Abstract
Animal appendages require a proximodistal (PD) axis, which forms orthogonally from the two main body axes, anteroposterior and dorsoventral. In this review, we discuss recent advances that begin to provide insights into the molecular mechanisms controlling PD axis formation in the Drosophila leg. In this case, two morphogens, Wingless (Wg) and Decapentaplegic (Dpp), initiate a genetic cascade that, together with growth of the leg imaginal disc, establishes the PD axis. The analysis of cis-regulatory modules (CRMs) that control the expression of genes at different positions along the PD axis has been particularly valuable in dissecting this complex process. From these experiments, it appears that only one concentration of Wg and Dpp are required to initiate PD axis formation by inducing the expression of Distal-less (Dll), a homeodomain-encoding gene that is required for leg development. Once Dll is turned on, it activates the medially expressed gene dachshund (dac). Cross-regulation between Dll and dac, together with cell proliferation in the growing leg imaginal disc, results in the formation of a rudimentary PD axis. Wg and Dpp also initiate the expression of ligands for the EGFR pathway, which in turn induces the expression of a series of target genes that pattern the distal-most portion of the leg.
1. Introduction
Animal appendages are external projections from the body wall that are used for very diverse functions including locomotion, grooming, and feeding. In the thorax of diptera, such as the fruit fly Drosophila melanogaster, there are dorsal appendages required for flight—a pair of wings in the second thoracic (T2) segment and a pair of halteres in T3—and three pairs of legs used for walking and grooming. The fly leg, the subject of this review, is composed of 10 morphologically unique segments: coxa, trochanter, femur, tibia, tarsal segments 1–5, and the claw. Together, these segments comprise the proximodistal (PD) axis, in which the proximal coxa is closest to the body and the claw is furthest from the body (Fig. 7.1).
Figure 7.1.
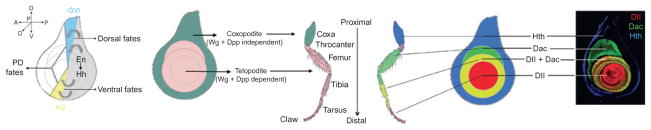
Overview of fly leg development. On the left shows the relationship between En, Hh, wg, and dpp and the definition of the telopodite (Hh, Wg, and Dpp-dependent domain) and the coxopodite (Hh, Wg, and Dpp-independent domain). On the right shows the relationship between the three primary PD gene expression domains established by Hth, Dac, and Dll.
Unlike the two other primary body axes (anteroposterior, AP; dorsoventral, DV), for each appendage, the PD axis is established during embryogenesis de novo. In contrast, at all stages of development, even in the unfertilized egg, rudimentary AP and DV axes exist. Thus, in this respect, the PD axis is unique among the main body axes. This topic, how so-called secondary developmental fields are established from preexisting developmental information, has been debated for decades both from theoretical perspectives and by classical developmental biologists (reviewed by Baker, 2011). Data generated over the past several years have provided novel mechanistic and molecular insights that build upon these earlier studies, providing interesting connections between cell division, secreted morphogens, and the use of dedicated cis-regulatory modules (CRMs) for transcriptional regulation of genes expressed along the PD axis. It is the goal of this review to summarize our current understanding of the intimate interplay between these components, orchestrated over developmental time, which establishes, elaborates, and fine-tunes the leg’s PD axis.
2. The Molecular Players in PD Axis Formation
As for much of the adult fly, fly legs are derived from imaginal discs, elliptical sheets of epithelia that are highly folded by the end of larval development. The fate map of the leg disc is such that cells at its center will give rise to distal-most structures, while cells further away from the center generate more proximal structures (Fig. 7.1). Imaginal discs do not only give rise to appendages: cells at the periphery of the leg disc, for example, generate the ventral portion of the adult body wall, the ventral and sterno pleura. Thus, in the leg imaginal disc, the PD axis—from distal claw to proximal body wall—is displayed as concentric rings within these elliptical epithelia (Fig. 7.1).
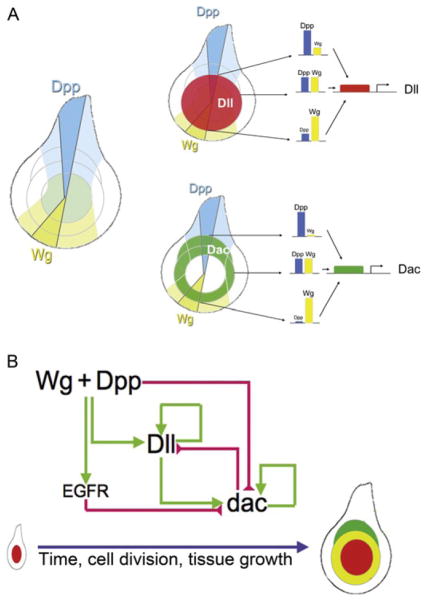
Many genes are expressed in rings or subdomains in the leg disc that mark distinct positions along the PD axis (Abu-Shaar and Mann, 1998; Campbell and Tomlinson, 1998; Diaz-Benjumea et al., 1994; Duncan et al., 1998; Emmons et al., 1999; Erkner et al., 1999; previously reviewed by Kojima, 2004; Kojima et al., 2000; Mardon et al., 1994). From this perspective, the problem of how the PD axis is established can be reformulated by asking the simpler question: how are these gene expression domains established? As will be discussed more below, much attention has been focussed on dissecting the regulation of two genes that are broadly expressed in distal and medial domains of the leg disc, Distal-less (Dll) and dachshund (dac), respectively (Fig. 7.1). Dll, in particular, is a critical player in leg development as it is one of the earliest known markers for the appendage, not just in flies, but also throughout the animal kingdom (Cohen, 1990; Cohen et al., 1989; Panganiban et al., 1997; reviewed in Panganiban and Rubenstein, 2002). Moreover, in flies both Dll and dac are required for the development of their respective distal and medial domains of the leg (Cohen and Jurgens, 1989; Mardon et al., 1994). homothorax (hth), encoding a homeodomain transcription factor, and teashirt (tsh), encoding a zinc-finger transcription factor, are coexpressed in an even more proximal domain (Abu-Shaar and Mann, 1998; Erkner et al., 1999; Rieckhof et al., 1997; Wu and Cohen, 1999). The domains resulting from the expression of hth/tsh (proximal), dac (medial), and Dll (distal), together with regions that have overlapping expression of these factors, broadly define the PD axis (Fig. 7.1). Other genes, also expressed at specific positions along the PD axis, for example, those expressed in the tarsal segments, are activated later in development in response to EGFR signaling and are required for forming the joints that separate each of the leg segments (see below).
Theoretical modeling and classical limb grafting experiments both led to the idea that the juxtaposition of three different cell types—in particular, posterior, anterior dorsal, and anterior ventral—leads to the induction of new PD axes (reviewed in Baker, 2011; Fig. 7.2). We now have a molecular understanding of this phenomenon, namely, that the juxtaposition of cells expressing Decapentaplegic (Dpp) next to cells expressing Wingless (Wg), two secreted morphogens used widely in animal development, is sufficient within the context of leg development to generate a new PD axis (Campbell et al., 1993; Diaz-Benjumea et al., 1994; Lecuit and Cohen, 1997). In the wild-type leg imaginal disc, dpp and wg are expressed along the AP compartment boundary in dorsal and ventral cells, respectively, both in response to Hedgehog (Hh) emanating from the posterior compartment (Basler and Struhl, 1994; Fig. 7.2). Accordingly, in the wild-type leg disc, cells expressing Wg and Dpp are adjacent to each other only at the center of the disc, which will give rise to the distal-most portion of the appendage. Thus, the expression patterns of Hh (posterior), Dpp (dorsal-anterior), and Wg (ventral-anterior) account for the three cell types initially postulated by the grafting and theoretical studies (Fig. 7.2).
Figure 7.2.
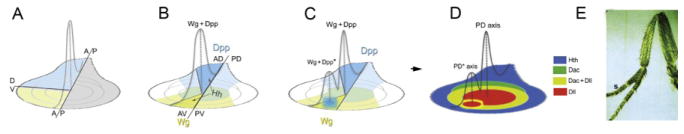
Wg+Dpp initiate the PD axis. (A) Meinhardt’s “three-sector” model for induction of the PD axis. (B) Hh, from the P compartment, induces Wg (yellow) in the anterior ventral (AV) domain and Dpp (blue) in the anterior dorsal (AD) domain; note that Wg- and Dpp-expressing cells are only adjacent in the center of the wild-type disc. (C–E) An ectopic source of Dpp (C) in the ventral domain induces an ectopic PD axis visualized in the disc (D) and in the adult appendage (E; from Campbell et al., 1993).
Significant effort over the past several years has attempted to connect these two sets of molecular players in PD axis formation. At the top of the hierarchy are Wg and Dpp, which together are sufficient for initiating a PD axis in the leg, inducing the correct expression domains of Dll, dac, and other genes expressed along this axis. How do these morphogens activate these PD genes? Is the activation direct or indirect? What is the relationship between the leg primordia and the dorsal appendage (e.g., wing) primordia? What genes provide the ventral identity of the appendage? Here, we provide an update on efforts to answer these questions and provide an initial view of the molecular architecture underlying PD axis specification.
3. The Initial Establishment of the PD Axis is Encoded in the cis-Regulatory Architecture of Dll
A common principle that has emerged from studying transcriptional regulatory mechanisms in many developmental genes is that dedicated CRMs drive small subsets of complex expression patterns (reviewed in Maeda and Karch, 2011). Dissection of the Dll locus revealed a similar level of CRM dedication, but with the additional finding that distinct Dll CRMs control Dll expression in cells that have different degrees of developmental potential (Fig. 7.3). The discovery of these CRMs has allowed lineage tracing experiments to be carried out, which helped redefine the fate map of the early appendage primordia. Below, we discuss this cis-regulatory architecture and its implications for leg development.
