-incongruent psychotic features in bipolar disorder: Familial aggregation and suggestive linkage to 2p11-q14 and 13q 21–33. Am J Psychiatry. 2007;164(2):236–247. doi: 10.1176/ajp.2007.164.2.236. [DOI] [PubMed] [Google Scholar]
- Grover D, Woodfield AS, Verma R, Zandi PP, Levinson DF, Potash JB. QuickSNP: An automated web server for selection of tagSNPs. Nucleic Acids Res. 2007;35(Web Server issue):W115–120. doi: 10.1093/nar/gkm329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs BW, Elashoff M, Richman S, Barci B. An online database for brain disease research. BMC Genomics. 2006;7:70. doi: 10.1186/1471-2164-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Xu X, Laird NM. The family based association test method: Strategies for studying general genotype-phenotype associations. Eur J Hum Genet. 2001;9(4):301–306. doi: 10.1038/sj.ejhg.5200625. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Isobe T, Okuyama T, Takahashi N, Araki K, Kuwano R, Takahashi Y. Molecular cloning of cDNA coding for brain-specific 14-3-3 protein, a protein kinase-dependent activator of tyrosine and tryptophan hydroxylases. Proc Natl Acad Sci USA. 1988;85(19):7084–7088. doi: 10.1073/pnas.85.19.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsoe JR, Spence MA, Loetscher E, Foguet M, Sadovnick AD, Remick RA, Flodman P, Khristich J, Mroczkowski-Parker Z, Brown JL, et al. A genome survey indicates a possible susceptibility locus for bipolar disorder on chromosome 22. Proc Natl Acad Sci USA. 2001;98(2):585–590. doi: 10.1073/pnas.011358498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Jang SW, Sung HJ, Lee HJ, Kim IS, Na DS, Ko J. Role of 14-3-3 eta as a positive regulator of the glucocorticoid receptor transcriptional activation. Endocrinology. 2005;146(7):3133–3140. doi: 10.1210/en.2004-1455. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, Williams NM, Schwab SG, Pulver AE, Faraone SV, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder. Part II. Schizophrenia. Am J Hum Genet. 2003;73(1):34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnis MG, Lan TH, Willour VL, McMahon FJ, Simpson SG, Addington AM, MacKinnon DF, Potash JB, Mahoney AT, Chellis J, et al. Genome-wide scan of bipolar disorder in 65 pedigrees: Supportive evidence for linkage at 8q24, 18q22, 4q32, 2p12, and 13q12. Mol Psychiatry. 2003;8(3):288–298. doi: 10.1038/sj.mp.4001277. [DOI] [PubMed] [Google Scholar]
- McQueen MB, Devlin B, Faraone SV, Nimgaonkar VL, Sklar P, Smoller JW, Abou Jamra R, Albus M, Bacanu SA, Baron M, et al. Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q. Am J Hum Genet. 2005;77(4):582–595. doi: 10.1086/491603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Peng L, Lewis DA, Levitt P, Mirnics K. Altered expression of 14-3-3 genes in the prefrontal cortex of subjects with schizophrenia. Neuropsychopharmacology. 2005;30(5):974–983. doi: 10.1038/sj.npp.1300674. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, DePaulo JR, Gershon ES, Reich T, Blehar MC, Edenberg HJ, Foroud T, Miller M, Bowman E, Mayeda A, et al. Genomic survey of bipolar illness in the NIMH genetics initiative pedigrees: A preliminary report. Am J Med Genet. 1997;74(3):227–237. doi: 10.1002/(sici)1096-8628(19970531)74:3<227::aid-ajmg1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 844–863. [DOI] [PubMed] [Google Scholar]
- Potash JB, Zandi PP, Willour VL, Lan TH, Huo Y, Avramopoulos D, Shugart YY, MacKinnon DF, Simpson SG, McMahon FJ, et al. Suggestive linkage to chromosomal regions 13q31 and 22q12 in families with psychotic bipolar disorder. Am J Psychiatry. 2003;160(4):680–686. doi: 10.1176/appi.ajp.160.4.680. [DOI] [PubMed] [Google Scholar]
- Potash JB, Toolan J, Steele J, Miller EB, Pearl J, Zandi PP, Schulze TG, Kassem L, Simpson SG, Lopez V, et al. The bipolar disorder phenome database: A resource for genetic studies. Am J Psychiatry. 2007;164(8):1229–1237. doi: 10.1176/appi.ajp.2007.06122045. [DOI] [PubMed] [Google Scholar]
- Pulver AE, Karayiorgou M, Wolyniec PS, Lasseter VK, Kasch L, Nestadt G, Antonarakis S, Housman D, Kazazian HH, Meyers D, et al. Sequential strategy to identify a susceptibility gene for schizophrenia: Report of potential linkage on chromosome 22q12-q13.1: Part 1. Am J Med Genet. 1994;54(1):36–43. doi: 10.1002/ajmg.1320540108. [DOI] [PubMed] [Google Scholar]
- Segurado R, Detera-Wadleigh SD, Levinson DF, Lewis CM, Gill M, Nurnberger JI, Jr, Craddock N, DePaulo JR, Baron M, Gershon ES, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder. Part III. Bipolar disorder. Am J Hum Genet. 2003;73(1):49–62. doi: 10.1086/376547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K, Nimgaonkar VL, McQueen MB, Faraone SV, Kirby A, et al. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008;13(6):558–569. doi: 10.1038/sj.mp.4002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: Rationale and reliability. Arch Gen Psychiatry. 1978;35(6):773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- Taya S, Shinoda T, Tsuboi D, Asaki J, Nagai K, Hikita T, Kuroda S, Kuroda K, Shimizu M, Hirotsune S, Iwamatsu A, Kaibuchi K. DISC1 regulates the transport of the NUDEL/LIS1/14-3-3epsilon complex through kinesin-1. J Neurosci. 2007;27(1):15–26. doi: 10.1523/JNEUROSCI.3826-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka K, Muratake T, Tanaka T, Igarashi S, Watanabe H, Takeuchi H, Hayashi S, Maeda M, Takahashi M, Tsuji S, et al. 14-3-3 protein eta chain gene (YWHAH) polymorphism and its genetic association with schizophrenia. Am J Med Genet. 1999;88(2):164–167. [PubMed] [Google Scholar]
- Vawter MP, Barrett T, Cheadle C, Sokolov BP, Wood WH, III, Donovan DM, Webster M, Freed WJ, Becker KG. Application of cDNA microarrays to examine gene expression differences in schizophrenia. Brain Res Bull. 2001;55(5):641–650. doi: 10.1016/s0361-9230(01)00522-6. [DOI] [PubMed] [Google Scholar]
- Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AH, Macciardi F, Klempan T, Kawczynski W, Barr CL, Lakatoo S, Wong M, Buckle C, Trakalo J, Boffa E, et al. Identification of candidate genes for psychosis in rat models, and possible association between schizophrenia and the 14-3-3eta gene. Mol Psychiatry. 2003;8(2):156–166. doi: 10.1038/sj.mp.4001237. [DOI] [PubMed] [Google Scholar]
- Wong AH, Likhodi O, Trakalo J, Yusuf M, Sinha A, Pato CN, Pato MT, Van Tol HH, Kennedy JL. Genetic and post-mortem mRNA analysis of the 14-3-3 genes that encode phosphoserine/threonine-binding regulatory proteins in schizophrenia and bipolar disorder. Schizophr Res. 2005;78(2–3):137–146. doi: 10.1016/j.schres.2005.06.009. [DOI] [PubMed] [Google Scholar]


