Abstract
The control of microtubule and actin mediated events that direct the physical arrangement and separation of chromosomes during meiosis is critical since failure to maintain chromosome organization can lead to germ cell aneuploidy. Our previous studies demonstrated a role for FYN tyrosine kinase in chromosome and spindle organization and cortical polarity of the mature mammalian oocyte. In addition to Fyn, mammalian oocytes express the protein tyrosine kinase (PTK) Fer at high levels relative to other tissues and the objective of the present study was to determine the function of this kinase in the oocyte. FER kinase protein was uniformly distributed in the ooplasm of small oocytes but became concentrated in the germinal vesicle during oocyte growth. After germinal vesicle breakdown, FER associated with the MI and MII spindles. Suppression of Fer expression by siRNA knockdown in germinal vesicle stage oocytes did not prevent activation of CDK1 activity or chromosome condensation during in vitro maturation, but did arrest oocytes prior to germinal vesicle breakdown or during MI. The resultant phenotype displayed condensed chromosomes trapped in the germinal vesicle, or condensed chromosomes poorly arranged in a metaphase plate but with an underdeveloped spindle microtubule structure or chromosomes compacted into a tight sphere. The results demonstrate that, FER kinase plays a critical role in oocyte meiotic spindle microtubule dynamics and may have an additional function in germinal vesicle breakdown.
Introduction
Maturation of the mammalian oocyte requires synchronous progression and integration of many signaling pathways. Historically, focus has been directed toward maturation promoting factor (MPF), mitogen activated protein kinases (MAPK) and other primary drivers that control meiosis (Masui and Markert 1971; Peng et al. 2007; Su et al. 2002b). Meiosis in oocytes also involves additional oocyte-specific mechanisms involved in sister chromatid cohesion (Hodges et al. 2005), chromosome condensation (Swain and Smith 2007), and spindle formation (Lindeman and Pelegri 2009) which are just now beginning to be understood. The precise control of the microtubule and actin mediated events that direct the physical arrangement and separation of chromosomes during meiosis is critical since failure to maintain chromosome organization can lead to germ cell aneuploidy, a major risk to fertility especially in ageing women (Hunt and Hassold 2008). Recent studies have demonstrated that oocytes are highly specialized for protein tyrosine kinase signaling with localized protein tyrosine kinase activity occurring in the vicinity of spindle poles of MII oocytes as well as in the cortex of fertilized eggs (McGinnis and Albertini 2010; McGinnis et al. 2007). Studies revealed that the SRC family kinase FYN plays an important role in maintaining meiotic spindle organization (Kinsey et al. 2003; Luo et al. 2009; McGinnis et al. 2007; McGinnis et al. 2009; Meng et al. 2006). Suppression of FYN in oocytes by chemical inhibition, siRNA knockdown, or gene knockout led to disorganization of MI and MII spindles which frequently correlated with meiotic arrest (McGinnis et al. 2009). In addition, Fynsuppressed oocytes exhibited defects in cortical actin organization and polarity which correlated with reduced developmental competence (Luo et al. 2009; McGinnis et al. 2007; Meng et al. 2006; Tomashov-Matar et al. 2008). The above findings highlighted the importance of identifying the control mechanisms that direct spindle function in oocytes because of their importance to oocyte quality and the objective of the present study was to determine whether other protein tyrosine kinases are critical to meiosis in oocytes.
One candidate family of protein kinases which may play a role in meiotic spindle function is the Fer/Fes family. Fer and Fes/Fps are the only known members of a distinct family of non-receptor tyrosine kinases (Smithgall et al. 1998). FER-like proteins have been identified in a diverse range of species including humans and other mammals, Drosophila, C. elegans, birds and marine sponges (Cetkovic et al. 1998; Feldman et al. 1986; Katzen et al. 1991; Paulson et al. 1997; Pawson et al. 1989; Putzke et al. 2005). The FER proteins consist primarily of 4 domains: FER-CIP1 Homology (FCH), 3 coiled-coils, SH2 and C-terminal kinase domains. The kinase region includes an atypical nuclear localization signal that requires the presence of the coiled-coil and SH2 domains for regulation of nuclear exit (Ben-Dor et al. 1999). This PTK family regulates numerous cellular processes including cytoskeletal organization, cell adhesion, vesicle transport and intracellular signaling cascades (Greer 2002). The close sequence homology between Fer and Fes suggests that these kinases have similar and sometimes redundant biological functions (Greer 2002; Smithgall et al. 1998). Several studies have described Fer (also called FerT2 in mouse) expression in male germ cell maturation (Chen et al. 2003; Hazan et al. 1993; Kierszenbaum et al. 2008), where a truncated form lacking the N-terminal FCH and coiled-coil domains participates in formation of the maturing sperm heads (Hazan et al. 1993; Kierszenbaum et al. 2008; Letwin et al. 1988; Pawson et al. 1989). FER has been shown to associate with spindle microtubules in somatic cells and can phosphorylate and promote elongation of microtubules in vitro (Kogata et al. 2003; Lee 2005; Shapovalova et al. 2007). In spite of the strong evidence obtained in vitro, a requirement for FER in spindle function has not been confirmed in intact cells or gene mutant models (Kogata et al. 2003; Senis et al. 2003; Shapovalova et al. 2007) and its contribution remains an important question.
Efforts to study FER following targeted gene knockout have not been successful to date, while a FES knockout proved to be embryonic lethal (Hackenmiller et al. 2000; Hackenmiller and Simon 2002). Successful generation of kinase dead mutant FER and FES mice however suggests that FER and FES perform some critical functions unrelated to kinase activity. Kinase dead FER or FES mutant females exhibit reduced fertility (Senis et al. 2003) and double mutant crosses exhibited reduced fertility and early reproductive senescence. Expression array data indicate that oocytes express Fer at unusually high levels compared to other tissues, while Fes is barely detectable, suggesting that Fer may play an important role in oocyte develoment. The objective of the present study was to determine the function of FER in oocytes and the results demonstrate that this kinase plays an important role in meiosis. Experiments designed to test the function of this kinase during oocyte maturation indicated that the kinase is required for germinal vesicle breakdown (GVBD) and separately, for assembly and function of the meiotic spindle. Suppression of Fer caused meiotic arrest, suggesting that FER activation may be a critical element of oocyte maturation and quality.
Results
Expression of Fer kinase in oocytes
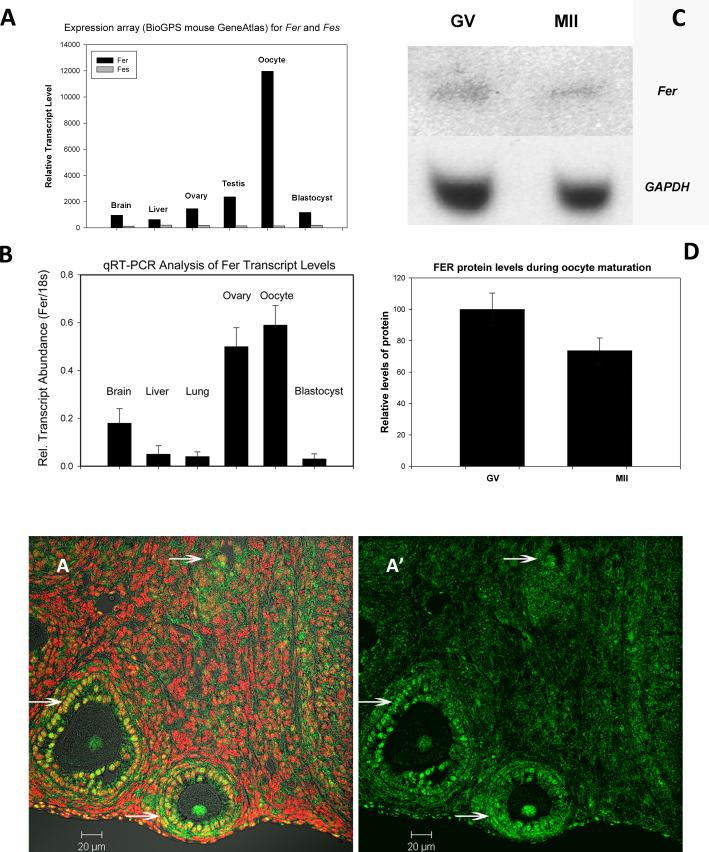
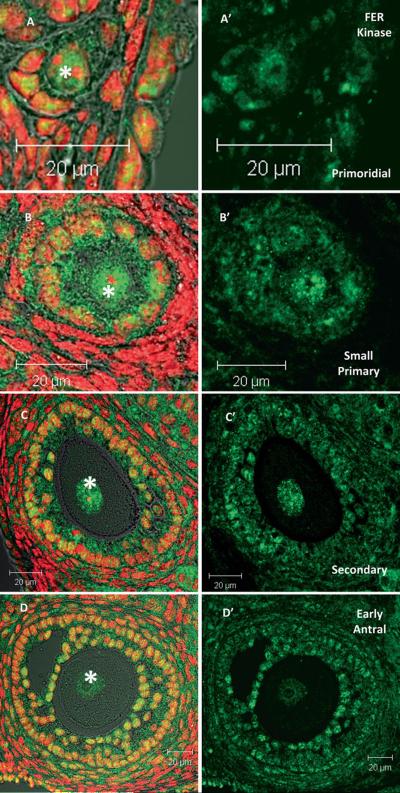
Fer kinase is expressed at a low level in most cell types (Greer 2002) however, analysis of expression array data (Novartis BioGPS, http://biogps.gnf.org (Su et al. 2002a) and http://amazonia.montp.inserm.fr (Assou et al. 2006; Assou et al. 2007; Wood et al. 2007) indicated that Fer transcripts are highly expressed (black bar Fig 1A) in human and mouse oocytes, while Fes transcripts were much lower in abundance (grey bar Fig 1A). Quantitative RT-PCR analysis confirmed elevated Fer expression in mouse oocytes (Fig 1B). Fer mRNA was easily detectable in MII oocytes (Fig. 1B) while, Fes kinase mRNA was barely detectable above background (not shown). This confirmed the expression array data and suggests that the oocyte is specialized for use of Fer and not Fes. Western blot analysis of whole ovarian tissue and oocytes demonstrated that both ovary (not shown) and oocytes (GV & MII) express FER protein (Fig. 1C). The FER antibody detected a single band at ~94 KDa, the predicted molecular weight of full length FER. FES protein was detected in whole ovary, but was not detectable in oocytes (not shown). The relative amount of FER protein in each oocyte was determined by ratio of FER to GAPDH within each set of oocytes. Although there appeared to be a slight decrease in FER during maturation from GV to MII, this change was not statistically significant (Fig. 1D; P>0.05; 4 replicates). Immunohistochemistry indicated that FER was consistently concentrated in oocytes and associated granulosa cells of ovarian follicles (Fig 1E arrows) and was less abundant in stromal components of the ovary and in the ovarian epithelium. Control sections (secondary antibody only) showed no labeling (data not shown). Detailed immunofluorescence analysis demonstrated that FER kinase undergoes subcellular redistribution during growth of the oocyte. Small oocytes enclosed in primordial and early primary follicles exhibited an even distribution of FER between the ooplasm and germinal vesicle (Fig. 2A, B). However, in growing oocytes of secondary and early antral follicles, FER was concentrated within the germinal vesicle (Fig. 2C*, D*).