Figure 7.3.
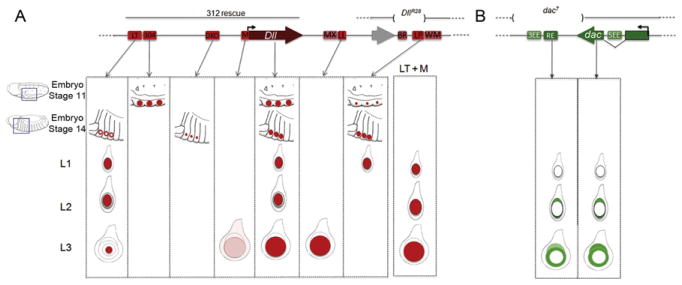
Dll and dac CRMs. (A,B) Schematic of the Dll (A) and dac (B) genomic regions showing the positions of identified CRMs (colored boxes) and transcription units (large arrows). The expression patterns driven by individual CRMs is indicated and compared to the intact genes. All CRMs are mentioned in the text except for DllMX, DllWM, dac3EE, and dac5EE, which are not active during leg development, or DllBR, which is active very late in leg development (Galindo et al., 2011; Pappu et al., 2005). A Dll rescue transgene (“312 rescue”) and a small Dll deficiency (DllR28) both result in a nearly complete PD axis, with defects primarily in the tarsal segments. dac7, which removes dacRE, is a deficiency that eliminates both dac expression and the medial Dac domain in the leg.
3.1. Dll304
The first sign of appendage formation in the Drosophila embryo is the activation of Dll at ~6h after egg laying (AEL; stage 11) in circular domains comprising ~20–30 cells per thoracic hemisegment (Cohen, 1990). At this stage, an early Dll regulatory element situated 11kb 5′ of the start of Dll transcription, called the Dll early enhancer or Dll304, is able to drive a pattern similar to Dll (Vachon et al., 1992). Genetically, Dll and Dll304 activity at this early stage depend on a positive input from Wg, but not from Dpp (Cohen, 1990; Cohen et al., 1993). In fact, Dll304 is repressed dorsally and ventrally by the Dpp and EGFR pathways, respectively (Goto and Hayashi, 1997; Kubota et al., 2000). Although a molecular dissection of the Wg, Dpp, or EGFR inputs into this enhancer has not yet been described, direct repressive inputs from the abdominal Hox proteins Ultrabithorax (Ubx) and Abdominal-A (AbdA) have been identified (Castelli-Gair and Akam, 1995; Gebelein et al., 2002, 2004; Mann, 1994; Vachon et al., 1992). By directly repressing Dll304, these Hox inputs block Dll expression and consequently limb development in the abdominal segments.
In the 1970s, Wieschaus and Gehring used X-ray somatic recombination and gynandromorphs to genetically follow marked cells by lineage analysis into the adult (Gehring et al., 1976; Wieschaus and Gehring, 1976). When induced at the blastoderm stage or earlier, marked clones often included both the T2 leg and wing, suggesting a common embryonic origin to both appendages. This conclusion has been corroborated by using the Dll304 element to carry out lineage tracing experiments, at first by following β-galactosidase perdurance from Dll304-lacZ reporter genes and later by genetic methods (Cohen et al., 1993; McKay et al., 2009). The results show that the cells that express Dll304 give rise not only to the entire ventral appendage (the entire leg) but also to all parts of the two dorsal appendages (the wing in T2 and haltere in T3; Fig. 7.4). In fact, together the ~20–30 early Dll304-expressing cells in each thoracic hemisegment have the potential to give rise to the entire adult thorax.
Figure 7.4.
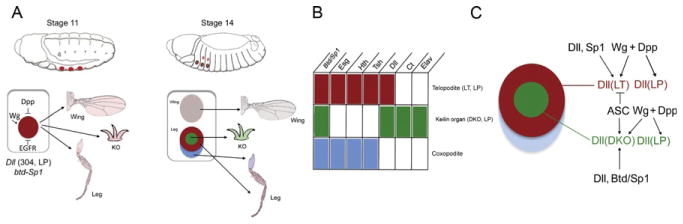
Embryonic appendage fate map. (A) Cells expressing Dll at stage 11 via the 304 CRM can give rise to the entire adult thorax, while those expressing Dll at stage 14 give rise to either the KO or telopodite, depending whether DKO or LT is driving expression, respectively. (B) Genes expressed in the progenitors to the telopodite, KO, and coxopodite. (C) Fate map and regulatory network defining the activity of Dll CRMs. The blue cells, expressing esg but not Dll, are fated to become coxopodite.
3.2. DllLT
The activity of Dll304 decays within a few hours, but Dll expression is maintained in a similarly positioned group of cells in each thoracic hemisegment, suggesting that other regulatory elements must assume control of Dll expression. One such element is the late-acting enhancer or “Leg Trigger” (DllLT), situated adjacent to Dll304 (Cohen et al., 1993; Estella et al., 2008; Vachon et al., 1992; Fig. 7.3). DllLT begins to be active at ~8h and is robustly expressed by ~10h AEL. This element is not active in all Dll-expressing cells but just in ~15 cells at the periphery of the Dll expression domain in each hemisegment (Cohen et al., 1993; McKay et al., 2009; Fig. 7.4). DllLT, in contrast to Dll304, requires positive inputs from both Wg and Dpp. Moreover, the Wg and Dpp input into DllLT is direct, mediated by several binding sites for their respective downstream transcription factors Pangolin (Pan) and Mothers against Dpp (Mad; Estella et al., 2008). Interestingly, DllLT also requires Dll for its activation, presumably derived from the earlier acting Dll304 element in the early primordia (Castelli-Gair and Akam, 1995; Estella and Mann, 2010; McKay et al., 2009).
Not only is there a temporal hand off from one Dll CRM to another, lineage tracing studies reveal that DllLT-expressing cells are dramatically more limited in developmental potential compared to Dll304-expressing cells. Specifically, the ~15 DllLT-expressing cells per hemisegment only give rise to the mature Dll and dac expression domains of the leg disc, but not to more proximal regions of the leg nor to any part of the dorsal appendages (McKay et al., 2009; Fig. 7.4). This restricted lineage is interesting for two reasons. For one, the combined Dll+Dac domain coincides with the so-called “telopodite” or “true leg” that was originally described by the American entomologist Robert Snodgrass (Snodgrass, 1935; Fig. 7.1). According to Snodgrass, the telopodite is evolutionarily distinct from the more proximal coxopodite, which evolved as a primitive and unsegmented extension from the body wall. Second, as shown more recently, the telopodite, but not the coxopodite, depends on Hh, Wg, and Dpp signaling (Diaz-Benjumea et al., 1994; Gonzalez-Crespo and Morata, 1996), and DllLT directly integrates the Wg and Dpp pathways for its activation (Estella et al., 2008). Thus, the telopodite/coxopodite subdivision concept gained significant molecular support by the observation that a single Dll CRM, DllLT, is dependent on Wg+Dpp signaling and is active in cells that give rise to the entire telopodite, and only the telopodite.
The telopodite/coxopodite subdivision idea is also supported by genetic analysis of the Dll gene, which encodes a homeodomain transcription factor: Dll null mutants lack the telopodite but retain the coxopodite (Cohen et al., 1993). There are two interesting follow-up points to be made here. First, even though Dll (via the Dll304 CRM) is expressed earlier, in the progenitors of the entire leg and wing, Dll function is only required for the development of the telopodite (Campbell et al., 1993; Cohen et al., 1993). Second, these genetic results imply that Dll is required for establishing the Dac domain, even though it is not expressed in part of the Dac domain by the end of larval development. As discussed below, these observations have recently been supported by a molecular analysis of a dac CRM, which requires direct Dll input for its activity (Giorgianni and Mann, 2011).
3.3. DllDKO
DllLT is active in only ~15 of the Dll-expressing cells in each thoracic hemisegment of stage 14 embryos. The remaining Dll-expressing cells have a neural identity as revealed by the expression of genes such as achaete (ac) and cut (ct; Bolinger and Boekhoff-Falk, 2005; Cohen and Jurgens, 1989; McKay et al., 2009). A third Dll CRM, named DllDKO (for Distal-less Keilin’s Organ) was identified ~3kb 5′ from the start of Dll transcription and is specifically activated in these Dll-expressing, DllLT-negative cells (Figs. 7.3 and 7.4). DllDKO receives positive input from members of the achaete–scute complex (ASC) and Dll, thus restricting its activity to the neurogenic cells in the limb primordia (McKay et al., 2009; Fig. 7.4).
At this point in embryogenesis, cells in the leg and wing primordia express the zinc-finger transcription factor escargot (esg), which is required to maintain diploidy and therefore an imaginal disc fate (Fuse et al., 1996; Hayashi et al., 1993). Notably, there is a gap in esg expression in the leg primordia that is filled by the expression of the neuronal genes ct and ac, suggesting that these cells do not contribute to the leg imaginal disc but instead are fated to form the Keilin’s Organ (KO), a larval sensory organ thought to be a vestige of larva legs present in more primitive insects (Bolinger and Boekhoff-Falk, 2005; Keilin, 1915). Lineage tracing experiments confirm that DllDKO-expressing cells do not give rise to any adult structures, consistent with the idea that they are dedicated to forming the KO (McKay et al., 2009; Fig. 7.4).
