Abstract
Contextual fear conditioning emerges around post-natal day (PD) 23 in the rat. This is thought to reflect hippocampus-dependent conjunctive learning, which binds the individual features of the context into a unified representation (Rudy, 1993). However, context conditioning can also be supported by hippocampus-independent, feature-based simple associations (Rudy, 2009) and these may operate at PD23–24 (Pugh & Rudy, 1996). To address this issue, we studied the ontogeny of a variant of contextual fear conditioning, termed the context-preexposure-facilitation-effect (CPFE), in which exposure to context and (immediate) foot shock occur on successive occasions. This variant requires conjunctive as opposed to feature-based simple associations (Rudy, 2009). We tested PD17, 24, and 31 rats on the CPFE vs. conventional context conditioning (Exp. 1) and on the CPFE with stronger reinforcement (Exp. 2). The CPFE emerged on PD24 regardless of reinforcer strength and in parallel with context conditioning. Infusions of the NMDA antagonist, MK-801, into the dorsal hippocampus just before preexposure on PD24 eliminated the CPFE, whereas infusions occurring after preexposure had no effect (Exp. 3). These findings demonstrate a role of hippocampal NMDA receptors in the CPFE as early as PD24 and implicate conjunctive learning mechanisms in the ontogeny of contextual fear conditioning.
Keywords: Hippocampus, Amygdala, Context, Memory, Infant rat, Development
Pavlovian fear conditioning has become a powerful paradigm for studying both the neurobiology of learning (Fanselow & Poulos, 2005; Kim & Fanselow, 1992; Kim et al., 1993; Maren, 2001; Phillips & LeDoux, 1992) and the ontogeny of learning (Hunt & Campbell, 1997; Richardson & Hunt, 2010; Rudy, 1992). This is especially true of contextual fear conditioning, because of its potential value for studying the ontogeny of hippocampal function from a multiple memory systems perspective (Fanselow & Rudy, 1998; Rudy, 1993, 2009; Rudy & O’Reilly, 1999; Stanton, 2000).
In the standard context conditioning procedure, rats are placed in a test chamber for a few minutes and then experience a brief foot-shock. When returned to the chamber the next day, they show a species-typical freezing response that reflects conditioned fear (e.g., Fanselow, 1990). This behavior is explained by a two-process model in which two distinct and competing associative systems can mediate contextual fear conditioning (Fanselow, 2000; Maren, 2001; Rudy, 2009; Rudy & O’Reilly, 2001; Rudy et al., 2004). Both associative systems represent the context in manner that can support the association with the shock. The first is a feature-based system where individual sensory elements of the context are each independently associated with the US and their additive strength determines conditioned fear in a subsequent test (e.g., Rudy & O’Reilly, 2001). The other associative system binds the separate features of the context into a new conjunctive representation. This representation is thought to depend on the hippocampal system (Rudy, 2009) and has also been referred to as a cognitive map (O’Keefe & Nadel, 1978), a unitary (Fanselow, DeCola, & Young, 1993) or unified (Anagnostaras et al., 2001), or an integrated “Gestalt” representation (Fanselow, 2000). This system supports rapid learning that automatically occurs simply as a consequence of the rat’s active exploration of the environment (Rudy, 2009; Nadel & Willner, 1980; Nadel et al., 1985). In addition to mediating context-shock associations, the conjunctive system also competitively inhibits the feature-based system so that conjunctive learning normally dominates during the acquisition of contextual fear (Fanselow, 2000; Maren et al., 1997; Rudy & O’Reilly, 2001; Rudy et al., 2004).
This theoretical framework accounts for the finding that anterograde neurotoxic lesions of dorsal hippocampal (DH) neurons do not affect standard contextual fear conditioning, while retrograde lesions produce deficits (e.g., Maren et al., 1997). Anterograde lesions free the feature-based associative system from competitive inhibition by the conjunctive system, allowing acquisition of contextual fear to be based on fear of individual features that comprise the context. However, if the hippocampus is intact during conditioning, feature-based learning is inhibited and retrograde lesions of the hippocampus impair retrieval of the contextual representation acquired solely by the conjunctive system (Fanselow, 2000; Maren et al., 1997, Rudy & O’Reilly, 2001).
There is a variant of contextual fear conditioning, termed the context preexposure facilitation effect (CPFE, Rudy et al., 2004) that selectively engages hippocampus-dependent conjunctive associations while minimizing the role of hippocampus-independent feature-based associations (Rudy, 2009; Rudy & O’Reilly, 2001). In the CPFE, context preexposure is followed a day later by brief chamber re-exposure and foot shock (Fanselow, 1986). This results in substantial conditioned freezing to the context in a subsequent test, relative to control rats that do not receive context preexposure (which show the immediate shock deficit, Fanselow, 1986). The CPFE occurs when rats are preexposed to various features of the training context in combination on a single occasion, but not when preexposure to the same features occurs on separate occasions, precluding their conjunctive representation (Rudy & O’Reilly, 1999). Thus, the CPFE depends critically on conjunctive associations (Fanselow, 2000; Rudy & O’Reilly, 1999; Rudy et al., 2004). The CPFE depends on hippocampal plasticity during the preexposure phase (Stote & Fanselow, 2004; Matus-Amat et al., 2007) and on hippocampal function during the training and test phases as well (Matus-Amat et al., 2004). Disruption of the CPFE by anterograde DH cell-body lesions (Rudy, Barrientos, O’Reilly, 2002) or muscimol infusion (Matus-Amat et al., 2004) indicates that, in contrast to conventional context conditioning, hippocampus-independent feature-based associations alone cannot support contextual fear conditioning in the CPFE paradigm (Rudy, 2009).
Auditory-cue and standard contextual fear conditioning dissociate during development. The emergence of auditory fear conditioning by PD 16–18, followed by contextual fear conditioning around PD 23, has been attributed to the ongoing maturation of the hippocampus and its cognitive functions (Dumas & Rudy, 2010; Burman, Murawski, Schiffino, Rosen, & Stanton, 2009; Rudy, 1993; Rudy & Morledge, 1994; Stanton, 2000). However, because standard context conditioning can be mediated by elemental learning processes when the hippocampus is not engaged, it remains unclear whether or not the ontogenetic emergence of context conditioning reflects the development of hippocampus-dependent conjunctive learning. Although spatial maze learning in the rat is generally absent or weak during the preweanling period (PD 16–20; summarized in Stanton, 2000, Table 1), there are many reports of context learning, or contextual modulation of learning, during this period, particularly when salient elemental stimuli such as odors, flashing lights, black vs. white walls, etc. are used to define the “context” (see findings and literature cited in Brasser & Spear, 2004; Esmoris-Arranz, Mendez & Spear, 2008). Indeed, attempts to determine whether context conditioning depends on conjunctive vs. feature-based learning suggest that it is feature-based in PD 18 and 23 rats (Pugh & Rudy, 1996).
A potentially important limitation of this previous work is that the training phase involved standard context conditioning (minutes of context exposure followed by shock) rather than immediate shock and this increases the potential role of feature-based conditioning processes. In contrast, conditioning with an immediate shock can only be supported by pattern completion processes of the hippocampus that operate to retrieve the preexposure memory (Rudy, 2009). Another limitation of this previous work is that the neurobiological literature on the CPFE (reviewed above) is based on immediate shock, making it hazardous to relate this literature to behavioral studies in developing rats involving delayed shock. Finally, none of this work has directly examined the role of hippocampal function in context conditioning.