Fig. 1.
Fer kinase is expressed in eggs and ovaries. Query of a mouse expression array database (BioGPS) showed that Fer kinase mRNA was expressed at very high levels in oocytes and zygotes while the closely related Fes kinase was not highly expressed in these cells (A). This was confirmed by qRT-PCR amplification of Fer transcripts from total RNA purified from different tissues presented in Panel B relative to the amplification of 18s mRNA. Western blot analysis of GV & ovulated MII stage oocytes (25 oocytes per lane) probed with anti-FER antibody detected a single protein band at 94 kDa (C). The original blot with anti-FER was stripped and reblotted for GAPDH. Metamorph software was used to determine a ratio of FER:GAPDH. This relative concentration of FER was not significantly different between GV & MII oocytes (D) The anti-FER antibody was also used to detect FER kinase in paraffin sections of ovaries from young female mice followed by alexa-488 coupled secondary antibody (green) and with Hoechst 33258 (red) to detect DNA. FER was present at a low level in most cells of the ovary (E) and was strongly expressed in follicles and oocytes (E arrows).
Fig. 2.
FER kinase localizes to the nucleus of growing oocytes. FER kinase was present in the nucleus and cytoplasm of oocytes and granulosa cells from primordial and small primary follicles (A & B). However, as oocytes grow, FER becomes localized specifically within the GV (C & D). The location of the GV in each oocyte is marked with an * (Green = FER; red = DNA. Bar = 20 μm.)
Redistribution of FER during meiotic maturation
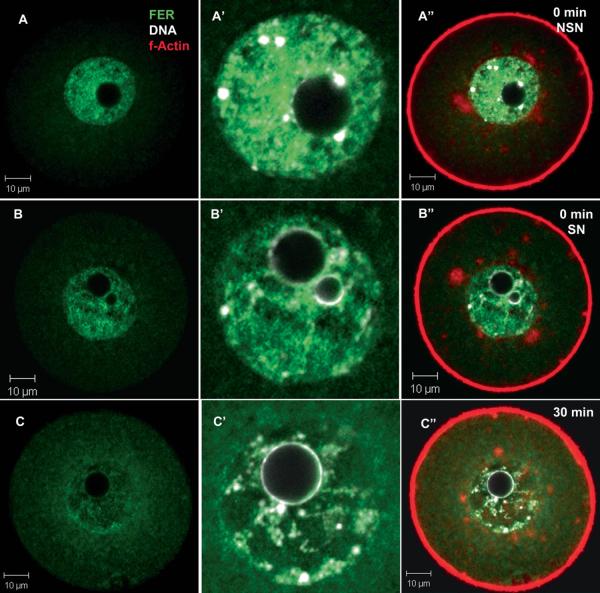
In order to study the pattern of expression of FER kinase during oocyte maturation, oocytes were collected from PMSG-primed females in medium containing cilostamide to maintain meiotic arrest at the GV stage, then washed free of the drug and allowed to mature. Confocal immunofluorescence analysis demonstrated that FER kinase was concentrated within the GV prior to acquisition of meiotic competence (nucleolus not surrounded by chromatin; NSN) (Fig. 3A) and after acquiring meiotic competence (nucleolus surrounded by chromatin; SN) (Fig. 3B). The FER-specific fluorescence in the GV quickly declined once the oocytes were washed free of cilostamide to allow maturation to proceed (Fig. 3C). During this period, FER was more easily detected in the ooplasm suggesting that some of the FER protein was released from the GV and dispersed. In addition, co-labeling with DNA indicated that FER protein within the GV seemed to surround condensing chromatin as maturation progressed (Fig. 3A’-C). While FER was seen to localize regionally along the chromatin, FER and DNA rarely overlapped (co-localized). Figure 3 also demonstrates the characteristic relationship between FER, chromatin, and peri-nuclear actin. FER was also specifically excluded from patches of peri-nuclear f-actin (detected with phalloidin) surrounding the GV (Fig. 3C”).
Fig. 3.
FER kinase is localized within the GV during meiotic arrest. Oocytes were collected from PMSG primed ovaries into FHM+cilostamide to maintain GV arrest. Incompetent NSN oocytes exhibited FER sequestered primarily within the GV (A). Competent SN oocytes also contain high levels of FER kinase located primarily within the GV (B), although they also had an increased level of FER in the cytoplasm as compared to the NSN oocytes. By 30 minutes of maturation, GV levels of FER were further reduced (C). The FER within the GV localize near to but no overlaying chromatin. (Green = FER; red = f-actin; white = DNA. Bar = 10 μm. A, B & C are confocal sections (2 μm thick) taken through the center of the chromatin and showing the entire oocyte. A’, B’ & C’ enlargements of the nuclear region within the same oocytes. A”, B” & C” are the same images as A, B & C but with red (actin) included to better show the outline of the oocytes and the actin matrix surrounding the chromatin (A” & B”).
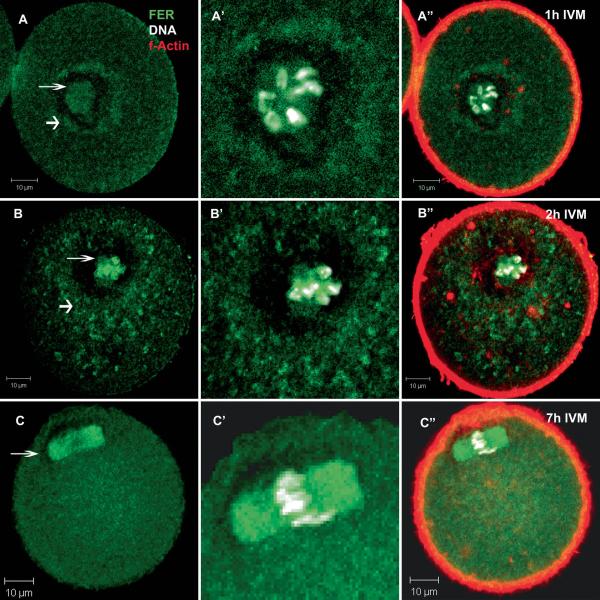
Once GVBD did occur, the pattern of FER kinase localization changed again. As the chromosomes began to associate with the forming spindle 1-2 hrs after release from meiotic arrest, FER-specific fluorescence was concentrated adjacent to or surrounding the condensed chromatin in a manner similar to that observed when the chromosomes were still retained within the GV (Fig 4A’, B’) . In addition, a ring of FER protein surrounded the filamentous actin fibers that encircled the forming spindle (Fig 4A”, B”). At metaphase I (Fig 4C) and II (not shown), FER kinase was strongly associated with the spindle itself and appeared distributed throughout the ooplasm with regular granularity.