3.4. Other Dll CRMs
Dll304, DllLT, and DllDKO are all located within a 14-kb region 5′ to the start of Dll transcription (Fig. 7.3). Recently, additional Dll CRMs 3′ to the Dll transcription unit that are also able to produce some aspects of the Dll expression pattern in the leg imaginal disc have been identified (Galindo et al., 2011; Fig. 7.3). One element, called DllLP for Leg Primordium, is active in a subset of Dll-expressing cells in stage 10 embryos and remains active until the end of first larval instar when its activity decays. DllLP, like DllLT, is activated by Wg and Dpp in the embryo, although it is not known if this activation is direct. A second 3′ element, called DllLL for Leg Late, is only active in mid-third instar larvae and depends, like DllLT, on Dll for its activity. Although specific deletions do not exist to assess the necessity (or sufficiency) of each individual CRM, the available data suggest that both the 5′ and 3′ enhancers are important for wild-type Dll expression and function. While Dll null mutants lack the entire telopodite, a Dll minigene that includes the 5′ CRMs (including Dll304 and DllLT) but not the 3′ CRMs significantly rescues telopodite development with the exception of the tarsal segments (Galindo et al., 2011; Vachon et al., 1992). This partial rescue, which could be due to inaccurate timing or levels of expression, is very similar to a Dll deletion allele that removes the DllLP element but leaves the 5′ elements intact (Galindo et al., 2011; Fig. 7.3). Given the complexity of Dll regulation during development, it is not surprising that it is governed by multiple CRMs with partially overlapping activities.
The lineage tracing experiments cited above using individual Dll CRMs, combined with additional gene expression studies, resulted in a revised fate map of the ventral appendage primordia (Bolinger and Boekhoff-Falk, 2005; McKay et al., 2009; Fig. 7.4). At stage 14, this fate map comprises three domains that correspond to a rudimentary PD axis: (1) an esg on, hth on, tsh on, and Dll off domain that is fated to form the coxopodite and the body wall, (2) an esg on, hth on, tsh on, and Dll on (DllLT on) domain will give rise to the entire telopodite, and (3) an esg off, hth off, tsh off, and Dll on (DllDKO on) domain fated to generate the KO (Fig. 7.4). This revised fate map is distinct from an earlier version in which the overlap between hth and Dll was not recognized, likely based on an analogy with third instar discs where the expression of these factors is largely nonoverlapping (Fig. 7.1; Gonzalez-Crespo and Morata, 1996; Gonzalez-Crespo et al., 1998). The overlapping expression of Dll, hth, and tsh in the embryonic telopodite precursors is surprising and is no longer observed by the second instar stage, when these cells begin to divide. Interestingly, the coxopodite precursors (hth on, tsh on, Dll off) begin to proliferate slightly earlier than the telopodite precursors (Bryant and Schneiderman, 1969; McKay et al., 2009). The different timing in proliferation between these two domains may be a consequence of the coexpression of hth and Dll in the telopodite precursors. Consistently, the forced expression of hth in Dll-expressing cells blocks cell proliferation and telopodite formation (Azpiazu and Morata, 2002; McKay et al., 2009). Why this temporal asynchrony in the start of proliferation exists between these two domains of the leg is unknown.
4. The Role of Sp1 in Distinguishing Ventral Appendage from Dorsal Appendage Fates
Although the above focus on Dll regulation reveals how the initial PD axis and leg fate map are established, several questions remain concerning this early stage of leg development. Some of these questions are answered by two paralogous genes, buttonhead (btd) and Sp1 (Estella and Mann, 2010; Estella et al., 2003; Wimmer et al., 1996). Both genes encode Sp family zinc-finger transcription factors that share a similar expression pattern throughout development. Despite their similar expression patterns, the lack of Sp1 function (but not btd) completely abolishes leg formation. Unlike Dll, which is required only for telopodite development, Sp1 is required for the development of both the coxopodite and telopodite. Also noteworthy is that the closest vertebrate homolog of Sp1 is Sp8, which is required for limb development in the mouse, suggesting an evolutionarily conserved role for these transcription factors (Bell et al., 2003; Kawakami et al., 2004; Treichel et al., 2003).
The Sp1 expression pattern is also consistent with an early role for this transcription factor in leg development. In parallel to Dll304, Sp1 is first activated at stage 10 in the initial appendage primordia. Sp1 activation requires Wg and is repressed dorsally by Dpp and in the abdomen by Ubx. However, in contrast to Dll, btd and Sp1 are both expressed throughout the entire leg primordia, coinciding with esg expression (Estella et al., 2003). In embryos without btd and Sp1 function, Dll is activated normally but decays as the embryo develops. This is likely because Dll304 does not require Sp1/btd function, but DllLT does (Estella and Mann, 2010). Thus, Sp1 is initially activated in parallel to Dll but is required for the maintenance of Dll expression and is required for both telopodite and coxopodite fates. disconnected (disco) has also been proposed to play a role in the maintenance of Dll expression (Dey et al., 2009).
In addition to being required for the entire ventral appendage, Sp1 also appears to be required for suppressing dorsal appendage fates. When clonal analysis was used to generate ventral appendages devoid of both Sp1 and btd, in some cases dramatic homeotic transformations from leg to wing were observed in the adult (Estella and Mann, 2010). Together, these experiments suggest that Sp1 is a ventral appendage selector gene, and that in its absence dorsal appendage fates become derepressed.
Although many questions remain concerning this early aspect of appendage fate specification, there are several other relevant and interesting observations in the literature. First, as noted above, the dorsal appendage primordia (wing and haltere) and ventral appendage primordia (leg) are derived from the same set of Dll304-expressing cells. As the embryo develops, a small number of cells leave this early ventral (Dll304-expressing) primordia, migrate dorsally, stop expressing Dll, and contribute to the establishment of the dorsal appendage primordia (Cohen et al., 1993; McKay et al., 2009). Second, the balance between the sizes of the ventral and dorsal appendage primordia is sensitive to two opposing signaling pathways: Dpp and EGFR. High levels of Dpp signaling promote dorsal appendage and coxopodite fates, while high levels of EGFR signaling repress dorsal appendage fates and promote ventral appendage fates (Goto and Hayashi, 1997; Kubota et al., 2000). Finally, the T-box transcription factor-encoding genes Dorsocross1 (Doc1), Doc2, and Doc3 are required for establishing the dorsal, but not the ventral, primordia (Hamaguchi et al., 2004; Reim et al., 2003). Although the relationships between these various inputs (Sp1, Dpp, EGFR, and Doc) are not understood, these observations provide tantalizing hints at a complex process that establishes the fates of these two appendage primordia from a common group of cells.
5. Elaboration of the PD Axis: The Role of brk
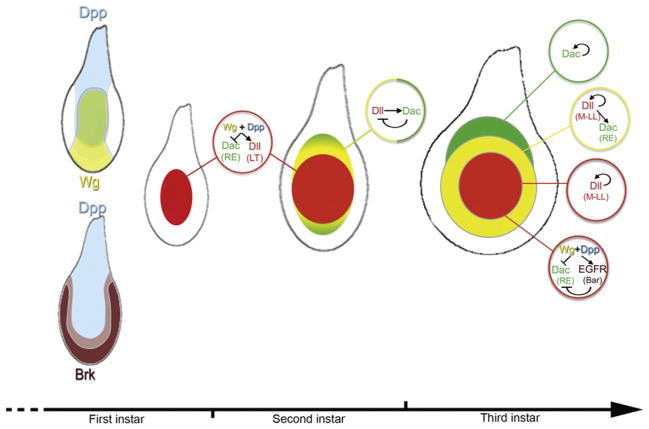
By the end of embryogenesis, a rudimentary PD axis of the leg is apparent in the expression patterns of Dll (via DllLT, DllDKO, and DllLP), hth, and tsh. How are these initial patterns elaborated upon to create the mature PD axis present in the third larval instar stage? The separation between these two time points is huge both in terms of time (96h) and tissue growth (from ~60 cells to ~10,000 cells). Also, at the end of embryogenesis, dac has not yet been activated but begins to be expressed in a circular medial domain in the second instar. Nevertheless, despite these dramatic changes, we are beginning to understand how the late embryonic patterns of gene expression evolve and are eventually stabilized during this phase of development.
As discussed above, many observations in the literature support the idea that the juxtaposition of Wg- and Dpp-expressing cells in the center of the leg disc leads to the formation of the PD axis, including the proper domains of Dll and dac expression. The activation of these genes by Wg and Dpp does not rely on a third signal, arguing that these two signals are both necessary and sufficient to induce the leg’s PD axis (Lecuit and Cohen, 1997). How can the obligate synergy between Wg and Dpp be explained at the molecular level? One answer is that, in the leg disc as in other places during fly development, Dpp functions mainly by repressing the transcriptional repressor, brinker (brk; Campbell and Tomlinson, 1999; Jazwinska et al., 1999; Minami et al., 1999). In contrast, Wg is an obligate activator of Dll and dac (Lecuit and Cohen, 1997). In the absence of Dpp, brk expands throughout the leg disc and Dll and dac fail to be activated. Importantly, however, in brk and dpp double mutants, Dll and dac expression domains are rescued, in patterns reminiscent to those in a wild-type leg (Estella and Mann, 2008). Thus, in the absence of brk, Dpp is not required for either Dll or dac activation. Further, by uncovering a role for Brk repression in dac and Dll regulation, they provided an explanation why both Wg and Dpp signals are required for PD axis formation: in the early disc, DllLT and Dll are only expressed in cells that (1) do not have Brk and (2) are exposed to high Wg levels. It was also suggested that different ratios of Wg (a positive input) and Brk (a negative input) determined whether dac or Dll would be activated (Estella and Mann, 2008). According to this idea, Dll is activated by high levels of Wg and very low or no Brk. In contrast, dac is activated by lower levels of Wg and is less sensitive to Brk repression than Dll.