Recently, this lab has shown that PD 23 rats demonstrate the CPFE when immediate shock is used during the training phase (Burman et al., 2009). We also showed that this CPFE is disrupted by systemic administration of the NMDA-receptor antagonist, MK-801, prior to the preexposure phase. This finding suggests that context learning that unequivocally depends on the hippocampus in adult rats emerges by PD23 and depends on NMDA receptor systems in the developing rat. The present study extended this finding by examining the ontogenetic profile of the CPFE in 17-, 24-, and 31-day-old rats, and by comparing this profile with standard contextual fear conditioning (Experiment 1), by re-examining the ontogeny of the CPFE with stronger reinforcement (Experiment 2), and by determining whether the CPFE depends on dorsal hippocampal NMDA receptors (Experiment 3). The findings demonstrate that the CPFE emerges between PD17 and 24 and depends on NMDA-receptor-mediated plasticity in dorsal hippocampus. The ontogeny of the CPFE is discussed in relation to current models of the neurobiology of context learning (Rudy, 2009).
Experiment 1
Conventional contextual fear conditioning emerges between PD 17 and PD 23 (Rudy, 1993; Rudy & Morledge, 1994; Stanton, 2000). Experiment 1 used identical procedural and parametric conditions to directly compare the ontogeny of the CPFE with that of conventional contextual fear conditioning across this same developmental period. At PD 17, 24, or 31, rats were exposed to either the training context (Pre) or an alternate context (No Pre) for 5 minutes. The following day rats received either an immediate shock or a shock delivered after a 120s placement-to-shock interval (PSI). Conditioning levels were assessed 24 hours later by exposing all groups to the training context for 5 minutes. Thus, a 2 (Pre-exposure: Pre vs. No Pre) × 2 (PSI: Immediate vs. 120s) × 3 (Age: PD 17 vs. PD 23 vs. PD 31) factorial design was used. The primary question of interest was whether the ontogenetic profiles of the CPFE and conventional contextual fear conditioning would be the same or different. If the two forms of context conditioning share common mechanisms, their profiles should be similar. Divergence of their developmental profiles would suggest divergence of underlying mechanisms. For example, standard context conditioning might be stronger at earlier stages of development because non-hippocampal, feature-based processes are engaged in a manner that is precluded in the CPFE (Rudy, 2009).
Methods
The methods for Experiment 1 were as described previously by this lab (Burman, Murawski, Schiffino, Rosen & Stanton, 2009).
Subjects
Subjects were 140 Long Evans weanling rats (69 male, 71 female) that were the offspring of 39 different mothers, bred in the University of Delaware Animal Facility from Harlan Long-Evans stock. Litters were weighed and culled to eight pups (usually 4 male, 4 female) on PD 3, and were weaned on PD 21. Dams were housed with their litters in clear polypropylene containers measuring 8" high × 18" long × 9" wide in a USDA-approved animal facility that was operated according to NIH guidelines. The housing facility was maintained on a 12:12 hour light/dark cycle with lights on at 7 a.m. The age of litters was determined by daily checks during the light part of the cycle. All births occurred on gestational day 22. After weaning from their mothers on PD 21, pups were housed with same sex littermates and continuously supplied with food and water except during actual experimentation. No more than a single same-sex littermate was assigned to a given experimental group.
Apparatus and Stimuli
We have described the apparatus in detail previously (Burman et al., 2009). Four Plexiglas conditioning chambers were situated in a fume hood. The sides and ceilings were clear except for one opaque side wall that prevented rats from seeing each other. Each had a grid floor (0.5-cm diameter bars placed 1.25 cm apart) and the US was a 1.5-mA, 2s, scrambled foot-shock. Pre-exposure occurred either in these chambers or alternate chambers (see Behavioral Procedure). The alternate chambers were wire mesh cages located in an entirely different room in the building and used for eyeblink conditioning (see Brown & Stanton, 2008).
Behavioral Procedure
The CPFE procedure took place in three phases: pre-exposure, training, and testing. Rats were pre-exposed either on PD 17, 24, or 31. Animals were trained the next day (PD 18, 25, or 32) and testing occurred on the day following training (PD 19, 26, or 33). PD 24 and 31 rats were weighed and transferred to individual white plastic cages (24 × 18 × 13 cm) two days before the pre-exposure phase. PD 17 rats remained in their home litter cages. All sessions occurred in the afternoon, beginning between 3:00 and 5:00 pm. Prior to each session, rats were weighed and placed into individual transport containers within an animal colony room situated in the laboratory as described previously (Burman et al., 2009). Rats were carted to a holding area across the hall from the training room and kept in the transport container while the conditioning chambers were cleaned with 5% ammonium hydroxide solution before the initiation of each phase. As a result, the rats spent between 2–4 minutes in the transport container before each phase (1 minute during transport to the holding area, and 1–3 minutes while conditioning chambers were cleaned.
For pre-exposure, rats were run in pairs matched for Pre-exposure and placed for 5 minutes into either the foot-shock chamber where they were to be conditioned the next day (Pre) or the completely separate and alternate context described above (No Pre) to control for handling and exposure to novelty.
For training, rats were transported in pairs matched for Age and PSI. Load order was counterbalanced across Pre-exposure and Sex. Rats in Group Pre were trained in the same chambers they were pre-exposed to the previous day. Rats received a single foot-shock (1.5-mA, 2s duration) immediately upon placement into the chamber or after a 120s delay. To ensure an immediate delivery of shock, animals in the Immediate Group were trained one at a time (approximately 3s placement-to-shock interval). Rats were removed from the chambers as quickly as possible following the foot-shock, returned to their home cages and left for approximately 24 hours until testing.
For testing, rats were run in identical circumstances as for training except that the testing session lasted for 300s and no shock was delivered. All rats were tested in the same chamber they were trained in.
Data Analysis and Statistics
The data were analyzed using FreezeFrame software (Actimetrics, Wilmette, IL) as described previously (Burman et al., 2009). Once percent freezing was determined, the data were imported into Statistica 7 data analysis software for analysis via a 2 (Sex) X 2 (Pre-exposure) X 2 (PSI) X 3 (Age) factorial ANOVA. A single rat was removed from analysis due to procedural error. The remaining subjects were distributed across the groups as follows: PD17-Imm-Pre (6 males, 5 females), PD17-Imm-No Pre (5 males, 6 females), PD17–120-Pre (6 males, 5 females), PD17–120-No Pre (6 males, 7 females), PD24-Imm-Pre (6 males, 8 females), PD24-Imm-No Pre (8 males, 7 females), PD24–120-Pre (6 males, 3 females), PD24–120-No Pre (3 males, 6 females), PD31-Imm-Pre (7 males, 6 females) and PD31-Imm-No Pre (7 males, 6 females), PD31–120-Pre (5 males, 5 females) and PD31–120-No Pre (4 males, 6 females).