Fig 4.
FER kinase associated with the forming meiotic spindle during prometaphase and metaphase-I. During maturation, FER kinase localized to the region surrounding condensing chromosomes and the forming spindle (A-B thin arrow). FER also was concentrated in the region immediately outside of the actin matrix which surrounds the chromatin (A-B thick arrow). By 7 hours of culture, the oocytes had reached metaphase-I with FER localized specifically to the spindle and diffusely throughout the cytoplasm (C arrow). (Green = FER; red = f-actin; white = DNA. Bar = 10 μm).
FER kinase is required for meiotic maturation
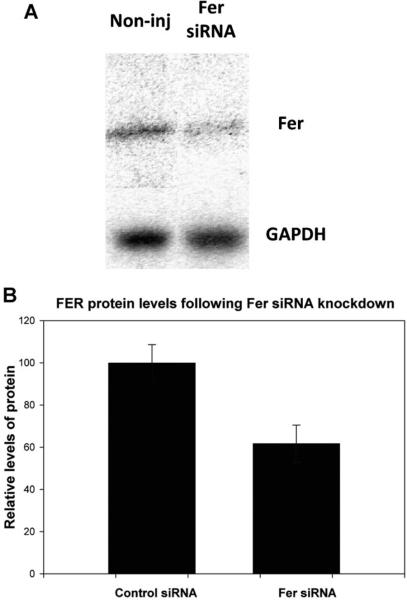
In order to determine whether FER kinase played a significant role in oocyte maturation and quality, Fer expression was suppressed by injection of siRNA complimentary to Fer during maturation in vitro. GV stage oocytes recovered from large follicles were injected with different concentrations (0.05-0.30 μM) of siRNA specific for the Fer sequence or with a scrambled control siRNA (see Materials and Methods). Western blot analysis of four experimental groups was performed to demonstrate the effectiveness of the knockdown procedure (Fig 5). The Fer siRNA (0.30 μM) reduced FER protein by ~40% in GV oocytes that were held arrested for 7h following siRNA injections (Fig 5B, P<0.05). Morphological analysis of the injected oocytes following 17h of maturation demonstrated that knockdown of FER strongly inhibited meiotic maturation while the control siRNA had no effect as seen in Table 1. Injection of Fer siRNA at all concentrations caused a delay in the rate of GVBD. Treatment with lower dosages (0.05-0.10 μM) Fer siRNA caused a concentration-dependent meiotic arrest during MI, but injection of 0.30 μM Fer siRNA also caused arrest prior to GVBD.
Fig. 5.
Western blot analysis shows a reduction in FER protein in siRNA injected eggs. Groups of 30 oocytes were injected with 0.3 μM of Fer-siRNA or a scrambled control siRNA, then allowed to mature in vitro as described in Materials and Methods. Oocytes were processed for western blot and probed for FER protein and GAPDH as described previously (A). Fer siRNA injection reduced the relative amount of Fer (B) in oocytes 40% (P=0.05; n=4 replicates).
TABLE I.
Fer siRNA Inhibition Meiotic Maturation in a Dose-Dependent Manner
| Treatment | Concentration (mM) | n | 1hr GV | 3 hr GV | 17 hr GV | MI-MII | MII |
|---|---|---|---|---|---|---|---|
| Non-injected | 0 | 36-41 | 5b | 0c | 0a | 100a | 82a |
| Control siRNA | 0.30 | 42-90 | 19b | 4c | 0a | 95ab | 76a |
| Fer siRNA | 0.05 | 39-50 | 81a | 44b | 2a | 78b | 35b |
| Fer siRNA | 0.10 | 38-45 | 98a | 60ac | 21a | 54c | 16bc |
| Fer siRNA | 0.30 | 44-53 | 100a | 93a | 54b | 31d | 2c |
Data presented as percentages of oocytes with a visible GV at 1, 3, or 17 hr of maturation or reached at least metaphase-I (MI p MII) and those that matured all the way to metaphase-II (MII) at 17 hr with 3-4 replicates per treatment. a,b,c Values in columns with different superscripts are significantly different at P < 0.05.
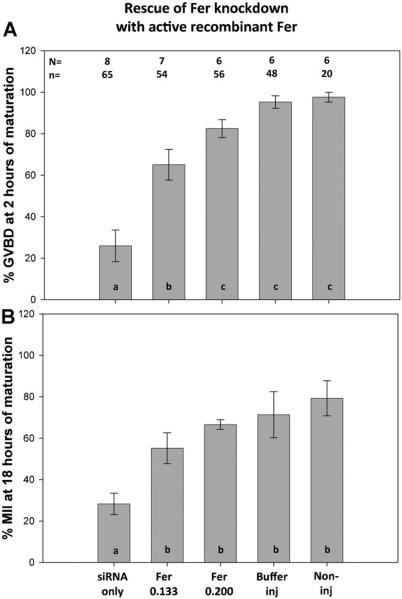
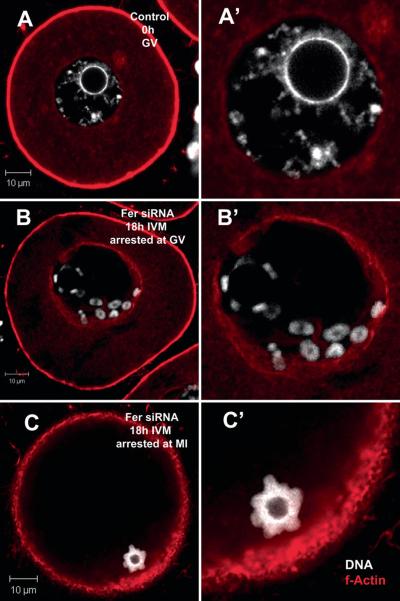
In order to demonstrate the specificity of the Fer siRNA knockdown methodology, we tested the ability of exogenous recombinant FER kinase protein to rescue the phenotype caused by siRNA suppression of Fer mRNA levels. In this experiment, Fer siRNA (0.1 μM) was co-injected with active recombinant FER kinase (0.133 μg/ml or 0.200 μg/ml). The addition of active FER protein produced a dose dependant recovery in the rate of GVBD by 2h of IVM (Fig. 6A) and the percentage of oocytes that matured to MII (Fig. 6B). MII oocytes appeared normal with spindles similar to those in control oocytes indicating that exogenous Fer kinase overcame the defect caused by siRNA knockdown of endogenous FER. In order to rule out the possibility that exogenous Fer kinase caused an independent stimulation of maturation, we injected oocytes with exogenous, active FER protein alone and compared to buffer control injected oocytes. The exogenous, active FER had no significant effect on the percentage of oocytes that matured to GVBD at 1 h (84% n=31: 89% n=22, respectively) nor on the percentage that reached MII (74%:88%, respectively) indicating that exogenous FER kinase did not produce an unregulated stimulation of maturation beyond that accomplished by the endogenous pool of native kinase. Immunofluorescence analysis revealed that the oocytes arrested as a result of Fer-specific siRNA injection had a characteristic morphology in which chromosome condensation had occurred without arrangement of the chromosomes on the spindle. Normal GV stage oocytes exhibit chromatin that is uniformly distributed within the GV with a thin strand surrounding the nucleolus (8A). Approximately 61% of the oocytes that arrested at the GV stage as a result of Fer siRNA injection exhibited condensed chromatids lying within the GV near the nuclear envelope and tightly enclosing the nucleolus (8B). In those cases where GVBD was not blocked by 0.30 μM Fer siRNA injection, 73% of the oocytes exhibited chromosomes condensed into a compact sphere of chromatids with a hollow core rather than arranged on a spindle (Fig. 7C). These chromatin structures were usually located at the oocyte cortex where the MI spindle would normally migrate, however, no spindle was observed.
Fig 6.
Rescue of the Fer siRNA phenotype by co-injection with active recombinant FER kinase. GV stage oocytes were injected either with Fer siRNA (0.1 μM) alone or coinjected with siRNA and active recombinant FER protein (0.133 or 0.200 μg/ml). Oocytes were held arrested at the GV stage for 12h then washed and allow to mature for 18h. The percentage of each group (N) of oocytes that successfully completed GVBD (A) or proceeded to MII (B) is indicated in the Y axis. Error bars indicate standard deviation of the mean. N=number of replicates; n=total number of oocytes per treatment. Data were analyzed by ANOVA with SIDAK post-hoc comparisons. Bars with different subscripts (a, b or c) are significantly different at P<0.05).
Fig. 7.