6. Elaboration of the PD Axis: The Role of a Transcription Factor Cascade and Cross-regulation
In addition to positing that Wg and Dpp are critical for initiating the PD axis, Lecuit and Cohen proposed a gradient model to account for the PD axis expression patterns of dac and Dll. According to this model, the expression of dac and Dll along the PD axis depends on the levels of Wg and Dpp a cell perceives: high concentrations of both Wg and Dpp activate Dll and repress dac in the center of the leg disc; intermediate levels activate dac but not Dll in medial regions of the disc; and low levels of these morphogens fail to activate either gene (Fig. 7.5A). Two main observations supported this model (Lecuit and Cohen, 1997): (1) ectopic expression of wg in the dorsal half of the leg (where Dpp levels are high) recapitulated the wild-type nested pattern of PD gene expression, with Dll expressed closest to the Wg source and dac further away and (2) mutant clones for mad or disheveled (dsh; essential components of the Dpp and Wg pathways, respectively) in the center of the leg disc derepressed dac. In addition, based on their expression patterns, the highest levels of Wg and Dpp would be located in the center of the leg disc where the expression of these two morphogens meet, and the levels would gradually diminish in cells closer to the periphery of the disc (Fig. 7.2).
Figure 7.5.
Gradient versus cascade models. (A) The gradient model, highlighting that, depending on a cell’s position in the disc, Dll and dac CRMs must interpret very different ratios of Dpp:Wg signaling. (B) The cascade model, in which Wg+ Dpp are only required to initiate PD axis formation by activating Dll and ligands for the EGFR pathway. Dll in turn activates dac, and both Dll and dac maintain their expression in a Wg+Dpp-independent manner. EGFR activity maintains dac repression, while Wg+Dpp repress dac in the center of the leg disc early in leg development.
Although very attractive, the gradient model is difficult to envision at the molecular level, where the inputs from the Wg and Dpp signaling pathways must converge onto the CRMs of Dll and dac (Fig. 7.5A). The model is also difficult to reconcile with the observation that brk dpp mutant discs, which do not have a Dpp gradient, have a PD axis (Estella and Mann, 2008). The dissection of Dll and dac CRMs has provided insights into how these genes respond to Wg and Dpp signaling. As described above, DllLT is first activated in the progenitors of the telopodite cells in late embryogenesis. Although DllLT remains active during larval development, in third instar discs, it is restricted to cells at the center of the disc, close to where the Wg and Dpp domains touch. This is a small subset of the overall Dll domain at this time (Fig. 7.3). Moreover, in contrast to Dll, DllLT continuously depends on Wg and Dpp signals and it integrates these inputs directly by the binding of the transcription factors Mad, Brk, and Pan (Estella et al., 2008). Although Wg and Dpp meet in other tissues such as the wing disc, DllLT activity is restricted to the leg imaginal disc by Sp1 and Dll, although it is not yet known if this regulation is direct (Estella and Mann, 2010; McKay et al., 2009).
As noted above, the dependency of Dll on Wg and Dpp inputs is only transient: by the second instar, these signals are no longer required for Dll expression in the leg disc. It has been suggested that the Wg- and Dpp-independent expression phase or “maintenance” is achieved in combination with another Dll cis-regulatory element that includes the Dll transcription start site. On its own, this element, named DllM for Maintenance, is weakly active in Dll-expressing cells. But when placed in cis with DllLT, it produces an accurate and robust Dll expression pattern in the leg disc (Fig. 7.3; Estella et al., 2008). Moreover, DllLT+M, like Dll, is able to maintain its expression in the absence of Wg and Dpp inputs, in part by a positive autoregulatory feedback loop. The M element contains Dll binding sites that are required for maintenance. Interestingly, the M element is also able to produce an accurate Dll pattern when placed close to other Dll CRMs that, on their own, do not drive a Dll-like expression pattern (Estella et al., 2008). Although it is very likely that additional CRMs are involved (Estella et al., 2008; Galindo et al., 2011; Vachon et al., 1992), the “trigger-maintenance” mechanism provides a molecular explanation for how Wg and Dpp activate Dll and how Dll expression is maintained as the disc grows, despite widely varying levels of Wg+Dpp signaling in Dll domain. Importantly, this mechanism does not require Dll CRMs to integrate gradients of Dpp and Wg inputs.
The molecular dissection of a dac CRM (dacRE for Ring Enhancer), which recapitulates most of the medial expression pattern of this gene (Fig. 7.3B), suggests that dac also does not need to interpret Wg and Dpp gradients for its activation in the medial leg domain (Giorgianni and Mann, 2011). Eliminating most of the putative Pan- and Mad-binding sites has no or very little effect on dacRE activity in third instar leg discs, suggesting that this element is not integrating intermediate levels of Wg and Dpp. Instead of being activated by Wg and Dpp, dacRE is directly activated by Dll, consistent with lineage tracing experiments showing that the Dac domain is derived from DllLT-expressing cells (McKay et al., 2009). Moreover, dacRE repression in the distal tip of the leg by Wg and Dpp is transient and is maintained by other transcription factors expressed later in development (see below). In summary, instead of using a gradient mechanism, these results suggest that this phase of PD axis formation depends on a genetic cascade, in which Wg+Dpp activate Dll, and Dll activates dac (Fig. 7.5B).
If gradients of Wg and Dpp signaling are not required for Dll and dac activation, how are these two genes differentially expressed along the PD axis of the leg? One plausible scenario is as follows (Fig. 7.6): During the first and second instar leg disc, high levels of Wg and Dpp activate Dll expression, in part via DllLT, and repress dac via the dacRE element. As the disc grows, dac is activated by Dll in cells where the levels of Wg and Dpp signaling are low enough to escape repression. As cells continue to divide, some Dll- and dac-expressing cells will end up removed from peak Wg and Dpp levels and lose Dll expression, generating the Dac-only domain. In this manner, the three primary PD domains of gene expression, Dll only, Dll+ Dac, and Dac only, have been specified. At about the same time, the expression of these genes starts to become independent of the activating signals and may be maintained, in part, by an autoregulatory mechanism (via the M element for Dll) or by a transcriptional memory mechanism (Kim et al., 2008). One key aspect to this model is that Wg+Dpp are only required to initiate the PD cascade: by initially activating Dll (and repressing dac) in the center of the imaginal disc, Wg+Dpp trigger the cascade but are no longer essential after this step (Figs. 7.5B and 7.6). Although there are many aspects to this model that need confirmation, such a mechanism, coupled to tissue growth, is sufficient to account for the generation of stable domains of gene expression along the PD axis.
Figure 7.6.
Summary of the dynamic network establishing the PD axis. The relationship between Dpp, Wg, and Brk triggers the expression of Dll and represses dac in the center of the disc. As the disc grows, Dll activates dac in cells that escape repression by Wg+Dpp. These initial domains are likely maintained by a combination of autoregulation, cross-regulation, and transcriptional memory systems.
Finally, we highlight the role of cross-regulation and additional repressive interactions for the maintenance of PD domains. As noted above, the coxopodite domain is defined by the expression of hth and tsh in the body wall and coxa (Abu-Shaar and Mann, 1998; Erkner et al., 1999; Gonzalez-Crespo and Morata, 1996; Rieckhof et al., 1997; Wu and Cohen, 1999, 2000). There is evidence that the combined input from Wg and Dpp signaling represses hth and tsh expression in the telopodite cells via activation of Dll and dac (Abu-Shaar and Mann, 1998; Gonzalez-Crespo et al., 1998) and by a Dll-independent mechanism (Wu and Cohen, 1999). This Dll-independent repression mechanism may be mediated via the elbow–no ocelli gene complex, which depends on Wg and Dpp signaling for expression and delimits the appendage field in the leg imaginal disc (Weihe et al., 2004). In addition, repression of dac by the homeodomain proteins Bar (BarH1/BarH2) is one way in which dac repression is maintained in distal leg cells (Giorgianni and Mann, 2011; Kojima et al., 2000). Additional cross-regulation between PD genes is also observed among other transcription factors expressed in the tarsal segments, downstream of EGFR signaling (see below).
7. Patterning the DV Axis
If gradients of Wg and Dpp are not used to establish the PD axis, what purpose might they serve? The posterior expression of the homeodomain transcription factors engrailed (en) and invected (inv) divides the leg into anterior and posterior compartments, which have distinct cell lineages (Morata and Lawrence, 1975). In contrast to strict lineage restrictions along the AP axis, the distinction between dorsal and ventral fates is controlled by the secreted molecules Wg and Dpp in a nonlineage-dependent manner. As noted above, in the leg imaginal disc, Hh signals from the posterior compartment to anterior compartment cells to activate the expression of wg and dpp in the ventral and dorsal halves of the disc, respectively (Basler and Struhl, 1994). The anterior dorsal and anterior ventral expression of these two genes in the leg imaginal disc is maintained by a mutual antagonism that prevents the expression and/or signaling of these pathways in the other half of the disc. Dpp specifies dorsal fates and represses ventral ones, whereas Wg specifies ventral fates and represses dorsal ones (Brook and Cohen, 1996; Jiang and Struhl, 1996; Johnston and Schubiger, 1996; Morimura et al., 1996; Penton and Hoffmann, 1996; Struhl and Basler, 1993; Theisen et al., 1996; Wilder and Perrimon, 1995). Hypomorphic mutations in wg result in the mirror image duplication of the dorsal leg pattern at the expense of the ventral pattern. Analogously, mutations in dpp have the opposite effects (Brook and Cohen, 1996; Jiang and Struhl, 1996; Theisen et al., 1996). Thus, although gradients of Wg and Dpp signaling may not be required for specifying distinct PD axis fates, they appear to play a critical role in establishing positional information along the DV axis.