Results & Discussion
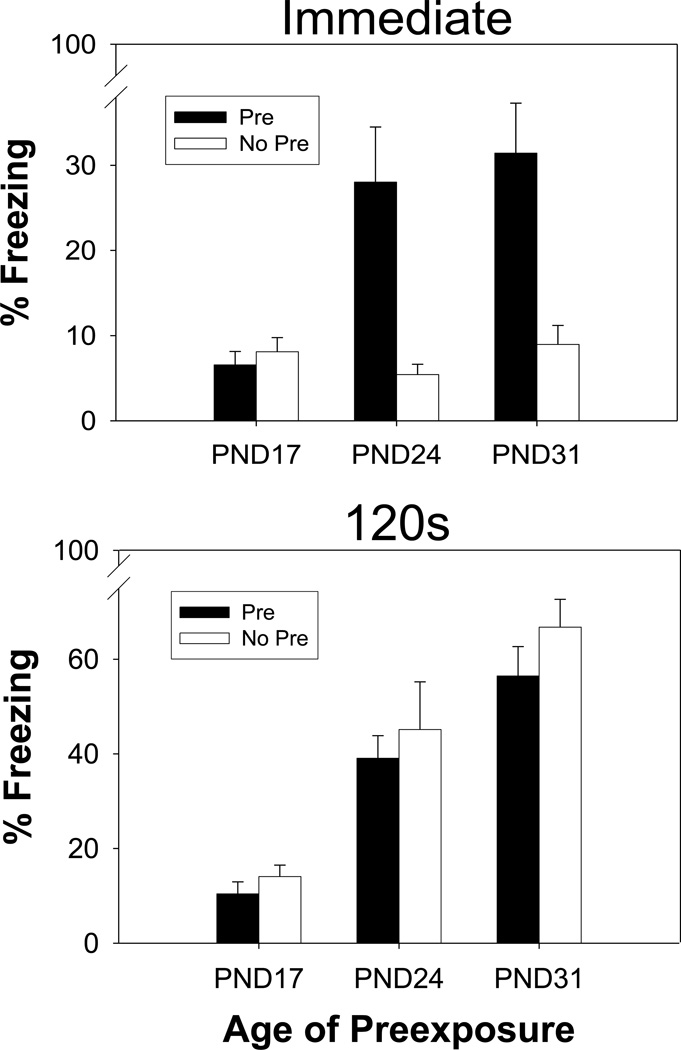
The results are depicted in Figure 1. There was no effect of sex or any interactions involving Sex (ps > .23) so data are shown collapsed across this variable. Both the CPFE and standard context conditioning (120 PSI) emerged between PD 17 and 24. Context conditioning continued to increase from PD24 to 31, whereas the CPFE did not. At both older ages, context preexposure enhanced conditioning when shock was immediate (CPFE) but not when shock was delayed for 120 sec.
Figure 1.
Mean percent freezing in Experiment 1 displayed for Pre (black) and No Pre (white) groups across Age (PND 17, 24, and 31) when trained with an Immediate shock (top) or one delivered after a 120s placement-to-shock interval (bottom). Bars represent standard errors of the mean. Facilitation of conditioning by context pre-exposure was only observed on PND 24 and 31 for animals in the Immediate shock condition.
Statistically, there were main effects of Age (F (2, 115) = 42.58, p < .0001) and PSI (F (1, 115) = 74.21, p < .0001) but not of Pre-exposure (F (1, 115) = 1.40, p > .23). There were significant interactions of Age × PSI (F (2, 115) = 14.42, p < .0001) and PSI × Pre-exposure (F (1, 115) = 16.45, p < .0001). More importantly, the Age × PSI × Pre-exposure interaction was significant (F (2, 115) = 3.35, p < .039). Post-hoc Newman-Keuls test revealed a number of interesting contrasts. First, in the immediate shock condition, none of the No Pre groups differed across age (ps > .90), while both the PD 24 and PD 31-Pre groups froze significantly more than Group PD 17-Pre (ps < .026) and these PD24 and 31 groups did not differ from one another (p > .61). As a result, the CPFE is only observed in the immediate condition on PD 24 or PD 31, with Groups PD 24 and 31-Pre freezing significantly more than their No Pre counterparts (ps < .022). This pattern of results demonstrates that the CPFE emerges between PD 17 and PD 24 and does not seem to develop further between PD 24 and PD 31. A markedly different ontogenetic profile is observed for the 120s PSI condition. Here there was no effect of Pre-exposure at any Age (ps > 0.13) and, regardless of Pre-exposure, each of the Age groups differed significantly from each other (all ps < .034). Therefore, with a 120s PSI, contextual fear conditioning seems to emerge between PD 17 and PD 24 and developed further through PD 31 regardless of Pre-exposure. The longer PSI also seems to facilitate conditioning for Pre animals on PD 31 when compared to the immediate shock condition (p < .003), where this enhancement is not seen for the other age groups (ps > .24). The CPFE can be compared against conventional contextual fear conditioning by contrasting the Pre-Immediate Groups with the No Pre-120s PSI Groups. There is no difference between the groups in question on PD 17 (p > .80) or PD 24 (p > .06). On PD 31, the No Pre-120s PSI Group did freeze at significantly higher levels than its CPFE counterpart (p < .0002).
Experiment 1 sought to determine whether the ontogenetic emergence of the CPFE and conventional contextual fear conditioning is the same or different. The findings indicate that both forms of fear conditioning emerge between PD 17 and 24. Experiment 1 also showed that conventional contextual fear conditioning continues to increase through PD 31 whereas the CPFE does not. There was no support for our hypothesis that the ontogeny of the CPFE would be more protracted than for conventional context conditioning. Rather, the CPFE appears to be a weaker form of conditioning, regardless of age. Accordingly, Experiment 2 reexamined the CPFE with stronger training parameters to determine whether this would alter the developmental profiles of this variant of fear conditioning.
Experiment 2
The purpose of Experiment 2 was to reexamine the ontogeny of the CPFE under stronger training parameters. It is possible that the low levels of conditioning for animals pre-exposed on PD 17 reflect weak training parameters, as opposed to an inability to learn the CPFE per se. It is also possible that stronger parameters would cause the CPFE to continue to increase in strength beyond PD24. To address these issues, Experiment 2 replicated Experiments 1 but used two shocks as the reinforcer instead of one (Landeira-Fernandez, DeCola, Kim, & Fanselow, 2006). Thus, a 2 (Pre-exposure: Pre vs. No Pre) × 3 (Age: PD 17 vs. PD 24 vs. PD 31) factorial design was used. In summary, Experiment 2 asked whether the increased number of shocks would alter the developmental profile of the CPFE.
Methods
Subjects
A total of 67 Long Evans weanling rats (34 male, 33 female) that were the offspring of 21 different mothers were used in this study. Rats were bred, culled, reared, etc. as described previously (Experiment 1). No more than a single same-sex littermate was assigned to a given experimental group.
Apparatus and Stimuli
The apparatus and training context were the same as in Experiment 1.
Behavioral Procedure
Context pre-exposure (PD 17, 24, or 31) and testing (PD 19, 25, or 33) followed identical procedures as Experiment 1. Training (PD 18, 25, or 32) was identical to Experiment 1, except each rat received two foot-shocks (separated by 1s) immediately after placement in the training context instead of one.
Data Analysis and Statistics
Data analysis and statistical tests were performed using the same programs as previous experiments. A 2 (Sex) × 2 (Pre-exposure) × 3 (Age) factorial ANOVA was run on the data. A single male rat was removed from analysis for being an extreme outlier relative to the other animals in the group (4.8 standard deviations away from the mean of the other rats in Group PD 17-Pre). The remaining rats were distributed across the groups as follows: PD17-Pre (3 males, 4 females), PD17-No Pre (4 males, 4 females), PD24-Pre (5 males, 4 females), PD 24-No Pre (4 males, 5 females), PD31-Pre (9 males, 9 females) and PD31-No Pre (8 males, 7 females). Newman-Keuls post-hoc analysis was used to further examine trends found in the ANOVAs.
