Abstract
Specification and maturation of insulin+ cells accompanies a transition in expression of Maf family of transcription factors. In development, MafA is expressed after specification of insulin+ cells that are expressing another Maf factor, MafB; after birth, these insulin+ MafA+ cells stop MafB expression and gain glucose responsiveness. Current differentiation protocols for deriving insulin-producing β-cells from stem cells result in β-cells lacking both MafA expression and glucose-stimulated insulin secretion. So driving expression of MafA, a β-cell maturation factor in endocrine precursors could potentially generate glucose-responsive MafA+ β cells. Using inducible transgenic mice, we characterized the final stages of β-cell differentiation and maturation with MafA pause/release experiments. We found that forcing MafA transgene expression, out of its normal developmental context, in Ngn3+ endocrine progenitors blocked endocrine differentiation and prevented the formation of hormone+ cells. However, this arrest was reversible such that with stopping the transgene expression, the cells resumed their differentiation to hormone+ cells, including α-cells, indicating that the block likely occurred after progenitors had committed to a specific hormonal fate. Interestingly, this delayed resumption of endocrine differentiation resulted in a greater proportion of immature insulin+MafB+ cells at P5, demonstrating that during maturation the inhibition of MafB in β-cell transitioning from insulin+MafB+ to insulin+MafB- stage is regulated by cell-autonomous mechanisms. These results demonstrate the importance of proper context of initiating MafA expression on the endocrine differentiation and suggest that generating mature Insulin+MafA+ β-cells will require the induction of MafA in a narrow temporal window to achieve normal endocrine differentiation.
Keywords: Differentiation of endocrine progenitors, Transcription factor MafA, β-Cell maturation
INTRODUCTION
Insulin+ cells specified during pancreatic development undergo a maturation process by transitioning from being MafB+MafA-Insulin+ to MafB+MafA+Insulin+ and then to MafB-MafA+Insulin+ cells (Artner et al., 2010; Artner et al., 2006; Nishimura et al., 2006). The induction of MafA after the initiation of insulin expression indicates that MafA regulates β-cell maturation/function rather than β-cell specification. This is consistent with MafA knockout mice having normal-looking islets at birth but developing β-cell dysfunction and hyperglycemia gradually with age (Artner et al., 2010; Zhang et al., 2005). Both MafB and MafA bind Maf Response Elements (Nishimura et al., 2006), and most MafA-regulated genes are first regulated by MafB during embryonic development (Artner et al., 2010). Yet β-cell mass is reduced only in MafB-deficient and not MafA knockout mice (Artner et al., 2007; Artner et al., 2010; Nishimura et al., 2008). In addition to demonstrating a critical role of MafA in β-cell maturation, these observations emphasize a unique temporal role for Maf factors during commitment to β-cell fate and the importance of correct context of their initiation on differentiation of β-cells.
The goal of β-cell replacement therapy for type 1 diabetes is to achieve insulin independence by restoring the functional β-cell mass. Yet differentiation protocols for deriving functional β-cells from embryonic stem (ES) cells and induced pluripotent stem (iPS) cells (D'Amour et al., 2006; Kroon et al., 2008; Maehr et al., 2009; McKnight et al., 2010; Rezania et al., 2012) still only result in immature cells with limited insulin content and lacking glucose-stimulated insulin secretion (GSIS) (Basford et al., 2012; Mfopou et al., 2010). To overcome these limitations it is vital to understand how insulin-producing cells are formed during embryonic development and how they mature into glucose-responsive β-cells.
It is likely that during ES cell differentiation protocols inappropriate control of the initiation of Maf factor expression prevents MafA induction and the maturation of insulin+ cells (Basford et al., 2012; D'Amour et al., 2006). One suggestion to generate glucose responsive β-cells has been to force MafA expression during the differentiation of ES and iPS cells. Our data on the detrimental effects of mistimed MafA expression in early Pdx1+ pancreatic progenitors, such that their proliferation and the differentiation of endocrine cells were impaired (Nishimura et al., 2009), demonstrate the narrowness of the effective window for initiation of MafA expression. To avoid these detrimental effects in Pdx1+ progenitors (Nishimura et al., 2009), one possibility would be to force MafA expression upon initiation of endocrine differentiation to force immature insulin+ cells into “mature” insulin+MafA+ cells.