In the tip of the leg, all cells are likely to perceive high levels of Wg and Dpp, so understanding how a cell is able to discriminate between these signals to promote dorsal or ventral fates is an important question. One possibility is that there are dorsal and/or ventral selector genes activated downstream of these signals. Recently, Svendsen et al. (2009) characterized the expression and function of two redundant Tbx20 transcription factors, H15 and midline (mid), two genes that can fulfil this role (Svendsen et al., 2009). These genes are expressed in the ventral half of the leg disc, in a broader domain than wg that coincides with the domain deleted in wg mutants. In H15 and mid mutants, ventral leg structures are transformed to the corresponding dorsal ones, without affecting wg or dpp expression, probably due to the low levels of Dpp signaling found in the ventral domain (Blackman et al., 1991). Moreover, ectopic mid and H15 expression is able to induce ventral fates in dorsal cells. Although Dpp-dependent dorsal fates have been suggested to be mediated by the dorsal-specific T-box gene optomotor-blind (omb; Grimm and Pflugfelder, 1996; Maves and Schubiger, 1998), omb is not derepressed in mid mutant clones (Svendsen et al., 2009). One possibility is that the DV “ground state” is dorsal, but this is argued against by the observation that lateral structures are formed in legs with reduced expression of both wg and dpp (Held et al., 1994). Thus, other factors in addition to omb are probably required to define dorsal fates.
8. EGFR Signaling Patterns the Tarsus
While Wg and Dpp play an important role in initiating the PD axis, by the early third instar, Wg and Dpp are no longer required for the PD axis and the role of further elaborating this axis is handed off to the EGFR signaling pathway (Campbell, 2002; Galindo et al., 2002; Fig. 7.7). Shifts of the temperature-sensitive mutant Egfr[ts] (Egfr[tsla]/Egfr[null]) to the restrictive temperature in the beginning of the third larval stage lead to development of legs without pretarsus and one or more tarsal segments depending on the severity of the restrictive temperature. Similar results were obtained when dominant-negative forms or inhibitors of EGFR signaling components were ectopically expressed in leg discs (Campbell, 2002; Galindo et al., 2002, 2005). Genetic experiments suggest that initiation of EGFR signaling in the center of leg discs is dependent on wg, dpp, and Dll (Galindo et al., 2002, 2005). Although it is not known how this occurs at a mechanistic level, it is plausible that in this case graded EGFR activity may be important for setting up distinct PD fates in the tarsus.
Figure 7.7.
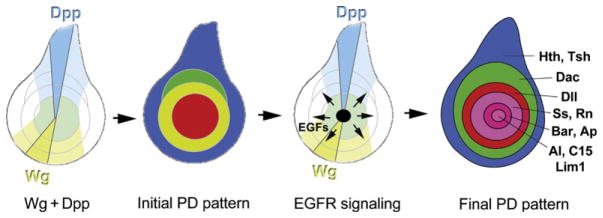
EGFR signaling patterns the tarsal segments. After the initial PD domains are established, EGFR ligands are produced at the center of the disc and activate a series of secondary PD targets in the progenitors to the tarsal segments.
EGFR signaling occurs in waves, by the consecutive activation of ligands, proteases, and inhibitors (reviewed in Shilo, 2005). In leg imaginal discs, EGFR signaling is initiated in early third instar larvae by expression of the secreted ligand Vein (Vn) in the central region of leg disc (Campbell, 2002; Galindo et al., 2002), which we refer to as the EGF Organizing Center (EOC). Shortly after vein is activated, another component of the EGFR signaling cascade is expressed: the protease Rhomboid (Rho; Campbell, 2002; Galindo et al., 2005). Rho is required for processing of the membrane-bound ligands Spitz (Spi), Keren (Krn), and Gurken (Grk); without Rho these ligands are not secreted (reviewed in Urban, 2006). Although none of the membrane-bound ligands have been detected in the center of leg discs, Vn cannot be the only ligand that plays a role in the EOC because vn single mutant leg discs do not phenocopy Egfr mutants (Campbell, 2002; Galindo et al., 2005). Only the triple mutant ru rho vn produces strong phenotypes that resemble medium Egfr[ts] leg mutants (roughoid (ru) encodes a paralog of rho). Small regions of wild-type tissue in the center of otherwise ru rho vn mutant leg discs can rescue tarsal formation (Campbell, 2002), providing additional evidence that the EOC serves as a source of secreted EGFR ligands that pattern the tarsus.
Drosophila EGFR signaling is a typical Ras–Raf–MAPK (Map kinase) signaling pathway. In the leg imaginal disc, phosphorylated MAPK is detected shortly after expression of vn in the EOC (Campbell, 2002). Moreover, the Ets transcription factor PointedP2 (PntP2), a common downstream effector of receptor tyrosine kinase (RTK) signaling in Drosophila (Brunner et al., 1994; O’Neill et al., 1994; Scholz et al., 1997), is also expressed in the EOC, and misexpression of a dominant-negative form of pntP2 (pntP2[DN]) abolishes tarsal segments 4 and 5 and pretarsus (Galindo et al., 2005). Although the truncation phenotype in this experiment is not as strong as that of Egfr[ts], it is possible that PntP2[DN] cannot completely block PntP2 activity or that additional EGFR downstream effectors participate in this process. Interestingly, gene repression by general repressors such as Capicua and Groucho was recently shown to be relieved by EGFR signaling in other developmental contexts (Ajuria et al., 2011; Cinnamon et al., 2008). Similar mechanisms might be involved in leg disc patterning by EGFR.
The expression of several transcription factors required for PD patterning is dramatically changed in Egfr mutants, Egfr mosaic clones, or ectopic EGFR activation. The results of several studies (Campbell, 2002, 2005; Galindo et al., 2002, 2005) show that high levels of EGFR signaling are required for activation of aristaless (al), C15 (clawless), and Lim1 in small overlapping circular domains in the very center of the leg discs (Fig. 7.7). These homeodomain proteins are required for the specification of the pretarsus (Campbell, 2005; Campbell and Tomlinson, 1998; Kojima et al., 2005; Pueyo and Couso, 2004; Pueyo et al., 2000; Tsuji et al., 2000). Lower levels of EGFR signaling are required for expression of another set of homeodomain-encoding genes, BarH1/BarH2 and apterous (ap), in rings surrounding the al/C15/Lim1 domain. Apterous and BarH1/BarH2 specify tarsal segments 4 and 5 (Kojima et al., 2000; Pueyo and Couso, 2004; Pueyo et al., 2000). tarsal-less (tal), spineless (ss), rotund (rn), and bric-a-brac (bab) are expressed in concentric rings just proximal to the rings of BarH1/BarH2 and ap. These genes are required for the proper development of tarsal segments 1–5 (Couderc et al., 2002; Duncan et al., 1998; Galindo et al., 2007; Godt et al., 1993; St Pierre et al., 2002). tal, ss, rn, and bab are repressed directly or indirectly by EGFR signaling in the EOC since mild loss of EGFR signaling abolishes al, C15, and Lim1 expression, while Bar, ap, ss, rn, and bab expression shifts toward the center of leg discs. Strong loss of EGFR signaling abolishes expression of most tarsal patterning genes while at the same time allows for dac expression in the center of leg discs (Campbell, 2002; Galindo et al., 2002). This latter observation is consistent with mutagenesis studies of the dacRE element, which suggested that Bar is a direct repressor of dac in third instar leg discs (Giorgianni and Mann, 2011). Spineless and the zinc-finger transcription factor Rotund are also involved in delimiting the distal margin of the dac expression domain in mid-third instar—ss; rn double mutant leg discs show expansion of the dac domain distally and the BarH1/H2 domain proximally until they juxtapose each other (Pueyo and Couso, 2008).
In addition to being targets of EGFR signaling, the tarsal PD patterning genes show complex interactions with each other. For example, Al and C15 form a protein complex and are required for the expression of Lim1, while Lim1 together with its cofactor Chip maintains the expression domain of al and C15 by repressing BarH1/H2 in the very center of leg discs (Campbell, 2005; Kojima et al., 2005; Pueyo and Couso, 2004). BarH1/H2, in turn, keeps dac off in the EOC (Giorgianni and Mann, 2011; Kojima et al., 2000) and also activates the polycistronic gene tal in a ring between its own expression domain and the dac domain (Pueyo and Couso, 2008). Interestingly, all of the above-mentioned EGFR targets in the tarsus encode transcription factors except for tal, which encodes four short peptides (Galindo et al., 2007). During embryogenesis and later during leg joint formation, these peptides act posttranslationally to cleave and modulate the activity (repressor vs. activator) of the zinc-finger transcription factor Shavenbaby (Svb; Kondo et al., 2010; Pueyo and Couso, 2011). As a result of Tal function, a cell-nonautonomous signal is released that triggers the expression of spineless and rotund in cells in between the domains of Dac and BarH1/H2 (Pueyo and Couso, 2008). bab (BTB/POZ pair of genes bab1/bab2) is expressed in a similar domain as rn and ss (Godt et al., 1993), but its early regulation seems to be independent of Tal and Ss. However, bab1/2 later expression is aberrant in ss or tal mutants suggesting that it is at least partially regulated by Ss (Chu et al., 2002; Pueyo and Couso, 2008).
9. Leg Segmentation and Growth
The process of leg segmentation, or forming the joints that separate each of the leg segments, is one critical downstream consequence of PD gene expression (Rauskolb, 2001). Several genes and pathways required for forming the joints have been defined (Bishop et al., 1999; Chu et al., 2002; Ciechanska et al., 2007; de Celis Ibeas and Bray, 2003; de Celis et al., 1998; Galindo et al., 2005; Greenberg and Hatini, 2009, 2011; Hao et al., 2003; Kerber et al., 2001; Mishra et al., 2001; Pueyo and Couso, 2011; Rauskolb and Irvine, 1999; Shirai et al., 2007). A key step in leg segmentation is the induction of the Notch ligands Delta and Serrate (Bishop et al., 1999; de Celis et al., 1998; Rauskolb and Irvine, 1999), which activate the Notch signal transduction pathway (reviewed by Greenwald, 1998; Kimble and Simpson, 1997). Notch activation at the presumptive borders between leg segments, in turn, induces the expression of several downstream genes, including d-AP2 and the odd-skipped family members, drumstick (drm), odd-skipped (odd), brother of odd with entrails limited (bowl), and sister of odd and bowl (sob; de Celis Ibeas and Bray, 2003; Hao et al., 2003; Kerber et al., 2001; Rauskolb and Irvine, 1999). Together with the nuclear protein Lines, which antagonizes Bowl, the activity of these genes establishes a feedback mechanism that stabilizes these borders, allowing joint morphogenesis to ensue (Greenberg and Hatini, 2009). In addition, EGFR signaling is induced in the interjoint regions. These waves of EGFR signaling prevent formation of supernumerary joints by antagonizing some of the Notch effector genes (Galindo et al., 2005; Shirai et al., 2007). Other Notch downstream genes, such as nubbin and d-AP2, play a role in leg growth via mechanisms that have not yet been established (Kerber et al., 2001; Rauskolb and Irvine, 1999).