Results & Discussion
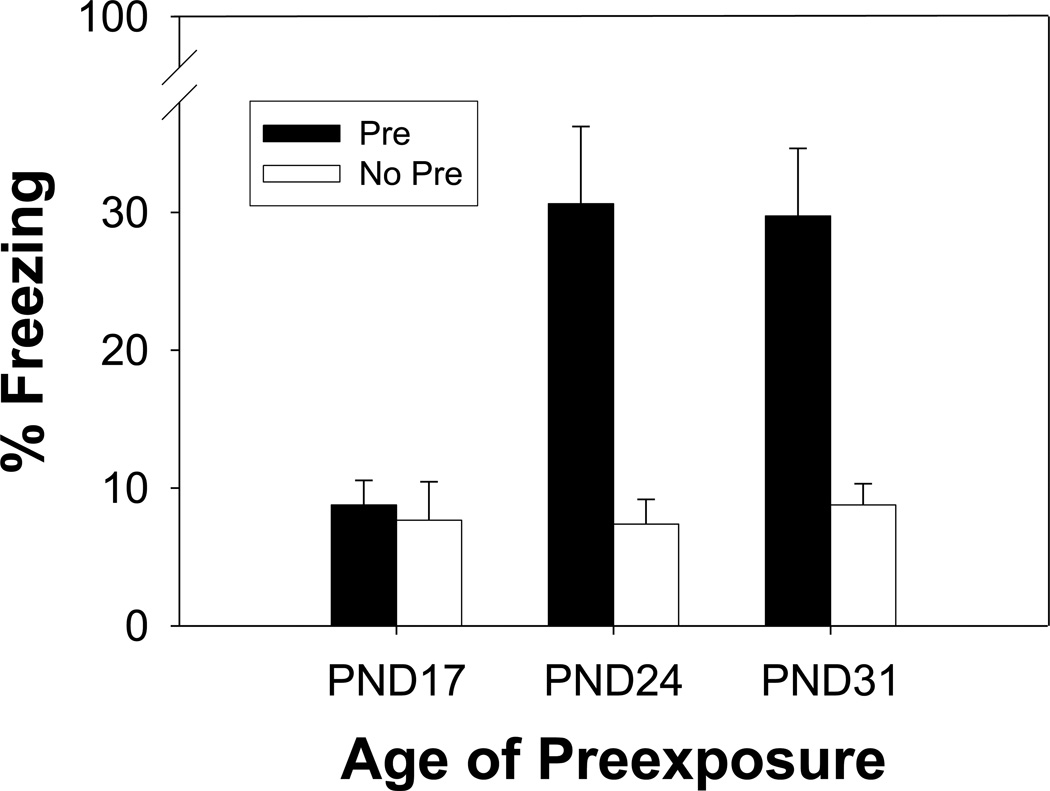
There was no main effect of or any interactions involving Sex (ps > .10), and so data are shown collapsed across that variable. The results of Experiment 2 appear in Figure 2, which again demonstrates the differential effect context pre-exposure had on conditioning across the three ages. ANOVA revealed significant main effects of Age (F (2, 54) = 3.42, p < .04) and Preexposure (F (1, 54) = 14.11, p < .0005) and an Age × Pre-exposure interaction (F (2, 54) = 3.78, p < .03). Newman-Keuls post-hoc analysis revealed that both Groups PD24-Pre and PD31-Pre differed from all other groups (ps < .018) except each other (p > .64), while none of the other groups differed (ps > .89). Therefore, when training consists of two shocks immediately delivered upon placement in the conditioning chamber, a significant CPFE was observed for both PD 24 and PD 31 groups. These results are consistent with the results from Experiment 1 and once again, Group PD24-Pre displays similar levels of conditioned freezing when compared to its PD 31 counterpart. Thus, there was no evidence for further development of the CPFE over this age range when a stronger reinforcer was used. By the same token, the PD 17 groups continued to demonstrate an absence of the CPFE, even after two-shock training.
Figure 2.
Mean percent freezing in Experiment 2 displayed for Pre (black) and No Pre (white) groups across Age (PND 17, 24, and 31). Bars represent standard errors of the mean. Two shocks were administered immediately upon placement. As in Experiment 1, low levels of conditioning were reported on PND 17 even with the stronger reinforcement while a comparable CPFE was observed on PND 24 and PND 31.
Experiment 3
The purpose of Experiment 3 was to determine the role of hippocampal NMDA receptors in the CPFE in weanling rats. Although contextual fear conditioning is thought to be mediated by the hippocampus during early ontogeny (Rudy, 1993; Rudy & Morledge, 1994; Stanton, 2000), there is scant empirical support for this view and it is possible that contextual fear conditioning in weanling rats is mediated by nonhippocampal systems (Pugh & Rudy, 1996; Rudy, 2009). As described previously (see Introduction), hippocampal plasticity during the pre-exposure phase plays a critical role in the CPFE in adult rats (Matus-Amat et al., 2007; Stote & Fanselow, 2004). Antagonism of NMDA-receptors by infusing MK-801 into the dorsal hippocampus (DH) of weanling rats has been shown to impair other spatial learning tasks, such as delayed alternation (Watson et al., 2009) and discrimination reversal (Watson & Stanton, 2009a). In addition, systemic administration of MK-801 on the pre-exposure day disrupts the CPFE in weanling rats (Burman et al., 2009). Based on these findings, Experiment 3a examined the effect of NMDA-receptor antagonism in the DH of weanling rats during the pre-exposure phase of the CPFE paradigm. Experiment 3b controlled for the timing of the drug infusion by administering MK-801 2 hr after the preexposure phase.
Experiment 3A
A 2 (Pre-exposure: Pre vs. No Pre) × 3 (Drug Treatment: MK-801 vs. Saline vs. Un-operated) factorial design was used. The Un-operated group was included to determine whether surgical installation of cannulas alters the CPFE in weanling rats. The training parameters were the same as in Experiment 2. A significant CPFE was anticipated when comparing freezing values of Pre vs. No Pre in the control groups. No increase in freezing was predicted in the MK-801-Pre group relative to the MK-801-No Pre group.
Methods
Subjects
A total of 46 Long Evans weanling rats (23 male, 23 female) that were the offspring of 11 different mothers were used in this study. Rats were bred, culled, reared, etc. as described previously (Experiment 1). No more than a single same-sex littermate was assigned to a given experimental group, except in a single inadvertent case in which the behavioral data of the two subjects were averaged and included in the statistical analysis as a single observation.
Surgery
Cannulation surgeries were performed as described in Watson et al. (2009). Rats were taken individually from post-weaning group housing on PD 22 and anesthetized with a ketamine/xylazine mixture in a volume of 1mg/kg prior to surgery. Once anesthetized, animals were prepared for surgery and placed into a stereotaxic frame apparatus. Stainless-steel guide cannulas (Plastics One, Roanoke, VA) were implanted bilaterally to terminate in the dorsal hippocampus (DH) using the following coordinates: anteroposterior (AP), +2.6 mm relative to interaural midline; mediolateral (ML), ± 2.3 mm and dorsoventral (DV), −2.0 mm relative to bregma. Cannulas were fixed in place using dental acrylic on two “skull hooks” (Stanton & Freeman, 1994; Watson et al., 2009). After surgery, dummy cannulas were inserted and rats were allowed to recover from anesthesia in individual white plastic cages (Experiment 1) with half the floor placed on electric heating pads (Watson et al., 2009). Rats were given a full day (PD 23) to recover from surgery before the pre-exposure phase of the behavioral protocol began on PD 24. Un-operated control rats were weighed and transferred to individual cages on PD 22 like the operated rats but were left undisturbed until the start of the experiment.
Apparatus and Stimuli
The apparatus and training context were the same as in Experiment 1.
Drug Infusion
On PD 24 (pre-exposure phase), rats received microinjections of either MK-801 or sterile saline in their home cages. Un-operated rats were handled in a similar manner and for an approximately equivalent duration as those rats receiving infusions. The pre-exposure phase began on average 32 minutes (± 3 min) after the infusion procedure. Details of the infusion procedure appear in Watson et al. (2009). MK-801 (10 µg/µL dissolved in sterile saline) was bilaterally infused at a rate of 0.25 µL per minute for a single minute, delivering 2.5 µg of MK-801 on each side. This infusion volume does not spread beyond the DH (unpublished observations involving autoradiography of labeled MK-801). Saline controls received equivalent volumes of sterile saline at an identical rate. Squads of behaviorally tested rats were counterbalanced by drug treatment, so that each group had at least one MK-801 infusion and one saline infusion. Animals remained in their home cages until the start of behavioral procedures. Once again, un-operated control rats were handled in a similar manner for a similar duration.