Here we demonstrate that out-of-context MafA expression in Ngn3+ (Neurog3+ Mouse Genome Informatics) endocrine progenitors does not affect their survival but blocks their differentiation and the formation of hormone+ cells. This block occurs after progenitors commit to a specific hormone-expressing fate. Importantly, removing MafA expression re-engages the ‘normal’ differentiation program in these cells, thereby driving committed precursors into hormone+ cells. Our experimental approach provides an important means to evaluate the effects of the on/off timing of MafA expression as a driver of differentiation/maturation of β-cells. Using this approach, we show the importance of the proper context of initiating MafA expression for endocrine differentiation and a role of cell-intrinsic mechanisms in postnatal suppression of MafB expression in insulin+ cells.
MATERIALS AND METHODS
Mice
All animal procedures were approved by Joslin Diabetes Center IACUC. A line of tetracycline-inducible transgenic mice driving expression of Myc-tagged human MafA (TetOMafA) under the control of pTRE-Tight promoter (Clontech) (Nishimura et al., 2009) (expressing 5 copies of transgene) was used. TTendo mice result from breeding Ngn3Cre mice (Schonhoff et al., 2004) with ROSA26 lox-stop-lox rtTA-IRES-EGFP mice (Rosa26rtTA) (Belteki et al., 2005) and our TetOMafA mice. For induction, 1g/L Doxycycline (Sigma) was added in the drinking water containing artificial sweetener; water was changed every second day.
Immunohistochemistry
Pancreases were fixed in 4% paraformaldehyde, processed through sucrose before enrobing in OCT and frozen. Immunostaining (see supplementary methods) was done on frozen sections. Images were taken either with Zeiss Axiocam or confocally Zeiss LSM 710 microscopes. Cell area quantification was performed with Volocity (PerkinElmer). Quantification of each antigen was performed on five sections separated by at least 50μm for at least 3 mice per group.
Antibodies: rabbit anti-MafA 1:100 (Bethyl, Montgomery, TX), rabbit anti-MafB 1:100 (Bethyl), rabbit anti-Myc 1:200 (Cell Signaling, Danvers, MA), guinea pig anti-Insulin 1:100 (Linco, Billerica, MA), mouse anti-Glucagon 1:100 (Sigma, Saint Louis, MO), mouse anti-Ngn3 1:100 (Hybridoma Bank, Iowa City, IA), mouse anti-Pdx1 1:1000 (Millipore, Temecula, CA), rabbit anti-GFP 1:100 (Invitrogen, Eugene, OR), biotinylated-DBA lectin (Vector, Burlingame, CA) and others were described earlier (Nishimura, et. al., 2009). In our hands, immunostaining with both Chromogranin-A 1:1000 (Immunostar, Hudson, WI) and cocktail of Ghrelin, Somatostatin, PP antibodies were technically incompatible, as was GFP co-staining with Myc-tag, MafA and MafB. To overcome these incompatibilities, adjacent sections were used with different combinations of antibodies.
qRT-PCR and Genotyping
Total RNA from 4 control and 3 TTendo P0 pancreases (DOX-ON from E7.5 to P0) was prepared using Trizol RNA isolation reagent followed by Qiagen RNA easy mini cleanup kit. cDNAs from individual RNA samples were used in quantitative RT-PCR. The results were quantified using comparative threshold cycle method (ΔΔCT), following normalizing expression of different genes with the expression of control 18S rRNA. Forward and Reverse primers used in the study are described in the Supplemental Table 1. PCR primers designed in 3’ un-translated region (UTR) of MafA detect expression of only endogenous MafA, while those amplifying the coding sequence (cds) quantify both endogenous and TetOMafA transgene.