10. Concluding Remarks
The above review reveals that a molecular framework of PD axis formation is now emerging. Yet, many questions remain. For one, the initial stages of dorsal and ventral primordia establishment are not well understood. How, for example, does Sp1 block dorsal appendage fates? Second, although graded levels of Wg and Dpp activities may not be relevant to elaborating the PD axis, it remains an open question whether a gradient of EGFR signaling is used to turn on its targets in the tarsus. Third, it is not clear how large a role transcriptional memory mechanisms play in maintaining PD gene expression domains once they are initially established, and how the transition from establishment to maintenance occurs. However, now that there are working hypotheses and a molecular framework for how the initial PD pattern is formed, it is likely that these and other questions will soon be answered.
Acknowledgments
We are grateful to members of the Mann, Struhl, and Johnston labs for comments, and funding from the NIH (GM058575). R. V. is a CDP Fellow of the Leukemia and Lymphoma Society.
References
- Abu-Shaar M, Mann RS. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development. 1998;125:3821–3830. doi: 10.1242/dev.125.19.3821. [DOI] [PubMed] [Google Scholar]
- Ajuria L, Nieva C, Winkler C, et al. Capicua DNA-binding sites are general response elements for RTK signaling in Drosophila. Development. 2011;138:915–924. doi: 10.1242/dev.057729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiazu N, Morata G. Distinct functions of homothorax in leg development in Drosophila. Mech Dev. 2002;119:55–67. doi: 10.1016/s0925-4773(02)00295-2. [DOI] [PubMed] [Google Scholar]
- Baker NE. Proximodistal patterning in the Drosophila leg: Models and mutations. Genetics. 2011;187:1003–1010. doi: 10.1534/genetics.111.127191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–214. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- Bell SM, Schreiner CM, Waclaw RR, et al. Sp8 is crucial for limb outgrowth and neuropore closure. Proc Natl Acad Sci USA. 2003;100:12195–12200. doi: 10.1073/pnas.2134310100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SA, Klein T, Arias AM, Couso JP. Composite signalling from Serrate and Delta establishes leg segments in Drosophila through Notch. Development. 1999;126:2993–3003. doi: 10.1242/dev.126.13.2993. [DOI] [PubMed] [Google Scholar]
- Blackman RK, Sanicola M, Raftery LA, et al. An extensive 3′ cis-regulatory region directs the imaginal disk expression of decapentaplegic, a member of the TGF-beta family in Drosophila. Development. 1991;111:657–666. doi: 10.1242/dev.111.3.657. [DOI] [PubMed] [Google Scholar]
- Bolinger RA, Boekhoff-Falk G. Distal-less functions in subdividing the Drosophila thoracic limb primordium. Dev Dyn. 2005;232:801–816. doi: 10.1002/dvdy.20329. [DOI] [PubMed] [Google Scholar]
- Brook WJ, Cohen SM. Antagonistic interactions between wingless and decapentaplegic responsible for dorsal-ventral pattern in the Drosophila Leg. Science. 1996;273:1373–1377. doi: 10.1126/science.273.5280.1373. [DOI] [PubMed] [Google Scholar]
- Brunner D, Ducker K, Oellers N, et al. The ETS domain protein pointed-P2 is a target of MAP kinase in the sevenless signal transduction pathway. Nature. 1994;370:386–389. doi: 10.1038/370386a0. [DOI] [PubMed] [Google Scholar]
- Bryant PJ, Schneiderman HA. Cell lineage, growth, and determination in the imaginal leg discs of Drosophila melanogaster. Dev Biol. 1969;20:263–290. doi: 10.1016/0012-1606(69)90015-3. [DOI] [PubMed] [Google Scholar]
- Campbell G. Distalization of the Drosophila leg by graded EGF-receptor activity. Nature. 2002;418:781–785. doi: 10.1038/nature00971. [DOI] [PubMed] [Google Scholar]
- Campbell G. Regulation of gene expression in the distal region of the Drosophila leg by the Hox11 homolog, C15. Dev Biol. 2005;278:607–618. doi: 10.1016/j.ydbio.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Campbell G, Tomlinson A. The roles of the homeobox genes aristaless and Distal-less in patterning the legs and wings of Drosophila. Development. 1998;125:4483–4493. doi: 10.1242/dev.125.22.4483. [DOI] [PubMed] [Google Scholar]
- Campbell G, Tomlinson A. Transducing the Dpp morphogen gradient in the wing of Drosophila: Regulation of Dpp targets by brinker. Cell. 1999;96:553–562. doi: 10.1016/s0092-8674(00)80659-5. [DOI] [PubMed] [Google Scholar]
- Campbell G, Weaver T, Tomlinson A. Axis specification in the developing Drosophila appendage: The role of wingless, decapentaplegic, and the homeobox gene aristaless. Cell. 1993;74:1113–1123. doi: 10.1016/0092-8674(93)90732-6. [DOI] [PubMed] [Google Scholar]
- Castelli-Gair J, Akam M. How the Hox gene Ultrabithorax specifies two different segments: The significance of spatial and temporal regulation within metameres. Development. 1995;121:2973–2982. doi: 10.1242/dev.121.9.2973. [DOI] [PubMed] [Google Scholar]
- Chu J, Dong PD, Panganiban G. Limb type-specific regulation of bric a brac contributes to morphological diversity. Development. 2002;129:695–704. doi: 10.1242/dev.129.3.695. [DOI] [PubMed] [Google Scholar]
- Ciechanska E, Dansereau DA, Svendsen PC, et al. dAP-2 and defective proventriculus regulate Serrate and Delta expression in the tarsus of Drosophila melanogaster. Genome. 2007;50:693–705. doi: 10.1139/g07-043. [DOI] [PubMed] [Google Scholar]
- Cinnamon E, Helman A, Ben-Haroush Schyr R, et al. Multiple RTK pathways downregulate Groucho-mediated repression in Drosophila embryogenesis. Development. 2008;135:829–837. doi: 10.1242/dev.015206. [DOI] [PubMed] [Google Scholar]
- Cohen SM. Specification of limb development in the Drosophila embryo by positional cues from segmentation genes. Nature. 1990;343:173–177. doi: 10.1038/343173a0. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Jurgens G. Proximal-distal pattern formation in Drosophila: Cell autonomous requirement for Distal-less gene activity in limb development. EMBO J. 1989;8:2045–2055. doi: 10.1002/j.1460-2075.1989.tb03613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Bronner G, Kuttner F, et al. Distal-less encodes a homoeodomain protein required for limb development in Drosophila. Nature. 1989;338:432–434. doi: 10.1038/338432a0. [DOI] [PubMed] [Google Scholar]
- Cohen B, Simcox AA, Cohen SM. Allocation of the thoracic imaginal primordia in the Drosophila embryo. Development. 1993;117:597–608. doi: 10.1242/dev.117.2.597. [DOI] [PubMed] [Google Scholar]
- Couderc JL, Godt D, Zollman S, et al. The bric a brac locus consists of two paralogous genes encoding BTB/POZ domain proteins and acts as a homeotic and morphogenetic regulator of imaginal development in Drosophila. Development. 2002;129:2419–2433. doi: 10.1242/dev.129.10.2419. [DOI] [PubMed] [Google Scholar]
- de Celis Ibeas JM, Bray SJ. Bowl is required downstream of Notch for elaboration of distal limb patterning. Development. 2003;130:5943–5952. doi: 10.1242/dev.00833. [DOI] [PubMed] [Google Scholar]
- de Celis JF, Tyler DM, de Celis J, Bray SJ. Notch signalling mediates segmentation of the Drosophila leg. Development. 1998;125:4617–4626. doi: 10.1242/dev.125.23.4617. [DOI] [PubMed] [Google Scholar]
- Dey BK, Zhao XL, Popo-Ola E, Campos AR. Mutual regulation of the Drosophila disconnected (disco) and Distal-less (Dll) genes contributes to proximal-distal patterning of antenna and leg. Cell Tissue Res. 2009;338:227–240. doi: 10.1007/s00441-009-0865-z. [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea FJ, Cohen B, Cohen SM. Cell interaction between compartments establishes the proximal-distal axis of Drosophila legs. Nature. 1994;372:175–179. doi: 10.1038/372175a0. [DOI] [PubMed] [Google Scholar]
- Duncan DM, Burgess EA, Duncan I. Control of distal antennal identity and tarsal development in Drosophila by spineless-aristapedia, a homolog of the mammalian dioxin receptor. Genes Dev. 1998;12:1290–1303. doi: 10.1101/gad.12.9.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons RB, Duncan D, Estes PA, et al. The spineless-aristapedia and tango bHLH-PAS proteins interact to control antennal and tarsal development in Drosophila. Development. 1999;126:3937–3945. doi: 10.1242/dev.126.17.3937. [DOI] [PubMed] [Google Scholar]
- Erkner A, Gallet A, Angelats C, et al. The role of Teashirt in proximal leg development in Drosophila: Ectopic Teashirt expression reveals different cell behaviours in ventral and dorsal domains. Dev Biol. 1999;215:221–232. doi: 10.1006/dbio.1999.9452. [DOI] [PubMed] [Google Scholar]