Fer knockdown caused meiotic arrest at GV or MI. Normal GV stage oocytes have diffuse chromatin throughout the nucleus and surrounding the nucleolus (A). Knock-down of Fer kinase with siRNA produced two primary phenotypes: One arrested at GV/GVBD and the other at MI. Those Fer siRNA-injected oocytes that were arrested at GVBD exhibited condensed chromatids lying close to the nuclear envelope and other chromatids surrounding the nucleolus (B). Those Fer siRNA-injected oocytes that were arrested at MI often contained compacted chromatids surrounding a spherical center without DNA. This sphere of chromatids migrated to the cell cortex as would be expected of a normal MI spindle (C). (Red = f-actin; white = DNA; A, B & C are 2 μm sections taken thru the center of the chromatin; A’, B’ & C’ are enlargements of the GV/chromatin of the same oocytes.
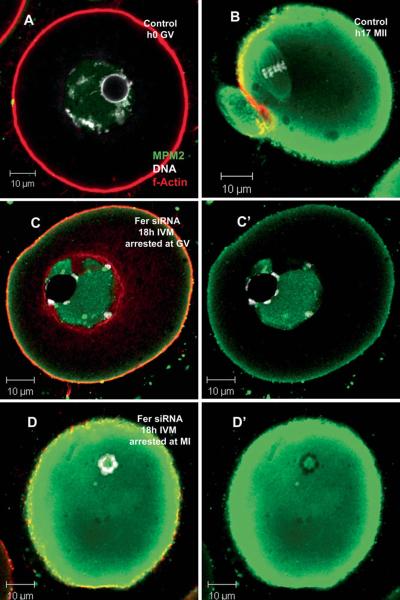
In order to determine whether the knockdown of FER kinase prevented activation of the cell cycle machinery involved in meiosis resumption, control and Fer knockdown samples were labeled with an antibody to the CDK1 target proteins (MPM2 epitope). The MPM2 antibody was designed to recognize a subset of proteins that are ser/thr phosphorylated by active CDK1 and is commonly used as an indicator of mitotic and meiotic cell cycle activation (Centonze and Borisy 1990; do Carmo Avides et al. 2001; Ito et al. 2008; Lee et al. 2006; McGinnis and Albertini 2010; Messinger and Albertini 1991; Vandre et al. 1984; Westendorf et al. 1994). The images presented in Figure 8 demonstrate that Fer kinase knockdown did not prevent CDK1 activation. Normal control GV arrested oocytes, in which CDK1 is inactive, show no MPM2 epitopes in the cytoplasm and only faint labeling within the GV (Fig. 8A). Fer knockdown oocytes that were arrested at the GV stage exhibited accumulation of MPM2 epitope within the GV and near the cell cortex indicating that CDK1 was activated in this compartment (Fig 8C). Control oocytes that have initiated maturation exhibit high levels of MPM2 epitopes throughout the cytoplasm with specific concentration in the subcortical region (Fig 8B). Those Fer knockdown oocytes that were arrested with condensed spheres of chromatin but no spindle exhibited intense MPM2 accumulation in the cytoplasm indicating that CDK1 was strongly activated in Fer siRNA-injected oocytes (Fig 8D).
Fig. 8.
Fer siRNA oocytes resume meiosis but fail to complete GVBD or to assemble an MI spindle. Oocytes injected with Fer siRNA were labeled with MPM2 antibody to detect activation of CDK1 and initiation of meiotic maturation. GV stage control oocytes have no MPM2 positive epitopes in the cytoplasm and only faint labeling within the GV (A). Control oocytes that resumed meiosis have high levels of MPM2 epitopes in the cytoplasm, especially in a sub-cortical region and associated with the metaphase spindle (B) demonstrating activation of CDK1. Fer siRNA injected oocytes that arrested at GVBD contained high levels of MPM2 epitopes within the GV and a region of the subcortex (C). Fer siRNA oocytes with condensed chromosome spheres contained high levels of MPM2 epitopes throughout the cytoplasm and especially near the cortex (D) similar to the MII stage control oocyte (B). (Green = MPM2, red = f-actin, white = DNA; C’ & D’ are the same oocytes as seen in C & D but with actin (red) turned-off to allow clearer observation of the MPM2 signal).
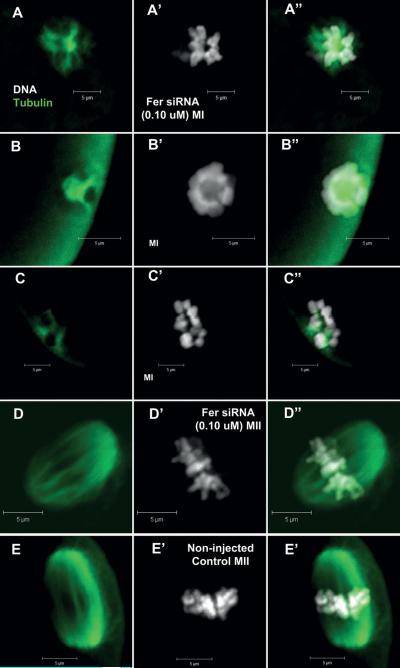
In order to determine whether the unusual morphology of MI chromosomes that resulted from Fer-knockdown might be associated with a failure of spindle formation, these oocytes were stained with anti-tubulin to visualize spindle microtubules (Fig 9). The Fer-knockdown oocytes arrested with condensed chromatids, exhibited very few microtubules which were poorly organized and short in length (Fig 9A, C) compared with controls (Fig 9E). Those Fer-knockdown oocytes that were arrested with a condensed sphere of chromosomes exhibited significant tubulin staining in the central cavity, but no evidence of organized microtubules (Fig 9B). The few Fer-knockdown oocytes with chromosomes aligned on a metaphase plate did exhibit a fairly normal spindle as detected with the anti-tubulin antibody (Fig 9D) suggesting that if Fer was not suppressed sufficiently, the spindle could form normally and remain organized.
Fig. 9.
FER depletion affects spindle formation. Oocytes injected with Fer siRNA (0.1 μM) were matured for 17h, then fixed, labeled for β-tubulin and imaged by confocal microscopy as described in Methods. Variation was seen in the presence and/or formation of microtubules that correlated with chromatin configurations. Oocytes arrested in MI with abnormal chromosomes had few or no detectable microtubules (A, B & C). The few oocytes that matured to MII exhibited apparently normal spindles (D) similar to non-injected control oocytes (E). (Green = β-tubulin; white = DNA; A=tubulin only; A’ = DNA only; A” = combined).
In summary, knockdown of Fer expression at the GV stage did not block CDK1 activation or chromosome condensation however, it had two major effects on the ability of the oocyte to mature. The first effect was to block GVBD resulting in an intact GV often containing condensed chromosomes. The second effect was apparent as a novel phenotype in which the distinct chromatids remained disorganized or grouped into a sphere, while the MI spindle failed to form or was unstable and dissociated after forming.
Discussion
Oocyte maturation is a critical step in preparing the oocyte for fertilization and defects in this process have profound implications for oocyte quality and developmental competence of the zygote. Mammalian oocytes are arrested at prophase of the first meiotic division until the signal for ovulation is received. Once released from the suppressive signals produced by follicle cells, the oocyte resumes meiosis which involves a complex series of intracellular events that are directed by protein kinases. In addition to the cell cycle kinases which act as the primary control for cell cycle resumption, functional studies using protein kinase inhibitors suggest that protein tyrosine kinases perform important steps during the complex intracellular events that are critical elements of meiosis (Zheng et al. 2007). For example, FYN is required for maintenance of spindle organization during chromosome segregation (McGinnis et al. 2009), and for maintaining cortical actin polarity once MII has been reached (Luo et al. 2009). Little is known about other protein tyrosine kinases that may perform critical steps during oocyte maturation.
The FER tyrosine kinase is highly expressed in oocytes relative to other cell types and therefore was a likely candidate for oocyte-specific functions. The results presented here demonstrate that FER is expressed in much of the ovary, but was particularly enriched in oocytes and the surrounding granulosa cells. FER was primarily concentrated within the germinal vesicle of growing and fully grown oocytes. At this stage, the level of FER kinase was more than 1-2 fold higher in the GV than in the surrounding cytoplasm. Once meiosis arrest was terminated following release from the follicle, the level of FER in the GV declined and the level in the ooplasm increased. Once the meiotic spindle formed, FER associated closely with the spindle microtubules or their associated matrix. The subcellular localization of FER in the oocyte has parallels in somatic cells. For example, FER kinase contains an atypical nuclear localization signal within the kinase region (Ben-Dor et al. 1999) and the closely related FES kinase was reported to translocate from the nucleus of interphase somatic cells to the cytoplasm during prometaphase (Carlson et al. 2005). In addition, GFP-FER and GFP-FES constructs have been localized to spindle microtubules in mitotic cells suggesting that this kinase family is capable of a specific interaction with microtubules (Kogata et al. 2003; Laurent et al. 2004). Structure function studies have implicated the FCH domain in binding to microtubules while the coiled-coil regions function in dimerization and are required for nuclear localization (Ben-Dor et al. 1999; Kim and Wong 1995; Smithgall et al. 1998).