Behavioral Procedure
Pre-exposure (PD24), training (PD 25) and testing (PD 26) followed identical behavioral procedures as for the PD 24 group in Experiment 3. During training, all rats received two foot-shocks (separated by 1s) immediately after placement (approximately 3s placement-to-shock delay). Order of placement in the training chambers was counterbalanced across Drug Treatment and Sex.
Histology
Within 24–48 hours after completion of behavioral testing, rats received a lethal overdose of the ketamine/xylazine mixture and were perfused under deep anesthesia. Animals were perfused intracardially with 0.9% saline for two minutes followed by perfusion of 10% neutral buffered formalin for eight minutes. The brains were removed and placed into vials containing 10% neutral buffered formalin to maximize tissue fixture. The following day, brains were placed in 30% sucrose in 10% buffered formalin. Coronal sections (40_m thick) were taken through the hippocampus using a microtome. Sections were mounted, and then counterstained with Neutral Red (1%). Slides were examined to confirm cannula tip placement in the DH.
Data Analysis and Statistics
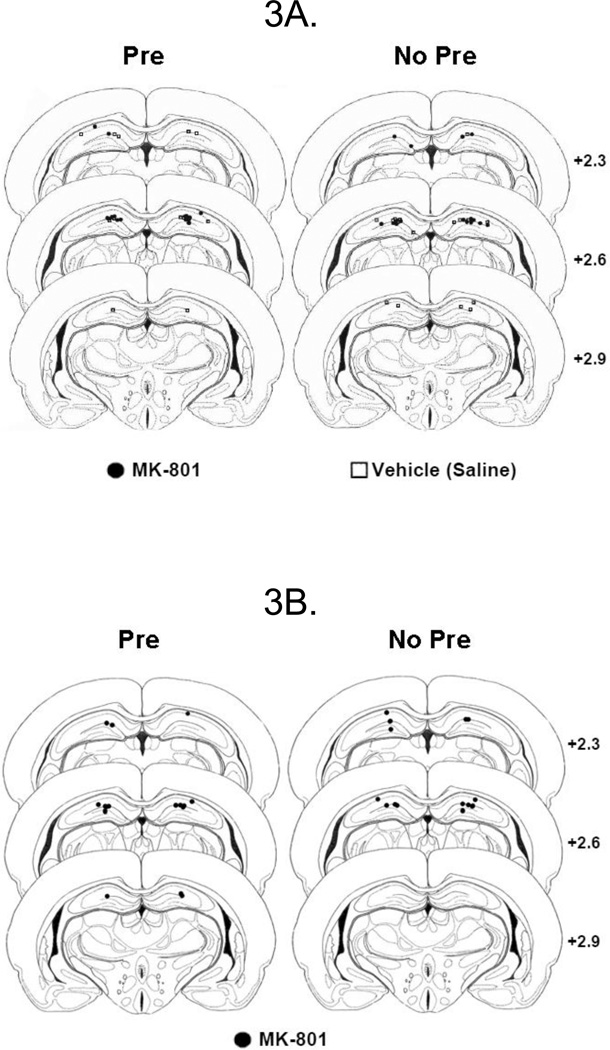
Data analysis and statistical tests were performed using the same procedures and programs as the previous experiments. All surgeries but one successfully implanted the cannula with the injector tip located in the DH and the tip placements for animals included in the analysis are shown in Figure 3A. Two rats from the Un-operated No Pre group and one rat from the Saline No Pre group were excluded due to procedural error. The remaining rats were distributed across the six groups as follows: MK-801-Pre (3 males, 6 females), MK-801-No Pre (4 males, 4 females), Saline-Pre (5 males, 3 females), Saline-No Pre (3 males, 3 females), Un-operated-Pre (3 males, 4 females), and Un-operated-No Pre (2 males, 1 female). Planned comparisons between the mean freezing levels for the saline and un-operated rats in the Pre groups did not differ, and likewise for their No Pre counterparts (ps > .37). Therefore, the data were collapsed across Saline and Un-operated rats, resulting in Groups Control-Pre (8 males, 7 females) and Control-No Pre (5 males, 4 females). Data were analyzed with a 2 (Sex) × 2 (Pre-exposure) × 2 (Drug Treatment: MK-801 vs. Control) factorial ANOVA and Newman-Keuls post-hoc tests.
Figure 3.
Schematic Representation of Injection Cannula Tip Placements in Dorsal Hippocampus in Experiment 3. Panel 3A shows placements for Pre (left) and No Pre (right) animals included in Experiment 3A. Panel 3B shows placements for Pre (left) and No Pre (right) animals included in Experiment 3B. The values to the right indicate the anterior position (in millimeters) of each section relative to interaural midline. Coronal brain images are adapted from the developing brain atlas of Sherwood & Timiras (1970).
Results & Discussion
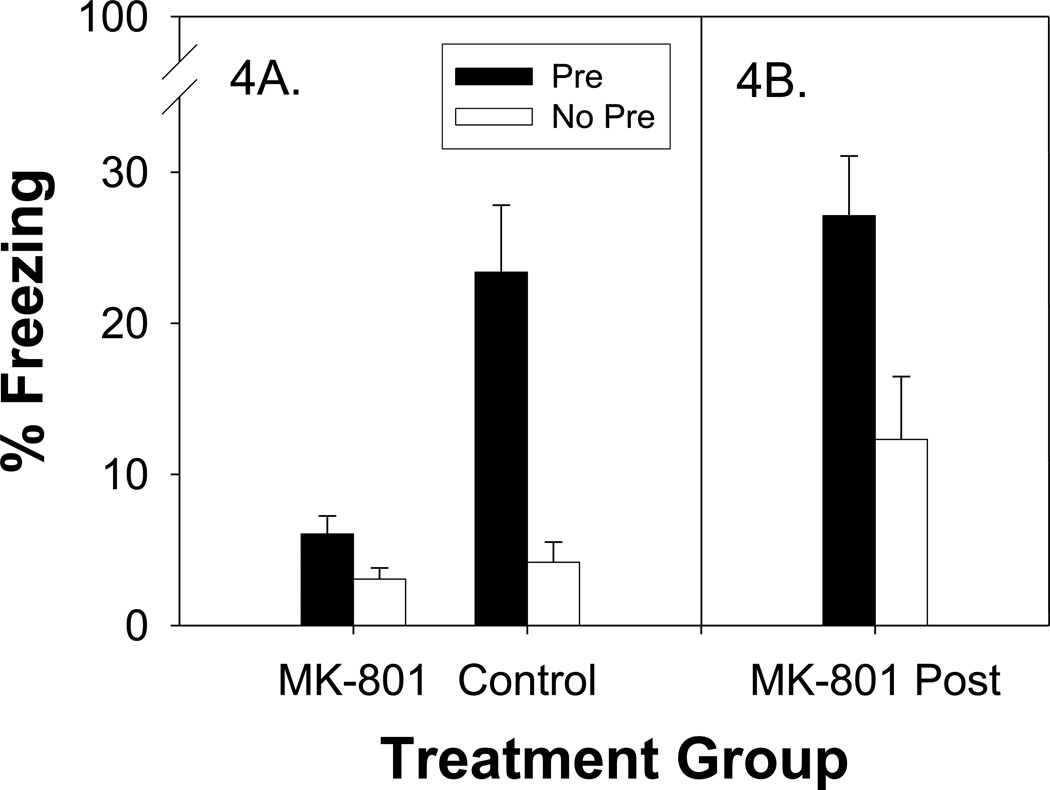
The behavioral results can be seen in Figure 4A. There were no main effects or interactions involving Sex (ps > .57) so data are shown combined across this factor. The findings demonstrate the critical role that NMDA-type glutamate receptors in the dorsal hippocampus play during the pre-exposure phase of the CPFE in PD24 rats. The control group exhibited the classic CPFE, with the Pre animals freezing at significantly higher levels than their No Pre counterparts. In contrast, the MK-801 Group failed entirely to show the CPFE.