Genotyping: Genomic DNA was extract from tail snip and used in PCR reaction to determine genotypes of different animals. Primers used in the PCR include MafA-transgene forward 5‘GTGCCAACTCCAGAGCCAGGTG3’ and reverse 5‘GTTTCAGGTTCAGGGGGAGGTGTG3’; Cre forward 5‘CCGGGCTGCCACGACCAA3’ and reverse 5‘GGCGCGGCAACACCATTTTT3’; and rtTA forward 5‘TGCCGCCATTATTACGACAAG3’ and reverse 5‘CCGCGGGGAGAAAGGAC3’. Additionally, genotyping to confirm wild type or knockin ROSA26 allele were performed as suggested by Jax Lab.
Western blot analysis
Adult control and TTendo mice, not exposed to DOX from conception to adult, were given DOX for 5 days in drinking water prior to sacrifice. Islets were isolated with collagenase digestions and whole islet protein extracts prepared (Kondo et al., 2009). Cell extracts of 293T cells infected with MafA adenovirus were prepared as positive controls. Protein extracts (20 μg) were boiled for 5 minutes in the presence of β-mercaptoethanol and resolved on 10% SDS-PAGE (polyacrylamide gel electrophoresis), transferred to PVDF membranes and probed with MafA (1:2000) (Nishimura et al., 2006), actin was used as a loading control. Primary antibodies were diluted in Tris-buffered saline containing 