Functionally, a role for FER/FES in microtubule dynamics and spindle assembly have been proposed (Kogata et al. 2003; Laurent et al. 2004; Shapovalova et al. 2007) and there is strong evidence for this in vitro. Functional analysis of FER has been hampered in many somatic cells by the tendency of FES and possibly SRC to compensate for the loss of FER. For example, Fer and Fes mutant mice expressing kinase dead FER or FES do not exhibit overt defects in microtubule function (Craig et al. 2001; Senis et al. 2003). Double mutant females however, have fertility defects of unknown origin with reduced numbers of pups per litter and early reproductive senescence in addition to other compromised health issues (Senis et al. 2003). Knockdown of FER within the fully grown oocyte immediately before maturation caused a dramatic inhibition of meiosis (current studies). When an mRNA is depleted immediately prior to induction of maturation, oocytes probably are unable to compensate for this sudden loss of an essential mRNA (Bachvarova 1985; Fourcroy 1982; Murchison et al. 2007). However, whole animal mutants survive because they can compensate for loss of FER kinase activity by substituting one or many of the other similarly functioning tyrosine kinases.
To determine if FER/FES may play a direct role in the oocyte, we queried online expression array databases and revealed that Fer kinase was expressed at higher levels in the oocyte than any other tissues reported for both human and mouse (Novartis BioGPS, http://biogps.gnf.org (Su et al. 2002a) and http://amazonia.montp.inserm.fr (Assou et al. 2006; Assou et al. 2007; Wood et al. 2007)). We confirmed this result by measuring mRNA and protein for FER/FES in mouse ovary and oocytes; oocytes expressed high levels of Fer mRNA and protein, while Fes was not detectable in oocytes by qRT-PCR or western blot. This suggested that the oocyte might rely heavily on FER kinase for some aspect of oocyte function. This is supported by our observation that knockdown of Fer in maturing oocytes had a very strong inhibitory effect on maturation. The siRNA injections reduced FER protein in GV arrested oocytes by ~ 40%. To ensure that the effects seen from Fer siRNA were not caused by a non-specific off target response, we conducted rescue experiments. This entailed the co-injection of active FER kinase with or without the Fer siRNA and resulted in a complete recovery of oocyte maturation to MII stage. FER kinase appears essential for progression through GVBD since Fer knockdown resulted in a high frequency of oocytes arrested with intact GVs, even though chromosome condensation had occurred. Fer knockdown also had a strongly negative effect on meiotic spindle formation or stability, with the result that most oocytes had few detectable spindle microtubules. Interestingly, Fer knockdown caused a disjunction between chromatin condensation, nuclear envelope breakdown and spindle assembly, events normally associated with activation of CDK1 (Brunet and Maro 2005). This unique stage of meiotic arrest seen in the absence of FER cannot be explained simply by the lack of spindle microtubules since Fer knockdown frequently resulted in atypical aggregation of well condensed chromosomes with distinct chromatids visible. While this might be expected in the absence of a spindle, the phenotype was subtly different from that reported for chemical suppression of spindle microtubules (FitzHarris et al. 2007) but was similar to the chromosome configuration that formed in consequence to spindle inhibition caused by TPX2 depletion (Brunet et al. 2008). The exact cause of this unusual phenotype remains an open question.
How FER kinase functions and the interacting partners involved in meiotic maturation are an intriguing unknown. Despite numerous studies reporting an association between FER/FES, spindle assembly and microtubule dynamics, no coherent model has yet evolved (Kogata et al. 2003; Laurent et al. 2004; Lee 2005; Shapovalova et al. 2007). Mechanisms by which FER could contribute to spindle assembly/organization include spindle pole associated tyrosine phosphoproteins that have been detected but have not yet been identified (McGinnis and Albertini 2010; McGinnis et al. 2007). The role of FER in GVBD may represent a process separate from its role in spindle assembly/organization. For example, FER may affect the process of nuclear envelope breakdown through regulation of actin associated and nuclear envelope proteins that are phosphorylated on tyrosine as part of the disassembly of the nuclear envelope (Otto et al. 2001; Schlosser et al. 2006; Tifft et al. 2009; Wang et al. 2008). Future identification of FER targets and associated proteins may help clarify a mechanism.
Our previous studies have focused on the SRC-family kinase FYN and its functions during oocyte maturation and fertilization (Luo et al. 2009; McGinnis et al. 2007; McGinnis et al. 2009; Sharma and Kinsey 2008; Talmor et al. 1998). Fyn, like Fer is highly expressed in mammalian oocytes. FER and FYN kinases are often part of the same signaling pathway since, FER kinase activity can be initiated following phosphorylation by FYN (Fan et al. 2004; Piedra et al. 2003). However, our studies suggest different mechanisms of action for these two kinases during meiotic maturation. Absence of Fyn kinase has no inhibitory effect on GVBD in our system (McGinnis et al. 2009), while Fer knockdown suppressed GVBD. Secondly, Fyn knockdown/knockout caused chromosome displacement on the meiotic spindle which exhibited disorganization, but had abundant microtubule content (McGinnis et al. 2009). However, Fer knockdown had a strongly negative effect on spindle formation or stability with greatly reduced microtubule formation.
Taken together, the results presented here demonstrate that the oocyte expresses Fer kinase at a high level while Fes is expressed at a low (undetectable) level. The oocyte appears to be heavily reliant on FER kinase for production or organization of the meiotic spindle and even partial reduction of the level of FER has drastic consequences for oocyte maturation. The Fer-knockdown oocyte may therefore represent an ideal model for study of the role of FER/FES kinases in microtubule dynamics which has been well characterized in vitro (Laurent et al. 2004; Smithgall et al. 1998). The combined contributions of FER and the SRC-family member FYN on meiotic spindle assembly, organization and function highlight the importance of protein tyrosine kinases on oocyte quality and defects in either of these signaling components appear to have drastic consequences for the oocyte.
Materials and methods
Oocyte collection
Cumulus-Oocyte-Complexes (COC) were collected from 6-7 week old CF1 female mice (Harlan Sprague-Dawley, Indianapolis IN). Mice were housed in a temperature and light-controlled room on a 14L:10D light cycle and experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences 1996). All experimental procedures were approved by the University of Kansas Medical Center IACUC committee. Mice were euthanized by isofluorothane inhalation anesthesia followed by cervical dislocation. Females were stimulated with 5 IU equine chorionic gonadotropin (eCG; Calbiochem, San Diego CA USA). Ovaries were collected at 42-46 hours (h) post-eCG. COC were released from large antral follicles into HEPES-buffered KSOM (FHM, Chemicon-Millipore, Billerica MA) supplemented with 4 mg/ml BSA (mFHM) and 0.1 μM Cilostamide to prevent GVBD. For experiments in which ovulated oocytes were used, female mice were stimulated with 5 IU eCG followed 48h later with 5 IU hCG and ovulated oocytes were collected from the oviducts 14-16 h post-hCG.
Fixation and immunohistochemical staining
Methods for fixation and immunohistochemistry of whole oocytes were similar to those previously reported (McGinnis et al. 2007). Briefly, oocytes were fixed for 30 min in 2% paraformaldehyde in PBS, washed twice (PBS + 4 mg/ml BSA + 0.01% Triton-X100) then permeabilized for 15 min in wash solution + 0.5% triton-X100. All fixatives and wash solutions were supplemented with 40 μM phenylarsine oxide, 100 μM sodium orthovanadate and 1 μM Calyculin-A to inhibit phosphatase activity. Antibodies used included β-tubulin (mouse monoclonal, Sigma, St Louis MO), MPM2 (mouse monoclonal, Upstate-Millipore, Billerica MA). FER kinase antibody is a rabbit monoclonal antibody directed against a sequence (GFGSDLKNSHEAVLKC) from the N-terminal domain of Fer kinase (#ab52479, Abcam Inc, Cambridge, MA). This antibody is specific for FER and does not cross-react with FES (Abcam technical support). Secondary antibodies were Alexa 488 or Alexa 568 (goat anti-mouse or goat anti-rabbit depending on the source of the primary antibody; Molecular Probes-Invitrogen, Eugene OR). Oocytes were labeled with primary antibodies at 35°C for 2h followed by secondary antibody for 1h. After secondary labeling, oocytes were transferred to wash solution containing 1 μg/ml Hoechst 33258 and 1:100 Alexa 568-phalloidin for 30 min. Oocytes were mounted and imaged immediately after labeling (mounting medium consisted of 1:1 glycerol: PBS supplemented with 5 mg/ml sodium azide and 1 μg/ml Hoechst 33258). All chemicals, hormones and reagents were purchased from Sigma Chemical Company, (St. Louis, MO) unless otherwise stated. Ovaries were fixed overnight in 4% PFA in PBS-azide supplemented with phosphatase inhibitors as described above. Fixed ovaries were processed for paraffin embedding using standard techniques. Three ovaries, each from a different donor female were placed together within each block. Sections were mounted on slides followed by deparafinization and antigen retrieval. For staining, sections were blocked with PBS+10% Goat serum overnight at 4°C then labeled with FER antibody overnight at 4°C. Sections were then washed 3x and labeled with Alexa-488 goat anti-rabbit secondary for 1.5h at room temperature. Control sections were labeled with secondary and Hoechst only. Lastly, sections were labeled with Hoechst 33258 for 5 min, blotted dry and cover slipped with Fluoromount-G (Southern Biotech, Birmingham, AL) and sealed with clear nail polish.