Figure 4.
Mean percent freezing in Experiments 3a and 3b depicted for Pre (black) and No Pre (white) groups across drug treatment conditions when administered before (Panel 4A, MK-801 vs. Control) or after preexposure (Panel 4B, MK-801 Post). Bars represent standard errors of the mean. The CPFE was observed for Control and MK-801 Post groups, but not when MK-801 was infused prior to pre-exposure, implicating hippocampal NMDA receptors in the formation of the context memory early in ontogeny.
Significant main effects of Pre-exposure (F (1, 33) = 8.80, p < .006) and Drug Treatment (F (1, 33) = 6.09, p < .019) were observed. More importantly, a significant Pre-exposure × Drug Treatment interaction (F (1, 33) = 4.71, p < .038) confirmed the differential effect of context pre-exposure in the different drug treatment groups. Newman-Keuls tests revealed that Group Control-Pre differed from all the other groups (ps < .003); while none of the other groups differed from each other (ps > .79).
The pattern of results confirms the disruption of the CPFE by MK-801 infusion on the day of context pre-exposure. Administration of the drug into the DH blocked the facilitating effect of context pre-exposure. In fact, MK-801 infusion caused the preexposed animals to demonstrate conditioning levels that are identical to those observed for animals that did not receive context pre-exposure.
Experiment 3B
The findings of Experiment 3a suggest that disruption of the CPFE was due to the MK-801 drug action that interfered with the hippocampal plasticity required for the formation of the contextual representation. However, it is possible that MK-801 had lasting effects across different phases of the procedure that could interfere with association formation during training or with the performance of freezing behavior during testing. Experiment 3b tested this possibility by infusing MK-801 into the DH two hours after the completion of the pre-exposure phase. As in previous experiments, the animals were either pre-exposed to the training chamber (Group Pre) or pre-exposed to the alternate context (Group No Pre). If MK-801 selectively disrupted formation of the contextual representation in Experiment 3a, then post-training NMDA-receptor antagonism should not impair conditioning in Group Pre in the present experiment. However, if the results of Experiment 3a reflected lingering effects of the drug on task performance, then post-training MK-801 administration would impair the CPFE in the same way that pre-training administration did.
Subjects
A total of 17 Long Evans weanling rats (9 male, 8 female) that were the offspring of 5 different mothers were used in this study. Rats were bred, culled, reared, etc. as described previously (Experiment 1). No more than a single same-sex littermate was assigned to a given experimental group, with 5 male and 4 female in Group Pre, and 4 male and 4 female rats in Group No Pre.
Surgery, Apparatus, Behavioral Procedures, and Histology
These were all exactly as described in Experiment 3a.
Drug Infusion
On PD 24, rats received bilateral microinjections of MK-801 into the dorsal hippocampus approximately two hours after completion of the pre-exposure phase. The infusion procedure was the same as Experiment 3a, except only MK-801 was infused. Infusion groups were counterbalanced by Pre-exposure, so that each group had at least one animal from Group Pre and one from Group No Pre.
Data Analysis and Statistics
A 2 (Sex) × 2 (Pre-exposure) factorial ANOVA was performed as in the previous experiments. All surgeries successfully implanted the cannula with the injector tip located in the DH and placements are depicted in Figure 3B.
Results & Discussion
The behavioral results are shown in Figure 4B and confirm that the findings from Experiment 3a cannot reflect lingering effects of the drug during training or testing. There were no main effects or interactions involving sex (ps > .15), so data were collapsed across this factor. A significant main effect of Pre-exposure (F (1, 12) = 7.70, p < .023) was observed, indicative of a significant CPFE (despite the statistically nonsignificant trend toward higher freezing in the No-Pre group relative to its counterparts in Experiment 3a). These results indicate the abolition of the CPFE by DH MK-801 administration in Experiment 3a reflects disruption of context learning during the pre-exposure phase.
Taken as a whole, the results of Experiment 3 indicate that the hippocampus plays an NMDA-receptor-dependent role in the formation of conjunctive representations of context on PD 24, just as it does in adult rats (Stote & Fanselow, 2004; Matus-Amat et al., 2007). They also suggest that drug action on neurons of the dorsal hippocampus is sufficient to account for the effects of systemic MK-801 injections on the CPFE in our previous study (Burman et al., 2009).
General Discussion
These experiments investigated the ontogeny and neural mechanisms of the context preexposure facilitation effect across the period of development when conventional contextual fear conditioning emerges in the rodent. Experiment 1 compared the ontogeny of the CPFE versus conventional contextual fear conditioning and found that both emerged between PD 17 and 24, whereas conventional conditioning but not the CPFE continued to increase between PD24 and 31. Experiment 2 reexamined the ontogeny of the CPFE with stronger reinforcement (two immediate shocks) and again found that the effect was absent on PD 17 and was present at equivalent levels on PD 24 and 31. Experiment 3 showed that blockade of NMDA-type glutamate receptors in the dorsal hippocampus during context preexposure abolished the CPFE whereas blockade occurring 2 hr following preexposure had no effect on the CPFE. Taken together, these findings demonstrate that a form of context conditioning that depends on hippocampal plasticity in adult rats emerges ontogenetically between PD 17 and 24 and also depends on hippocampal plasticity during the weanling period.
The present study compared the ontogeny of standard contextual fear conditioning, which can be learned either with hippocampus-dependent conjunctive processes or non-hippocampal, feature-based processes, with the ontogeny of the CPFE, which requires conjunctive processes (Rudy, 2009). We hypothesized that feature-based learning might cause standard context conditioning to be stronger at earlier stages of development than the CPFE. Our findings failed to support this hypothesis. Although standard context conditioning generated more freezing than the CPFE on PD31, as it does in adulthood (e.g., Matus-Amat et al., 2007), conditioned freezing on both tasks was absent on PD17 and emerged at comparable levels on PD 24. Our previous study also found that the CPFE and standard context conditioning generate comparable levels of freezing on PD 23 (Burman et al., 2009). Parsimony holds that standard context conditioning employing the types of procedures used in this study is also likely to be mediated by hippocampus-dependent conjunctive processes (Burman et al., 2009; Rudy, 1993; Rudy & Morledge, 1994; Stanton, 2000), although this remains to be confirmed empirically and it is also possible that the neural systems underlying feature-based representations of context develop in parallel with the hippocampus (see below).