0.2% Tween 20 (TBST). Membranes were washed in TBST and incubated with either anti-rabbit or anti-mouse secondary antibodies conjugated to horseradish peroxidase. Membranes were further processed using either chemiluminescence SuperSignal West Dura reagent or West Dura Femto (Pierce).
RESULTS
Mistimed expression of endocrine maturation factor MafA in Ngn3+ cells prevents access to endocrine ontogeny
We generated Triple Transgenic mice to induce MafA expression in progeny of endocrine progenitors (TTendo) by breeding Ngn3Cre mice (Schonhoff et al., 2004) with ROSA26 lox-Stop-lox rtTA-IRES-EGFP mice (Rosa26rtTA) (Belteki et al., 2005) and TetOMafA mice (Nishimura et al., 2009) (Fig. 1A). In the presence of Doxycycline (DOX), reverse tetracycline transactivator (rtTA) induced Myc-tagged MafA transgene (TetOMafA, subsequently referred to as MafAMyc) expression in the progeny of Ngn3+ cells. Independent of DOX, Cre-mediated excision of the lox-Stop-lox cassette should mark endocrine progenitors and their progeny with GFP.
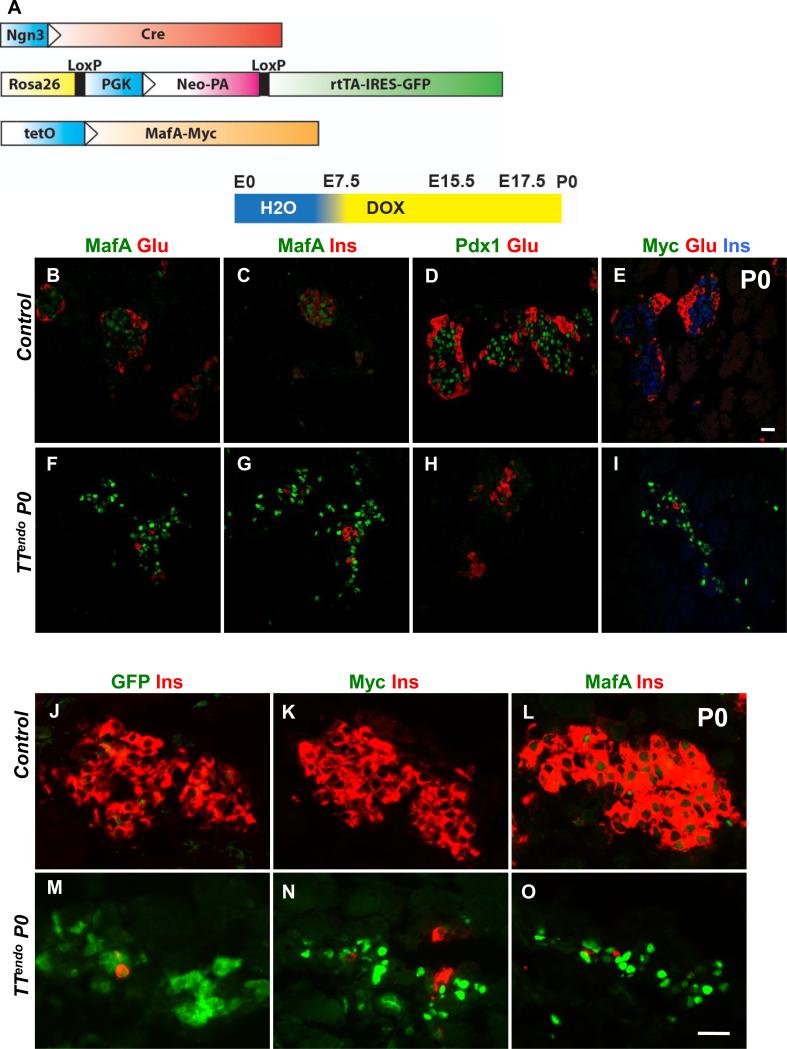
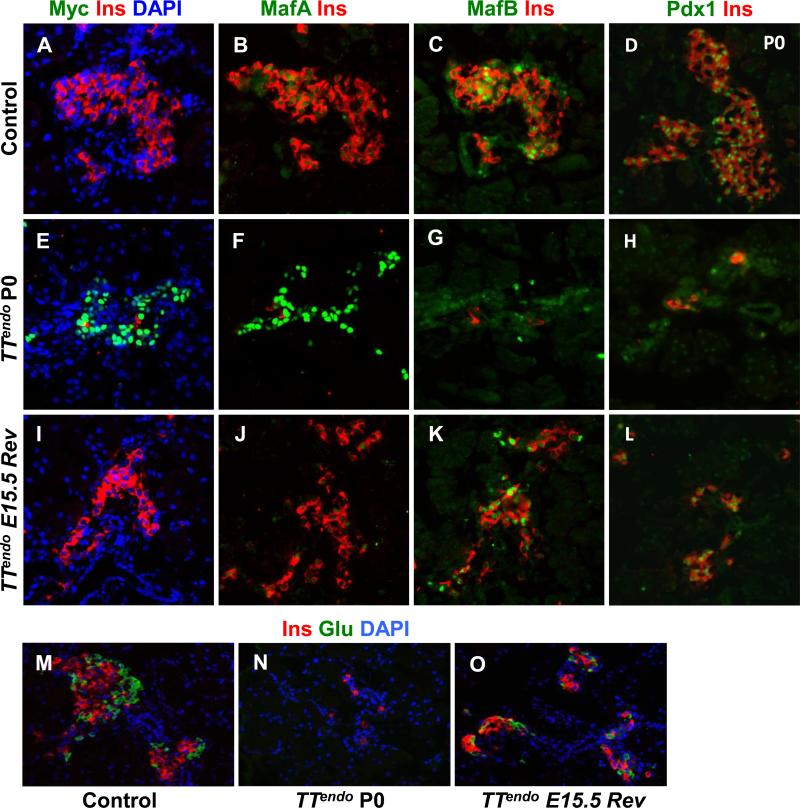
Figure 1. Inducing MafA expression in endocrine progenitors selectively inhibited endocrine differentiation.