siRNA Knock-down of Fer PTK
siRNA knock-down was performed as previously described (McGinnis et al. 2009). Briefly, COC were collected in mFHM with 0.1 μM Cilostamide as described above. Most of the cumulus cells were removed by brief exposure to 0.3 mg/ml hyaluronidase and gentle pipetting with a fine glass pipet. Fer siRNA (#sc39022, Santa Cruz Biotechnologies, Inc, Santa Cruz, CA) and a 20-25 nt non-targeting scrambled control siRNA (#sc37007 & sc36869, Santa Cruz Biotechnologies, Inc, Santa Cruz, CA) were prepared with supplied diluent at a concentration of 100 μM. The siRNA was thawed, diluted 1:3 with water (final concentration = 33.3 μM) and centrifuged at 16,000g for 15 min at 4°C then back loaded into 0.3 μm Egg-Jek needles (MicroJek, Kansas City, KS). Injections were performed on an inverted Nikon TE200 with an Eppendorf Cell Tram Vario micro-injector. The concentrations used were determined based on our previous studies with control siRNA. The maximum volume injected was approximately twice the volume of the nucleolus. The final oocyte concentrations of Fer siRNA with the oocyte were determined as 0.30 μM, 0.10 μM, 0.05 μM Following completion of siRNA microinjections, oocytes were cultured for 5-7 h in KSOM-MAT (KSOMaa medium(Invitrogen) supplemented with 1 mM glycylglutamine, 0.23 mM pyruvate, 4 mg/ml BSA, 0.6 mM L-cysteine, 0.02 μM ascorbate, 0.1% insulin-transferrin-selenium (ITS; Sigma Corp, St Louis MO), and 10 ng/ml EGF (Calbiochem, San Diego CA); IVMh) + cilostamide to maintain GV arrest and to allow for siRNA inhibition of endogenous mRNA. Oocytes were examined at the beginning and end of this culture and graded for presence of a visible GV. Following this culture, oocytes were washed without cilostamide and matured 17h in KSOM-MAT. After IVM, oocytes were fixed and imaged with immunofluorescence as described below. Three-four sets of injected and matured eggs were processed for western blot analysis to confirm the knockdown of FER protein.
Rescue of Fer mRNA knockdown
In order to prove that Fer siRNA knockdown phenotype can be rescued by replacement with active protein, we performed a rescue experiment. Fer siRNA knockdown was performed as described above (0.1 μM). In addition, one set of Fer siRNA were co-injected with active FER kinase (GST-tagged active FER (542-end), cat #F7805, lot #039K0621, Sigma-Aldrich Co., St Louis MO). Active FER kinase was supplied at 0.1 μg/μl in buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.25 mM DTT, 0.1 mM EGTA, 0.1 mM EDTA, 0.1 mM PMSF and 25% glycerol). For consistency of injection solution, the same buffer solution was added in equal volume to Fer siRNA and control injection solutions. Final amount of active FER kinase injected into oocytes was (0.133 μg/ml and 0.200 μg/ml). The experiment was repeated 3 times with 2-4 replicates of each treatment within each repeat. Preliminary tests were conducted to determine the possible toxicity of the injection buffer and active FER alone (0.133 μg/ml). GV stage oocytes were injected with buffer equivalent to the highest dose of buffer being injected in the experiments (64% buffer diluted in water). All oocytes were held arrested in cilostamide for 12h to allow for knockdown of the mRNA, then washed, transferred to fresh media without the PDE3 inhibitor and allowed to mature for 18h. No toxic effects were observed and oocytes matured normally.
Gene expression array data
Data from the expression array database at BioGPS.gnf.org was used for the initial determinations and comparisons of Fer and Fes gene expression. The site was searched for gene expression of Fer or Fes; species Mm (murine); select GeneAtlas GNF1M, gcrma, gnf1m12658_a_at; click tab for “Downloads”. The result is a table showing the relative expression levels for each of the different tissues in this array. SigmaPlot was used to display some of these values as shown in Fig. 1A.
Quantitative RT-PCR
Groups of 10 oocytes (Fer siRNA, control siRNA and non-injected) were dissolved into 500 μl of TRIzol-Reagent (Invitrogen) and stored at −80 °C. A known concentra on of Rabbit αGlobin mRNA was added to each tube of oocytes (1.0 pg/oocyte) in TriReagent for normalization of results. The methods for cDNA preparation and qRT-PCR were as previously published (McGinnis et al. 2009). Primers for Fer and Fes kinases were generated using Primer Express 3.0 from Applied Biosystems Fer 5’-GCTCTTAATTATGGGAGATACAGTTCTGA-3’; Fer 3’ GGCAGACTCCTAGGCTGAAGG T-5’; Fes 5’GTCTCACCGATGCCGCTTGCG GAT-3’ ; Fes 3’ ACCATTATGGGCTTCTCTTCAGAGCTGTGC 5’. These primers were purchased from Integrated DNA Technologies (Coralville, IA). For comparison of Fer and Fes expression in tissues, 20 zygotes, 10 blastocysts or small pieces of tissue (brain, liver, lung and ovary) collected from the embryo donors at the time of embryo collection were dissolved in TRIzol-Reagent and processed as described (Fiedler et al. 2008). The relative levels of mRNA determined by qRT-PCR were statistically compared by t-test. P-value of less than 0.05 were considered significant.
Western Blot
MII oocytes were solubilized (10 or 20/μl) in 2x sample buffer (2x SDS–gel sample buffer containing 40 μM phenylarsine oxide and 100 μM sodium orthovanadate) and stored at −20 °C. Ovaries were collected from cycling adult CF1 females. Each ovary was transferred into 100 ul of 1x sample buffer, homogenized, centrifuged and the supernatant collected for western blotting and stored at −20 °C. Samples of oocytes (20-30 per lane) and ovaries (0.5-1.0 ul per lane) were resolved on a 10% SDS–PAGE with a 4% stacking gel. The wells in the stacking gel were formed with a comb containing teeth 1 mm in width, which facilitated analysis of very small sample volumes (1-2.5 μl). Proteins were detected with FER rabbit monoclonal antibody (described above) and Fes polyclonal goat anti-mouse antibody (sc-7671 (N19), Santa Cruz Biotechnologies, Inc, Santa Cruz, CA). Immunoblotting and detection was performed as previously described (Sharma and Kinsey 2006).
Imaging and data analysis
Oocytes were imaged by serial z-sections (2 μm thick) on a Zeiss LSM5 Pascal confocal microscope. Statistical analysis of meiotic maturation & protein content was performed using SPSS software (SPSS Inc, Chicago IL). Data were analyzed by oneway ANOVA followed by Sidek post-hoc comparisons for experiments with 3 or more treatments. P-value of less than 0.05 was considered significant. All experiments were repeated at least 3 times. Metamorph imaging software was used to determine values for FER protein expression following knockdown and rescue. For each set of oocytes run on the western blot, the ratio of FER protein to GAPDH was determined. Four to six sets of oocytes were measured for each treatment and relative values were compared by ANOVA.
Acknowledgements
This work was supported by NICHD 14846 to W.H.K., and P20RR024214 to L.K.C. from the National Center for Research Resources. LKM is supported by T32HD007455. We would like to thank Dr. David Albertini and the Live Imaging Core at KUMC for the use of the Zeiss LSM5 Pascal confocal microscope, the Albertini Lab's Nikon Eclipse microscope. We would also like to thank Lily Zhang (Kinsey lab) and Jing Huang (KUMC Histology core) for assistance with processing and labeling of ovarian sections.