There are several reports that context preexposure facilitates context conditioning that is otherwise poor in weanling-juvenile rats (Rudy, 1996; Rudy & Morledge, 1994; Rudy & O’Reilly, 1999; Rudy & Pugh, 1998; Pugh & Rudy, 1996). All of these studies used a standard context conditioning procedure (2 minutes of context exposure paired with shock) but conditioned freezing was poor either because testing occurred after a short (10 minute) retention interval (Rudy & Morledge, 1994; Pugh & Rudy, 1996; Rudy & O’Reilly, 1999), training occurred at a particular time of day (Rudy & Pugh, 1998), or because socially-housed rats experienced isolation stress shortly after training (Rudy, 1996). Two studies asked whether facilitation by context preexposure was based on combined versus separate exposure to elemental features of the context (Pugh & Rudy, 1996; Rudy & O’Reilly, 1999). Rudy & O’Reilly (1999) tested 30-day-old rats in clear Plexiglas chambers in which cage geometry, floor texture and distal cues were the separable features and found that preexposure facilitated standard context conditioning when the features were experienced together but not when they were experienced on separate occasions. Pugh & Rudy (1996) tested 18- and 23-day-old rats in an analogous study in which wall color (black vs. clear Plexiglas) and floor texture were the separable features. This study, in contrast, found evidence only of features-based context learning at both PD 18 and 23 (plus possible “performance effects” of black test chambers). These studies suggest that context conditioning is not based on conjunctive processes until around 30 days of age. However, a potentially important limitation of these studies is that the training phase involved standard context conditioning (120-s of context followed by shock) rather than immediate shock. In addition, the test of fear conditioning occurred 10 minutes following training, when short-term context memories have decayed and long-term context memories have not yet consolidated in non-preexposed controls. In this CPFE paradigm, preexposure is thought to facilitate test performance because it allows long-term context memories to be consolidated at the time of training and testing (Rudy & Morledge, 1994). However, delayed shock increases the potential role of feature-based learning during the training phase and it is possible that preexposure enhances this learning, particularly in developing animals (Hall, 1979; Hoffman & Spear, 1989; Stanton, Fox & Carter, 1998). In contrast, with immediate shock, context exposure is so brief that the CPFE can only be supported by pattern completion processes of the hippocampus that operate to retrieve the conjunctive preexposure memory (Rudy, 2009). The present findings indicate that the CPFE established with immediate shock is absent during the preweanling period (PD17). This suggests that the type of feature-based context learning observed on PD18 by Pugh & Rudy (1996) does not occur under our training conditions. This may reflect either our use of immediate shock during training and/or our use of test chambers with clear Plexiglas walls, which may encourage processing of distal cues. Pugh & Rudy (1996) used test chambers with black vs. clear walls, and thus involved a brightness cue that preweanling rats can readily associate with shock (Jagielo et al., 2003). Another limitation of previous developmental work on the CPFE involving delayed shock is that it may be difficult to relate this work to neurobiological studies of the CPFE, that uniformly involve immediate shock. The present study shows that the CPFE established with immediate shock depends on hippocampal NMDA receptor function in weanling rats, just as it does in adult rats (Matus-Amat et al., 2007). That other variants of the CPFE used in weanling-juvenile rats (Pugh & Rudy, 1996; Rudy & Morledge, 1994; Rudy & O’Reilly, 1999) share this property is quite possible but remains to be determined. A noteworthy strength of these studies is that they have attempted to determine whether the CPFE depends on combined versus separate exposure to the component features of the context. To our knowledge, this question has not been explored in any studies of the CPFE that involve training with immediate shock. Theories of this variant of the CPFE suggest that it should not be feature-based under any circumstances (Rudy, 2009). This remains an important future direction both for our developmental work and for research on the neurobiology of context conditioning in adult animals.
That the CPFE involving immediate shock depends on the hippocampus has been shown with anterograde neurotoxic lesions (Rudy, Barrientos, & O’Reilly, 2002), intrahippocampal administration of muscimol (Matus-Amat et al., 2004), and infusions of NMDA-receptor antagonists into the ventricles (Stote & Fanselow, 2004) or the hippocampus (Matus-Amat et al., 2007). Inactivating the dorsal hippocampus with muscimol before each individual phase of the CPFE (preexposure, immediate shock training, or testing) or across all three phases (to control for state-dependent effects) significantly reduced the CPFE compared to vehicle controls. In contrast, infusions of muscimol immediately after context preexposure have no effect on the CPFE. Therefore, the hippocampus seems to be required for (1) the formation of the conjunctive contextual representation during the preexposure phase, (2) activation of the context memory via pattern completion during the training phase so that the conjunctive representation can be associated with immediate shock, and (3) activation of the context memory that retrieves the context-shock association and produces fear behavior during the testing phase (Matus-Amat et al., 2004). Although hippocampal activity is required during all three phases of the CPFE, hippocampal plasticity is required only during the preexposure phase. The CPFE is disrupted by intrahippocampal infusions of NMDA-receptor antagonists during preexposure but not during training with immediate shock or during testing (Matus-Amat et al., 2007). That anterograde DH cell body lesions or muscimol infusions spare conventional context conditioning but not the CPFE indicates that hippocampus-independent feature-based associations can support the former but not the latter; and is foundational for the two-process model of context conditioning (Rudy, 2009).
This two-process model holds that contexts can be represented via two neural systems that have different psychological properties (Nadel & Willner, 1980; Nadel et al., 1985; Rudy, 2009). The system that processes feature-based associations depends on parahippocampal regions such as perirhinal, postrhinal, and entorhinal cortex. This system learns slowly, is “reinforcement driven,” is not capable of pattern completion, is more susceptible to associative interference, and its associations sum algebraically (Rescorla & Wagner, 1972) to influence learned behavior (Rudy, 2009). In contrast, the system that processes conjunctive associations depends on interactions between the hippocampal formation and the aforementioned parahippocampal regions. This system learns very rapidly and in an “incidental” rather than “reinforcement-driven” manner (Rudy, 2009). Associations in this system do not sum algebraically but are bound together synergistically into an integrated map-like representation that is subject both to pattern completion (exposure to any feature activates the entire representation) and to pattern separation (conjunctive representations of different but overlapping features are easier to discriminate than are representations based on mere summation of feature-based associations) (Rudy, 2009). Finally, the two systems compete, with the conjunctive system dominating (inhibiting) the feature-based system under normal conditions (Maren et al., 1997; Rudy, 2009).
This model suggests a number of possible explanations for the developmental trends reported in the present study and in the broader developmental literature on fear conditioning. The first explanation concerns “performance effects,” i.e., that context conditioning emerges because the ability to process sensory cues or to perform the target freezing response develops between PND 17 and 24. An inability to show freezing can be rejected on the basis of the large, longstanding literature showing that conditioned freezing to a wide range of discrete olfactory, auditory and visual cues is comparable in pre- vs. postweanling rats (e.g., see reviews of Richardson & Hunt, 2010; Stanton, 2000; and reports by Brasser & Spear, 2004; Rudy, 1993; Rudy & Morledge, 2004). Sensory perception of contextual cues can be ruled out because post-shock freezing after conventional context conditioning occurs at comparable levels in pre- and postweanling rats (Rudy & Morledge, 2004). This freezing reflects a short-term context-shock association that does not depend on hippocampus (Kim, Fanselow, DeCola & Landeira-Fernandez, 1992). These studies also rule out the possibility that developmental changes in emotional learning per se (e.g., the ability of the amygdala to acquire fear) explain the ontogeny of contextual fear conditioning in the present study. It is more likely that the present findings reflect developmental changes in the hippocampus, in parahippocampal cortical areas, or in interactions of these systems with each other or with the amygdala. The possibility that the hippocampus itself is not functional on PD 17 in a general sense is unlikely because there are many hippocampus-dependent behaviors that are impaired by hippocampal damage in PD16–18 rats (Stanton, 2000; Table 1; Stanton, Jensen & Pickens, 1991). Indeed, recent neurophysiological studies