- Estella C, Mann RS. Logic of Wg and Dpp induction of distal and medial fates in the Drosophila leg. Development. 2008;135:627–636. doi: 10.1242/dev.014670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estella C, Mann RS. Non-redundant selector and growth-promoting functions of two sister genes, buttonhead and Sp1, in Drosophila leg development. PLoS Genet. 2010;6:e1001001. doi: 10.1371/journal.pgen.1001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estella C, Rieckhof G, Calleja M, Morata G. The role of buttonhead and Sp1 in the development of the ventral imaginal discs of Drosophila. Development. 2003;130:5929–5941. doi: 10.1242/dev.00832. [DOI] [PubMed] [Google Scholar]
- Estella C, McKay DJ, Mann RS. Molecular integration of wingless, decapentaplegic, and autoregulatory inputs into Distalless during Drosophila leg development. Dev Cell. 2008;14:86–96. doi: 10.1016/j.devcel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse N, Hirose S, Hayashi S. Determination of wing cell fate by the escargot and snail genes in Drosophila. Development. 1996;122:1059–1067. doi: 10.1242/dev.122.4.1059. [DOI] [PubMed] [Google Scholar]
- Galindo MI, Bishop SA, Greig S, Couso JP. Leg patterning driven by proximal-distal interactions and EGFR signaling. Science. 2002;297:256–259. doi: 10.1126/science.1072311. [DOI] [PubMed] [Google Scholar]
- Galindo MI, Bishop SA, Couso JP. Dynamic EGFR-Ras signalling in Drosophila leg development. Dev Dyn. 2005;233:1496–1508. doi: 10.1002/dvdy.20452. [DOI] [PubMed] [Google Scholar]
- Galindo MI, Pueyo JI, Fouix S, et al. Peptides encoded by short ORFs control development and define a new eukaryotic gene family. PLoS Biol. 2007;5:e106. doi: 10.1371/journal.pbio.0050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo MI, Fernandez-Garza D, Phillips R, Couso JP. Control of Distal-less expression in the Drosophila appendages by functional 3′ enhancers. Dev Biol. 2011;353:396–410. doi: 10.1016/j.ydbio.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebelein B, Culi J, Ryoo HD, et al. Specificity of Distalless repression and limb primordia development by abdominal Hox proteins. Dev Cell. 2002;3:487–498. doi: 10.1016/s1534-5807(02)00257-5. [DOI] [PubMed] [Google Scholar]
- Gebelein B, McKay DJ, Mann RS. Direct integration of Hox and segmentation gene inputs during Drosophila development. Nature. 2004;431:653–659. doi: 10.1038/nature02946. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Wieschaus E, Holliger M. The use of ‘normal’ and ‘transformed’ gynandromorphs in mapping the primordial germ cells and the gonadal mesoderm in Drosophila. J Embryol Exp Morphol. 1976;35:607–616. [PubMed] [Google Scholar]
- Giorgianni MW, Mann RS. Establishment of medial fates along the proximodistal axis of the Drosophila leg through direct activation of dachshund by Distalless. Dev Cell. 2011;20:455–468. doi: 10.1016/j.devcel.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt D, Couderc JL, Cramton SE, Laski FA. Pattern formation in the limbs of Drosophila: Bric a brac is expressed in both a gradient and a wave-like pattern and is required for specification and proper segmentation of the tarsus. Development. 1993;119:799–812. doi: 10.1242/dev.119.3.799. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Crespo S, Morata G. Genetic evidence for the subdivision of the arthropod limb into coxopodite and telopodite. Development. 1996;122:3921–3928. doi: 10.1242/dev.122.12.3921. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Crespo S, Abu-Shaar M, Torres M, et al. Antagonism between extradenticle function and Hedgehog signalling in the developing limb. Nature. 1998;394:196–200. doi: 10.1038/28197. [DOI] [PubMed] [Google Scholar]
- Goto S, Hayashi S. Specification of the embryonic limb primordium by graded activity of Decapentaplegic. Development. 1997;124:125–132. doi: 10.1242/dev.124.1.125. [DOI] [PubMed] [Google Scholar]
- Greenberg L, Hatini V. Essential roles for lines in mediating leg and antennal proximodistal patterning and generating a stable Notch signaling interface at segment borders. Dev Biol. 2009;330:93–104. doi: 10.1016/j.ydbio.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg L, Hatini V. Systematic expression and loss-of-function analysis defines spatially restricted requirements for Drosophila RhoGEFs and RhoGAPs in leg morphogenesis. Mech Dev. 2011;128:5–17. doi: 10.1016/j.mod.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald I. LIN-12/Notch signaling: Lessons from worms and flies. Genes Dev. 1998;12:1751–1762. doi: 10.1101/gad.12.12.1751. [DOI] [PubMed] [Google Scholar]
- Grimm S, Pflugfelder GO. Control of the gene optomotor-blind in Drosophila wing development by decapentaplegic and wingless. Science. 1996;271:1601–1604. doi: 10.1126/science.271.5255.1601. [DOI] [PubMed] [Google Scholar]
- Hamaguchi T, Yabe S, Uchiyama H, Murakami R. Drosophila Tbx6-related gene, Dorsocross, mediates high levels of Dpp and Scw signal required for the development of amnioserosa and wing disc primordium. Dev Biol. 2004;265:355–368. doi: 10.1016/j.ydbio.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Hao I, Green RB, Dunaevsky O, et al. The odd-skipped family of zinc finger genes promotes Drosophila leg segmentation. Dev Biol. 2003;263:282–295. doi: 10.1016/j.ydbio.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Hirose S, Metcalfe T, Shirras AD. Control of imaginal cell development by the escargot gene of Drosophila. Development. 1993;118:105–115. doi: 10.1242/dev.118.1.105. [DOI] [PubMed] [Google Scholar]
- Held LIJ, Heup MA, Sappington JM, Peters SD. Interactions of decapentaplegic, wingless, and Distalless in the Drosophila leg. Rouxs Arch Dev Biol. 1994;203:310–319. doi: 10.1007/BF00457802. [DOI] [PubMed] [Google Scholar]
- Jazwinska A, Kirov N, Wieschaus E, et al. The Drosophila gene brinker reveals a novel mechanism of Dpp target gene regulation. Cell. 1999;96:563–573. doi: 10.1016/s0092-8674(00)80660-1. [DOI] [PubMed] [Google Scholar]
- Jiang J, Struhl G. Complementary and mutually exclusive activities of decapentaplegic and wingless organize axial patterning during Drosophila leg development. Cell. 1996;86:401–409. doi: 10.1016/s0092-8674(00)80113-0. [DOI] [PubMed] [Google Scholar]
- Johnston LA, Schubiger G. Ectopic expression of wingless in imaginal discs interferes with decapentaplegic expression and alters cell determination. Development. 1996;122:3519–3529. doi: 10.1242/dev.122.11.3519. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Esteban CR, Matsui T, et al. Sp8 and Sp9, two closely related buttonhead-like transcription factors, regulate Fgf8 expression and limb outgrowth in vertebrate embryos. Development. 2004;131:4763–4774. doi: 10.1242/dev.01331. [DOI] [PubMed] [Google Scholar]
- Keilin D. Recherches sur les larves de dipteres cyclorhaphes. Bull Sci Fr Belg. 1915;49:15–198. [Google Scholar]
- Kerber B, Monge I, Mueller M, et al. The AP-2 transcription factor is required for joint formation and cell survival in Drosophila leg development. Development. 2001;128:1231–1238. doi: 10.1242/dev.128.8.1231. [DOI] [PubMed] [Google Scholar]
- Kim SN, Jung KI, Chung HM, et al. The pleiohomeotic gene is required for maintaining expression of genes functioning in ventral appendage formation in Drosophila melanogaster. Dev Biol. 2008;319:121–129. doi: 10.1016/j.ydbio.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Kimble J, Simpson P. The LIN-12/Notch signaling pathway and its regulation. Annu Rev Cell Dev Biol. 1997;13:333–361. doi: 10.1146/annurev.cellbio.13.1.333. [DOI] [PubMed] [Google Scholar]
- Kojima T. The mechanism of Drosophila leg development along the proximodistal axis. Dev Growth Differ. 2004;46:115–129. doi: 10.1111/j.1440-169X.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- Kojima T, Sato M, Saigo K. Formation and specification of distal leg segments in Drosophila by dual Bar homeobox genes, BarH1 and BarH2. Development. 2000;127:769–778. doi: 10.1242/dev.127.4.769. [DOI] [PubMed] [Google Scholar]
- Kojima T, Tsuji T, Saigo K. A concerted action of a paired-type homeobox gene, aristaless, and a homolog of Hox11/tlx homeobox gene, clawless, is essential for the distal tip development of the Drosophila leg. Dev Biol. 2005;279:434–445. doi: 10.1016/j.ydbio.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Kondo T, Plaza S, Zanet J, et al. Small peptides switch the transcriptional activity of Shavenbaby during Drosophila embryogenesis. Science. 2010;329:336–339. doi: 10.1126/science.1188158. [DOI] [PubMed] [Google Scholar]
- Kubota K, Goto S, Eto K, Hayashi S. EGF receptor attenuates Dpp signaling and helps to distinguish the wing and leg cell fates in Drosophila. Development. 2000;127:3769–3776. doi: 10.1242/dev.127.17.3769. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Cohen SM. Proximal–distal axis formation in the Drosophila leg. Nature. 1997;388:139–145. doi: 10.1038/40563. [DOI] [PubMed] [Google Scholar]