Funding: NICHD 14846
Abbreviations
- FER
FES-related kinase
- FES
feline encephalitis virus
- KSOM
potassium simplex-optimized medium
- KSOM-MAT
KSOM medium with supplements for oocyte maturation
- FHM
flushing and holding medium
- PTK
protein tyrosine kinase
- CDK1
cyclin dependent kinase
- MI
metaphase-I
- MII
metaphase-II
- GV
germinal vesicle
- MPF
maturation promoting factor
- MAPK
mitogen activated protein kinase
- SH2
SRC homology domain
- FCH
FER-CIP1 homology domain
- GVBD
germinal vesicle breakdown
- eCG
equine chorionic gonadotropin
- hCG
human chorionic gonadotropin
- IVM
in vitro maturation
- GFP
green fluorescent protein
- COC
cumulus-oocyte complex
- IACUC
internal animal care and use committee
- PFA
paraformaldehyde
- ITS
insulin transferrin-selenium solution
- EGF
epidermal growth factor
- GST
glutathione S transferase
- PDE3
phosphodiesterase-3
- PIPES
piperazine-N,N′-bis[2-ethanesulfonic acid]
- EGTA
ethylene glycol-bis(β- aminoethyl ether)N,N,N,N-tetraacetic acid
- PBS
phosphate-buffered saline
- BSA
bovine serum albumin
- HEPES
(2hydroxyethyl)piperazine-N′- [2-ethanesulfonic acid])
- DMSO
dimethylsuphoxide
References
- Assou S, Anahory T, Pantesco V, Le Carrour T, Pellestor F, Klein B, Reyftmann L, Dechaud H, De Vos J, Hamamah S. The human cumulus-oocyte complex gene-expression profile. Hum Reprod. 2006;21:1705–1719. doi: 10.1093/humrep/del065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assou S, Le Carrour T, Tondeur S, Strom S, Gabelle A, Marty S, Nadal L, Pantesco V, Reme T, Hugnot JP, Gasca S, Hovatta O, Hamamah S, Klein B, De Vos J. A meta-analysis of human embryonic stem cells transcriptome integrated into a web-based expression atlas. Stem Cells. 2007;25:961–973. doi: 10.1634/stemcells.2006-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachvarova R. Gene expression oduring oogenesis and oocyte development in mammals. Dev Biol. 1985;1:453–524. doi: 10.1007/978-1-4615-6814-8_11. [DOI] [PubMed] [Google Scholar]
- Ben-Dor I, Bern O, Tennenbaum T, Nir U. Cell cycle-dependent nuclear accumulation of the p94fer tyrosine kinase is regulated by its NH2 terminus and is affected by kinase domain integrity and ATP binding. Cell Growth Differ. 1999;10:113–129. [PubMed] [Google Scholar]
- Brunet S, Dumont J, Lee KW, Kinoshita K, Hikal P, Gruss OJ, Maro B, Verlhac MH. Meiotic regulation of TPX2 protein levels governs cell cycle progression in mouse oocytes. PLoS ONE. 2008;3:e3338. doi: 10.1371/journal.pone.0003338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet S, Maro B. Cytoskeleton and cell cycle control during meiotic maturation of the mouse oocyte: integrating time and space. Reproduction. 2005;130:801–811. doi: 10.1530/rep.1.00364. [DOI] [PubMed] [Google Scholar]
- Carlson A, Yates KE, Slamon DJ, Gasson JC. Spatial and temporal changes in the subcellular localization of the nuclear protein-tyrosine kinase, c-Fes. DNA Cell Biol. 2005;24:225–234. doi: 10.1089/dna.2005.24.225. [DOI] [PubMed] [Google Scholar]
- Centonze VE, Borisy GG. Nucleation of microtubules from mitotic centrosomes is modulated by a phosphorylated epitope. J Cell Sci. 1990;95(Pt 3):405–411. doi: 10.1242/jcs.95.3.405. [DOI] [PubMed] [Google Scholar]
- Cetkovic H, Muller IM, Muller WE, Gamulin V. Characterization and phylogenetic analysis of a cDNA encoding the Fes/FER related, non-receptor protein-tyrosine kinase in the marine sponge sycon raphanus. Gene. 1998;216:77–84. doi: 10.1016/s0378-1119(98)00320-5. [DOI] [PubMed] [Google Scholar]
- Chen YM, Lee NP, Mruk DD, Lee WM, Cheng CY. Fer kinase/FerT and adherens junction dynamics in the testis: an in vitro and in vivo study. Biol Reprod. 2003;69:656–672. doi: 10.1095/biolreprod.103.016881. [DOI] [PubMed] [Google Scholar]
- Craig AWB, Zirngibl R, Williams K, Cole L-A, Greer PA. Mice Devoid of Fer Protein-Tyrosine Kinase Activity Are Viable and Fertile but Display Reduced Cortactin Phosphorylation. Mol Cell Biol. 2001;21:603–613. doi: 10.1128/MCB.21.2.603-613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo Avides M, Tavares A, Glover DM. Polo kinase and Asp are needed to promote the mitotic organizing activity of centrosomes. Nat Cell Biol. 2001;3:421–424. doi: 10.1038/35070110. [DOI] [PubMed] [Google Scholar]
- Fan L, Di Ciano-Oliveira C, Weed SA, Craig AWB, Greer PA, Rotstein OD, Kapus As. Actin depolymerization-induced tyrosine phosphorylation of cortactin: the role of Fer kinase. Biochem J. 2004;380:581–591. doi: 10.1042/BJ20040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman RA, Tam JP, Hanafusa H. Antipeptide antiserum identifies a widely distributed cellular tyrosine kinase related to but distinct from the c-fps/fes-encoded protein. Mol Cell Biol. 1986;6:1065–1073. doi: 10.1128/mcb.6.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler SD, Carletti MZ, Hong X, Christenson LK. Hormonal regulation of MicroRNA expression in periovulatory mouse mural granulosa cells. Biol Reprod. 2008;79:1030–1037. doi: 10.1095/biolreprod.108.069690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzHarris G, Marangos P, Carroll J. Changes in endoplasmic reticulum structure during mouse oocyte maturation are controlled by the cytoskeleton and cytoplasmic dynein. Dev Biol. 2007;305:133–144. doi: 10.1016/j.ydbio.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Fourcroy JL. RNA synthesis in immature mouse oocyte development. J Exp Zool. 1982;219:257–266. doi: 10.1002/jez.1402190214. [DOI] [PubMed] [Google Scholar]
- Greer P. Closing in on the biological functions of Fps/Fes and Fer. Nat Rev Mol Cell Biol. 2002;3:278–289. doi: 10.1038/nrm783. [DOI] [PubMed] [Google Scholar]
- Hackenmiller R, Kim J, Feldman RA, Simon MC. Abnormal Stat activation, hematopoietic homeostasis, and innate immunity in c-fes-/− mice. Immunity. 2000;13:397–407. doi: 10.1016/s1074-7613(00)00039-x. [DOI] [PubMed] [Google Scholar]
- Hackenmiller R, Simon MC. Truncation of c-fes via gene targeting results in embryonic lethality and hyperproliferation of hematopoietic cells. Dev Biol. 2002;245:255–269. doi: 10.1006/dbio.2002.0643. [DOI] [PubMed] [Google Scholar]
- Hazan B, Bern O, Carmel M, Lejbkowicz F, Goldstein RS, Nir U. ferT encodes a meiosisspecific nuclear tyrosine kinase. Cell Growth Differ. 1993;4:443–449. [PubMed] [Google Scholar]
- Hodges CA, Revenkova E, Jessberger R, Hassold TJ, Hunt PA. SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat Genet. 2005;37:1351–1355. doi: 10.1038/ng1672. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad. Trends in Genetics. 2008;24:86. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Ito J, Yoon S-Y, Lee B, Vanderheyden V, Vermassen E, Wojcikiewicz R, Alfandari D, Smedt HD, Parys JB, Fissore RA. Inositol 1,4,5-trisphosphate receptor 1, a widespread CA2+ channel, is a novel substrate of polo-like kinase 1 in eggs. Dev Biol. 2008;320:402–413. doi: 10.1016/j.ydbio.2008.05.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzen AL, Montarras D, Jackson J, Paulson RF, Kornberg T, Bishop JM. A gene related to the proto-oncogene fps/fes is expressed at diverse times during the life cycle of Drosophila melanogaster. Mol Cell Biol. 1991;11:226–239. doi: 10.1128/mcb.11.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum AL, Rivkin E, Tres LL. Expression of Fer testis (FerT) tyrosine kinase transcript variants and distribution sites of FerT during the development of the acrosomeacroplaxome- manchette complex in rat spermatids. Dev Dyn. 2008;237:3882–3891. doi: 10.1002/dvdy.21789. [DOI] [PubMed] [Google Scholar]
- Kim L, Wong TW. The cytoplasmic tyrosine kinase FER is associated with the cateninlike substrate pp120 and is activated by growth factors. Mol Cell Biol. 1995;15:4553–4561. doi: 10.1128/mcb.15.8.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey WH, Wu W, Macgregor E. Activation of Src-family PTK activity at fertilization: role of the SH2 domain. Dev Biol. 2003;264:255–262. doi: 10.1016/j.ydbio.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Kogata N, Masuda M, Kamioka Y, Yamagishi A, Endo A, Okada M, Mochizuki N. Identification of Fer tyrosine kinase localized on microtubules as a platelet endothelial cell adhesion molecule-1 phosphorylating kinase in vascular endothelial cells. Mol Biol Cell. 2003;14:3553–3564. doi: 10.1091/mbc.E03-02-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent CE, Delfino FJ, Cheng HY, Smithgall TE. The Human c-Fes Tyrosine Kinase Binds Tubulin and Microtubules through Separate Domains and Promotes Microtubule Assembly. Mol Cell Biol. 2004;24:9351–9358. doi: 10.1128/MCB.24.21.9351-9358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Vermassen E, Yoon S-Y, Vanderheyden V, Ito J, Alfandari D, De Smedt H, Parys JB, Fissore RA. Phosphorylation of IP3R1 and the regulation of [Ca2+]i responses at fertilization: a role for the MAP kinase pathway. Development. 2006;133:4355–4365. doi: 10.1242/dev.02624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH. Interaction of nonreceptor tyrosine-kinase Fer and p120 catenin is involved in neuronal polarization. Mol Cells. 2005;20:256–262. [PubMed] [Google Scholar]