have demonstrated head-direction cells in subiculum and place cells in hippocampus as early as PD16; with entorhinal grid cells emerging a few days later (Langston et al., 2010; Wills, Cacucci, Burgess, & O'Keefe, 2010). Although developmental changes in neural function or plasticity that is intrinsic to the hippocampus (Dumas & Rudy, 2010) cannot be ruled out, it seems more likely that changing interactions of the hippocampal formation with parahippocampal regions and/or the amygdala determine when contextual fear conditioning emerges during ontogeny. Parahippocampal regions show protracted neuroanatomical development during the weanling period (e.g., Furtak, Moyer & Brown, 2007; Ulfig, 1993). This development may determine the point in ontogeny when the conjunctive learning and pattern completion processes of the hippocampus are able to mediate context conditioning. It is possible that these processes emerge at different points in ontogeny. As a conditioning paradigm, the CPFE offers several analytical advantages that should help address these types of questions in future studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11(1):8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Brasser SM, Spear NE. Contextual conditioning in infants, but not older animals, is facilitated by CS conditioning. Neurobiol Learn Mem. 2004;81(1):46–59. doi: 10.1016/s1074-7427(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Brown KL, Stanton ME. Cross-modal transfer of the conditioned eyeblink response during interstimulus interval discrimination training in young rats. Dev Psychobiol. 2008;50(7):647–664. doi: 10.1002/dev.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman MA, Murawski NJ, Schiffino FL, Rosen JB, Stanton ME. Factors governing single-trial contextual fear conditioning in the weanling rat. Behav Neurosci. 2009;123(5):1148–1152. doi: 10.1037/a0016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas TC, Rudy JW. Development of the hippocampal memory system: Creating networks and modifiable synapses. In: Blumberg MS, Freeman JH Jr, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York, NY: Oxford University Press; 2010. pp. 587–606. [Google Scholar]
- Esmoris-Arranz FJ, Mendez C, Spear NE. Contextual fear conditioning differs for infant, adolescent, and adult rats. Behav Processes. 2008;78(3):340–350. doi: 10.1016/j.beproc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow Associative vs topographical accounts of the immediate shock-freezing deficit in rats: Implications for the response selection rules governing species-specific defensive reactions. Learning & Motivation. 1986;17:16–39. [Google Scholar]
- Fanselow MS. Factors governing one-trial contextual conditioning. Animal Learning & Behavior. 1990;18(3):264–270. [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110(1–2):73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, DeCola JP, Young SL. Mechanisms responsible for reduced contextual conditioning with massed unsignaled unconditional stimuli. J Exp Psychol Anim Behav Process. 1993;19(2):121–137. [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Rudy JW. Convergence of experimental and developmental approaches to animal learning and memory processes. In: Carew T, Menzel R, Shatz C, editors. Mechanistic relationships between development and learning: Beyond metaphor. New York, NY: Wiley; 1998. pp. 15–28. [Google Scholar]
- Furtak SC, Moyer JR, Jr, Brown TH. Morphology and ontogeny of rat perirhinal cortical neurons. Journal of Comparative Neurology. 2007;505:493–510. doi: 10.1002/cne.21516. [DOI] [PubMed] [Google Scholar]
- Hall G. Exposure learning in animals. Psychol Bull. 1979;27:535–550. [Google Scholar]
- Hoffman H, Spear NE. Facilitation and impairment of conditioning in the pre-weanling rat after prior exposure to the conditioned stimulus. Anim Learn Behav. 1989;17:63–69. [Google Scholar]
- Hunt PS, Campbell BA. Developmental dissociation of the components of conditioned fear. In: Bouton ME, Fanselow MS, editors. Learning, Motivation and Cognition: The Functional Behaviorism of Robert C. Bolles. Washington, DC: American Psychological Association; 1997. pp. 53–74. [Google Scholar]
- Jagielo JA, Miller JS, Spear Smith J, Spear NE. Ontogenetic differences in the expression of conditioned visual aversions. Dev Psychobiol. 2003;42(2):123–130. doi: 10.1002/dev.10081. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS, DeCola JP, Landeira-Fernandez J. Selective impairment of long-term but not short-term conditional fear by the N-methyl-D-aspartate antagonist APV. Behav Neurosci. 1992;106:591–596. doi: 10.1037//0735-7044.106.4.591. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107(6):1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Landeira-Fernandez J, DeCola JP, Kim JJ, Fanselow MS. Immediate shock deficit in fear conditioning: effects of shock manipulations. Behavioral Neuroscience. 2006;120(4):873–879. doi: 10.1037/0735-7044.120.4.873. [DOI] [PubMed] [Google Scholar]
- Langston RF, Ainge JA, Couey JJ, Canto CB, Bjerknes TL, Witter MP, Moser EI, Moser MB. Development of the spatial representation system in the rat. Science. 2010;328(5985):1576–1580. doi: 10.1126/science.1188210. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88(2):261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24(10):2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behav Neurosci. 2007;121(4):721–731. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- Nadel L, Willner J. Context and conditioning: A place for space. Physiology & Behavior. 1980;8:218–228. [Google Scholar]
- Nadel L, Willner J, Kurz EM. Cognitive maps and environmental context. In: Balsam P, Tomie A, editors. Context and learning. Hillsdale, NJ: Erlbaum; 1985. pp. 385–406. [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. New York: Oxford University Press; 1978. [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Rudy JW. A developmental analysis of contextual fear conditioning. Dev Psychobiol. 1996;29(2):87–100. doi: 10.1002/(SICI)1098-2302(199603)29:2<87::AID-DEV1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WK, editors. Classical conditioning II: Current research and theory. New York, NY: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Richardson R, Hunt PS. Ontogeny of Fear Conditioning. In: Blumberg MS, Freeman JH Jr, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York, NY: Oxford University Press; 2010. pp. 527–545. [Google Scholar]
- Rudy JW. Development of learning: from elemental to configural associative networks. In: Rovee-Collier C, Lipsitt LP, editors. Advances in Infancy Research. Norwood, NJ: ABLEX Publishing Corporation; 1992. pp. 247–289. [Google Scholar]
- Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behav Neurosci. 1993;107(5):887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Postconditioning isolation disrupts contextual conditioning: an experimental analysis. Behav Neurosci. 1996;110(2):238–246. doi: 10.1037//0735-7044.110.2.238. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learn Mem. 2009;16(10):573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O'Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116(4):530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28(7):675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behav Neurosci. 1994;108(2):227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O'Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav Neurosci. 1999;113(5):867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O'Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn Affect Behav Neurosci. 2001;1(1):66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Pugh CR. Time of conditioning selectively influences contextual fear conditioning: further support for a multiple-memory systems view of fear conditioning. J Exp Psychol Anim Behav Process. 1998;24(3):316–324. doi: 10.1037//0097-7403.24.3.316. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Timiras PS. A Stereotaxic Atlas of the Developing Rat Brain. Berkeley: University of California Press; 1970. [Google Scholar]
- Stanton ME. Multiple memory systems, development and conditioning. Behav Brain Res. 2000;110(1–2):25–37. doi: 10.1016/s0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Fox GD, Carter CS. Ontogeny of the conditioned eyeblink response in rats: acquisition or expression? Neuropharmacology. 1998;37(4–5):623–632. doi: 10.1016/s0028-3908(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Freeman JH., Jr Eyeblink conditioning in the infant rat: an animal model of learning in developmental neurotoxicology. Environ Health Perspect. 1994;102(Suppl 2):131–139. doi: 10.1289/ehp.94102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stote DL, Fanselow MS. NMDA receptor modulation of incidental learning in Pavlovian context conditioning. Behav Neurosci. 2004;118(1):253–257. doi: 10.1037/0735-7044.118.1.253. [DOI] [PubMed] [Google Scholar]
- Watson DJ, Herbert MR, Stanton ME. NMDA receptor involvement in spatial delayed alternation in developing rats. Behav Neurosci. 2009;123(1):44–53. doi: 10.1037/a0013633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DJ, Stanton ME. Intrahippocampal administration of an NMDA-receptor antagonist impairs spatial discrimination reversal learning in weanling rats. Neurobiol Learn Mem. 2009;92(1):89–98. doi: 10.1016/j.nlm.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TJ, Cacucci F, Burgess N, O'Keefe J. Development of the hippocampal cognitive map in preweanling rats. Science. 2010;328(5985):1573–1576. doi: 10.1126/science.1188224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfig N. Ontogeny of the entorhinal cortex. Hippocampus. 1993;3(Spec No):27–32. [PubMed] [Google Scholar]