- Maeda RK, Karch F. Gene expression in time and space: Additive vs hierarchical organization of cis-regulatory regions. Curr Opin Genet Dev. 2011;21:187–193. doi: 10.1016/j.gde.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Mann RS. Engrailed-mediated repression of Ultrabithorax is necessary for the parasegment 6 identity in Drosophila. Development. 1994;120:3205–3212. doi: 10.1242/dev.120.11.3205. [DOI] [PubMed] [Google Scholar]
- Mardon G, Solomon NM, Rubin GM. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development. 1994;120:3473–3486. doi: 10.1242/dev.120.12.3473. [DOI] [PubMed] [Google Scholar]
- Maves L, Schubiger G. A molecular basis for transdetermination in Drosophila imaginal discs: Interactions between wingless and decapentaplegic signaling. Development. 1998;125:115–124. doi: 10.1242/dev.125.1.115. [DOI] [PubMed] [Google Scholar]
- McKay DJ, Estella C, Mann RS. The origins of the Drosophila leg revealed by the cis-regulatory architecture of the Distalless gene. Development. 2009;136:61–71. doi: 10.1242/dev.029975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami M, Kinoshita N, Kamoshida Y, et al. brinker is a target of Dpp in Drosophila that negatively regulates Dpp-dependent genes. Nature. 1999;398:242–246. doi: 10.1038/18451. [DOI] [PubMed] [Google Scholar]
- Mishra A, Agrawal N, Banerjee S, et al. Spatial regulation of DELTA expression mediates NOTCH signalling for segmentation of Drosophila legs. Mech Dev. 2001;105:115–127. doi: 10.1016/s0925-4773(01)00387-2. [DOI] [PubMed] [Google Scholar]
- Morata G, Lawrence PA. Control of compartment development by the engrailed gene in Drosophila. Nature. 1975;255:614–617. doi: 10.1038/255614a0. [DOI] [PubMed] [Google Scholar]
- Morimura S, Maves L, Chen Y, Hoffmann FM. decapentaplegic over-expression affects Drosophila wing and leg imaginal disc development and wingless expression. Dev Biol. 1996;177:136–151. doi: 10.1006/dbio.1996.0151. [DOI] [PubMed] [Google Scholar]
- O’Neill EM, Rebay I, Tjian R, Rubin GM. The activities of two Ets-related transcription factors required for Drosophila eye development are modulated by the Ras/MAPK pathway. Cell. 1994;78:137–147. doi: 10.1016/0092-8674(94)90580-0. [DOI] [PubMed] [Google Scholar]
- Panganiban G, Rubenstein JL. Developmental functions of the Distal-less/Dlx homeobox genes. Development. 2002;129:4371–4386. doi: 10.1242/dev.129.19.4371. [DOI] [PubMed] [Google Scholar]
- Panganiban G, Irvine SM, Lowe C, et al. The origin and evolution of animal appendages. Proc Natl Acad Sci USA. 1997;94:5162–5166. doi: 10.1073/pnas.94.10.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappu KS, Ostrin EJ, Middlebrooks BW, et al. Dual regulation and redundant function of two eye-specific enhancers of the Drosophila retinal determination gene dachshund. Development. 2005;132:2895–2905. doi: 10.1242/dev.01869. [DOI] [PubMed] [Google Scholar]
- Penton A, Hoffmann FM. Decapentaplegic restricts the domain of wingless during Drosophila limb patterning. Nature. 1996;382:162–164. doi: 10.1038/382162a0. [DOI] [PubMed] [Google Scholar]
- Pueyo JI, Couso JP. Chip-mediated partnerships of the homeodomain proteins Bar and Aristaless with the LIM-HOM proteins Apterous and Lim1 regulate distal leg development. Development. 2004;131:3107–3120. doi: 10.1242/dev.01161. [DOI] [PubMed] [Google Scholar]
- Pueyo JI, Couso JP. The 11-aminoacid long Tarsal-less peptides trigger a cell signal in Drosophila leg development. Dev Biol. 2008;324:192–201. doi: 10.1016/j.ydbio.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Pueyo JI, Couso JP. Tarsal-less peptides control Notch signalling through the Shavenbaby transcription factor. Dev Biol. 2011;355:183–193. doi: 10.1016/j.ydbio.2011.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueyo JI, Galindo MI, Bishop SA, Couso JP. Proximal-distal leg development in Drosophila requires the apterous gene and the Lim1 homologue dlim1. Development. 2000;127:5391–5402. doi: 10.1242/dev.127.24.5391. [DOI] [PubMed] [Google Scholar]
- Rauskolb C. The establishment of segmentation in the Drosophila leg. Development. 2001;128:4511–4521. doi: 10.1242/dev.128.22.4511. [DOI] [PubMed] [Google Scholar]
- Rauskolb C, Irvine KD. Notch-mediated segmentation and growth control of the Drosophila leg. Dev Biol. 1999;210:339–350. doi: 10.1006/dbio.1999.9273. [DOI] [PubMed] [Google Scholar]
- Reim I, Lee HH, Frasch M. The T-box-encoding Dorsocross genes function in amnioserosa development and the patterning of the dorsolateral germ band downstream of Dpp. Development. 2003;130:3187–3204. doi: 10.1242/dev.00548. [DOI] [PubMed] [Google Scholar]
- Rieckhof GE, Casares F, Ryoo HD, et al. Nuclear translocation of extradenticle requires homothorax, which encodes an extradenticle-related homeodomain protein. Cell. 1997;91:171–183. doi: 10.1016/s0092-8674(00)80400-6. [DOI] [PubMed] [Google Scholar]
- Scholz H, Sadlowski E, Klaes A, Klambt C. Control of midline glia development in the embryonic Drosophila CNS. Mech Dev. 1997;64:137–151. doi: 10.1016/s0925-4773(97)00078-6. [DOI] [PubMed] [Google Scholar]
- Shilo BZ. Regulating the dynamics of EGF receptor signaling in space and time. Development. 2005;132:4017–4027. doi: 10.1242/dev.02006. [DOI] [PubMed] [Google Scholar]
- Shirai T, Yorimitsu T, Kiritooshi N, et al. Notch signaling relieves the joint-suppressive activity of Defective proventriculus in the Drosophila leg. Dev Biol. 2007;312:147–156. doi: 10.1016/j.ydbio.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Snodgrass RE. Principles of Insect Morphology. McGraw-Hill; New York: 1935. [Google Scholar]
- St Pierre SE, Galindo MI, Couso JP, Thor S. Control of Drosophila imaginal disc development by rotund and roughened eye: Differentially expressed transcripts of the same gene encoding functionally distinct zinc finger proteins. Development. 2002;129:1273–1281. doi: 10.1242/dev.129.5.1273. [DOI] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Svendsen PC, Formaz-Preston A, Leal SM, Brook WJ. The Tbx20 homologs midline and H15 specify ventral fate in the Drosophila melanogaster leg. Development. 2009;136:2689–2693. doi: 10.1242/dev.037911. [DOI] [PubMed] [Google Scholar]
- Theisen H, Haerry TE, O’Connor MB, Marsh JL. Developmental territories created by mutual antagonism between Wingless and Decapentaplegic. Development. 1996;122:3939–3948. doi: 10.1242/dev.122.12.3939. [DOI] [PubMed] [Google Scholar]
- Treichel D, Schock F, Jackle H, et al. mBtd is required to maintain signaling during murine limb development. Genes Dev. 2003;17:2630–2635. doi: 10.1101/gad.274103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Sato A, Hiratani I, et al. Requirements of Lim1, a Drosophila LIM-homeobox gene, for normal leg and antennal development. Development. 2000;127:4315–4323. doi: 10.1242/dev.127.20.4315. [DOI] [PubMed] [Google Scholar]
- Urban S. Rhomboid proteins: Conserved membrane proteases with divergent biological functions. Genes Dev. 2006;20:3054–3068. doi: 10.1101/gad.1488606. [DOI] [PubMed] [Google Scholar]
- Vachon G, Cohen B, Pfeifle C, et al. Homeotic genes of the Bithorax complex repress limb development in the abdomen of the Drosophila embryo through the target gene Distal-less. Cell. 1992;71:437–450. doi: 10.1016/0092-8674(92)90513-c. [DOI] [PubMed] [Google Scholar]
- Weihe U, Dorfman R, Wernet MF, et al. Proximodistal subdivision of Drosophila legs and wings: The elbow-no ocelli gene complex. Development. 2004;131:767–774. doi: 10.1242/dev.00979. [DOI] [PubMed] [Google Scholar]
- Wieschaus E, Gehring W. Clonal analysis of primordial disc cells in the early embryo of Drosophila melanogaster. Dev Biol. 1976;50:249–263. doi: 10.1016/0012-1606(76)90150-0. [DOI] [PubMed] [Google Scholar]
- Wilder EL, Perrimon N. Dual functions of wingless in the Drosophila leg imaginal disc. Development. 1995;121:477–488. doi: 10.1242/dev.121.2.477. [DOI] [PubMed] [Google Scholar]
- Wimmer EA, Frommer G, Purnell BA, Jackle H. buttonhead and D-Sp1: A novel Drosophila gene pair. Mech Dev. 1996;59:53–62. doi: 10.1016/0925-4773(96)00575-8. [DOI] [PubMed] [Google Scholar]
- Wu J, Cohen SM. Proximodistal axis formation in the Drosophila leg: Subdivision into proximal and distal domains by Homothorax and Distal-less. Development. 1999;126:109–117. doi: 10.1242/dev.126.1.109. [DOI] [PubMed] [Google Scholar]
- Wu J, Cohen SM. Proximal distal axis formation in the Drosophila leg: Distinct functions of teashirt and homothorax in the proximal leg. Mech Dev. 2000;94:47–56. doi: 10.1016/s0925-4773(00)00311-7. [DOI] [PubMed] [Google Scholar]