- Letwin K, Yee SP, Pawson T. Novel protein-tyrosine kinase cDNAs related to fps/fes and eph cloned using anti-phosphotyrosine antibody. Oncogene. 1988;3:621–627. [PubMed] [Google Scholar]
- Lindeman RE, Pelegri F. Vertebrate maternal-effect genes: Insights into fertilization, early cleavage divisions, and germ cell determinant localization from studies in the zebrafish. Mol Reprod Dev. 2009;77:299–313. doi: 10.1002/mrd.21128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, McGinnis LK, Kinsey WH. Fyn kinase activity is required for normal organization and functional polarity of the mouse oocyte cortex. Mol Reprod Dev. 2009;76:819–831. doi: 10.1002/mrd.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971;177:129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- McGinnis LK, Albertini DF. Dynamics of protein phosphorylation during meiotic maturation. J Assist Reprod Genet. 2010;27:169–182. doi: 10.1007/s10815-010-9391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis LK, Albertini DF, Kinsey WH. Localized activation of Src-family protein kinases in the mouse egg. Dev Biol. 2007;306:241–254. doi: 10.1016/j.ydbio.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis LK, Kinsey WH, Albertini DF. Functions of Fyn kinase in the completion of meiosis in mouse oocytes. Dev Biol. 2009;327:280–287. doi: 10.1016/j.ydbio.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Luo J, Li C, Kinsey WH. Role of Src homology 2 domain-mediated PTK signaling in mouse zygotic development. Reproduction. 2006;132:413–421. doi: 10.1530/rep.1.01151. [DOI] [PubMed] [Google Scholar]
- Messinger SM, Albertini DF. Centrosome and microtubule dymanics during meiotic progression in the mouse oocyte. J Cell Sci. 1991;100:289–298. doi: 10.1242/jcs.100.2.289. [DOI] [PubMed] [Google Scholar]
- Murchison EP, Stein P, Xuan Z, Pan H, Zhang MQ, Schultz RM, Hannon GJ. Critical roles for Dicer in the female germline. Genes Dev. 2007;21:682–693. doi: 10.1101/gad.1521307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto H, Dreger M, Bengtsson L, Hucho F. Identification of tyrosine-phosphorylated proteins associated with the nuclear envelope. Eur J Biochem. 2001;268:420–428. doi: 10.1046/j.1432-1033.2001.01901.x. [DOI] [PubMed] [Google Scholar]
- Paulson R, Jackson J, Immergluck K, Bishop JM. The DFer gene of Drosophilamelanogaster encodes two membrane-associated proteins that can both transform vertebrate cells. Oncogene. 1997;14:641–652. doi: 10.1038/sj.onc.1200875. [DOI] [PubMed] [Google Scholar]
- Pawson T, Letwin K, Lee T, Hao QL, Heisterkamp N, Groffen J. The FER gene is evolutionarily conserved and encodes a widely expressed member of the FPS/FES protein-tyrosine kinase family. Mol Cell Biol. 1989;9:5722–5725. doi: 10.1128/mcb.9.12.5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng A, Lewellyn AL, Maller JL. Undamaged DNA transmits and enhances DNA damage checkpoint signals in early embryos. Mol Cell Biol. 2007;27:6852–6862. doi: 10.1128/MCB.00195-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedra J, Miravet S, Castano J, Palmer HG, Heisterkamp N, Garcia de Herreros A, Dunach M. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction. Mol Cell Biol. 2003;23:2287–2297. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzke AP, Hikita ST, Clegg DO, Rothman JH. Essential kinase-independent role of a Ferlike non-receptor tyrosine kinase in Caenorhabditis elegans morphogenesis. Development. 2005;132:3185–3195. doi: 10.1242/dev.01900. [DOI] [PubMed] [Google Scholar]
- Schlosser A, Amanchy R, Otto H. Identification of tyrosine-phosphorylation sites in the nuclear membrane protein emerin. FEBS J. 2006;273:3204. doi: 10.1111/j.1742-4658.2006.05329.x. [DOI] [PubMed] [Google Scholar]
- Senis YA, Craig AWB, Greer PA. Fps/Fes and Fer protein-tyrosinekinases play redundant roles in regulating hematopoiesis. Exp Hematol. 2003;31:673–681. doi: 10.1016/s0301-472x(03)00107-3. [DOI] [PubMed] [Google Scholar]
- Shapovalova Z, Tabunshchyk K, Greer PA. The Fer tyrosine kinase regulates an axon retraction response to Semaphorin 3A in dorsal root ganglion neurons. BMC Dev Biol. 2007;7:133. doi: 10.1186/1471-213X-7-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Kinsey WH. Fertilization triggers localized activation of Src-family protein kinases in the zebrafish egg. Dev Biol. 2006;295:604–614. doi: 10.1016/j.ydbio.2006.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Kinsey WH. Regionalized calcium signaling in zebrafish fertilization. Int J Dev Biol. 2008;52:561–570. doi: 10.1387/ijdb.072523ds. [DOI] [PubMed] [Google Scholar]
- Smithgall TE, Rogers JA, Peters KL, Li J, Briggs SD, Lionberger JM, Cheng H, Shibata A, Scholtz B, Schreiner S, Dunham N. The c-Fes family of protein-tyrosine kinases. Crit Rev Oncog. 1998;9:43–62. doi: 10.1615/critrevoncog.v9.i1.40. [DOI] [PubMed] [Google Scholar]
- Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, Patapoutian A, Hampton GM, Schultz PG, Hogenesch JB. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci U S A. 2002a;99:4465–4470. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y-Q, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ. Mitogen-Activated Protein Kinase Activity in Cumulus Cells Is Essential for Gonadotropin-Induced Oocyte Meiotic Resumption and Cumulus Expansion in the Mouse. Endocrinology. 2002b;143:2221–2232. doi: 10.1210/endo.143.6.8845. [DOI] [PubMed] [Google Scholar]
- Swain JE, Smith GD. Reversible phosphorylation and regulation of mammalian oocyte meiotic chromatin remodeling and segregation. Soc Reprod Fertil Suppl. 2007;63:343–358. [PubMed] [Google Scholar]
- Talmor A, Kinsey WH, Shalgi R. Expression and immunolocalization of p59c-fyn tyrosine kinase in rat eggs. Dev Biol. 1998;194:38–46. doi: 10.1006/dbio.1997.8816. [DOI] [PubMed] [Google Scholar]
- Tifft KE, Bradbury KA, Wilson KL. Tyrosine phosphorylation of nuclear-membrane protein emerin by Src, Abl and other kinases. J Cell Sci. 2009;122:3780–3790. doi: 10.1242/jcs.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomashov-Matar R, Levi M, Shalgi R. The involvement of Src family kinases (SFKs) in the events leading to resumption of meiosis. Mol Cell Endocrinol. 2008;282:56–62. doi: 10.1016/j.mce.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Vandre DD, Davis FM, Rao PN, Borisy GG. Phosphoproteins are components of mitotic microtubule organizing centers. Proc Natl Acad Sci U S A. 1984;81:4439–4443. doi: 10.1073/pnas.81.14.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Chen L, Ding Y, Jin J, Liao K. Centrosome separation driven by actin microfilaments during mitosis is mediated by centrosome-associated tyrosinephosphorylated cortactin. J Cell Sci. 2008;121:1334–1343. doi: 10.1242/jcs.018176. [DOI] [PubMed] [Google Scholar]
- Westendorf JM, Rao PN, Gerace L. Cloning of cDNAs for M-phase phosphoproteins recognized by the MPM2 monoclonal antibody and determination of the phosphorylated epitope. Proc Natl Acad Sci U S A. 1994;91:714–718. doi: 10.1073/pnas.91.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JR, Dumesic DA, Abbott DH, Strauss JF., III. Molecular Abnormalities in Oocytes from Women with Polycystic Ovary Syndrome Revealed by Microarray Analysis. J Clin Endocrinol Metab. 2007;92:705–713. doi: 10.1210/jc.2006-2123. [DOI] [PubMed] [Google Scholar]
- Zheng K-G, Meng X-Q, Yang Y, Yu Y-S, Liu D-C, Li Y-L. Requirements of Src family kinase during meiotic maturation in mouse oocyte. Mol Reprod Dev. 2007;74:126–131. doi: 10.1002/mrd.20613. [DOI] [PubMed] [Google Scholar]