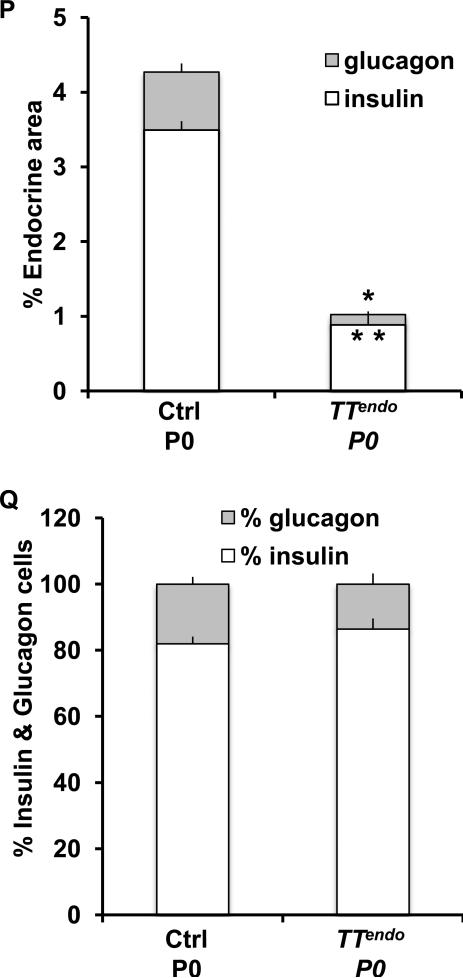
(A) Schematic of alleles used to generate the TTendo triple transgenic mice for DOX-dependent induction of MafAMyc in Ngn3+ cells and their progeny. (B-I) P0 pancreas from TTendoP0 (DOX-ON [yellow in schematic] from E7.5 to P0) and control littermates immunostained for MafA, Pdx1, transgene MafAMyc, insulin and glucagon. MafAMyc+ hormone- cells are likely the progeny of endocrine progenitors (GFP+) as shown on consecutive sections of control (J-L) and TTendoP0 (M-O) pancreas stained for GFP, MafA, Myc (green) and insulin (red). In TTendo P0 pancreas cells corresponding to GFP+ cells (M) also express transgene Myc (N) and MafA (O). (P) At P0, TTendo had significantly reduced insulin+ or glucagon+ areas compared to control littermates. (Q) Relative proportions (%) of insulin+ and glucagon+ cells in P0 islets were comparable in all groups. Mean ± s.e.m., n=3 animals per group. *p<0.05, **p<0.001. Images of control animals in Fig. 1B-E were from double transgenic Ngn3Cre/+;Rosa26rtTA/+, while those for Fig. 1J-L were from double transgenic TetOMafAMyc;Rosa26rtTA/+.
MafAMyc expression in the progeny of endocrine progenitors throughout embryonic development [DOX-ON from before Ngn3 expression (E.7.5) until P0] had no effect on the gross appearance of P0 TTendo pancreas, but it dramatically reduced the number of insulin- and glucagon-expressing, as well as Pdx1-expressing cells (Fig. 1F-I). During pancreatic development, MafA expression is initiated after a cell is already expressing insulin (Nishimura et al., 2009). However, many MafA+ cells in P0 TTendo pancreas did not express insulin (nor glucagon) (Fig. 1F-G). α-Myc and α-MafA antibodies were used to detect MafAMyc (transgene) and total MafA (endogenous and transgene) expression, respectively. Analysis of consecutive sections of TTendo P0 pancreas showed expression of lineage marker GFP, Myc and MafA in the same area (Fig. 1M-O), suggesting that MafA+Ins-Glu- cells (Fig. 1F,G, O) were expressing the MafAMyc transgene; consistent with the presence of many hormone- Myc+ cells in the TTendo P0 pancreas (Fig. 1I). qRT-PCR data from P0 control and TTendo pancreases showed a dramatic reduction in endogenous MafA (reduced levels of MafA 3’UTR PCR product) but a 5.7-fold increase in total MafA (MafA cds product) expression (Table 1) demonstrating that the MafA expression seen in the P0 pancreas (Fig. 1) results mainly from the MafAMyc transgene expression. MafAMyc expression in the endocrine progenitor inhibited the proportion of insulin and glucagon expressing cells in P0 TTendo pancreas by ~80% (Fig. 1P). However, this inhibition did not alter the relative proportion of α- and β-cells (~20% and 80%, respectively) in either group (Fig. 1Q).
Table 1.
Quantitative RT-PCR results showing relative expression of transcription factors and hormone genes in control and TTendo P0 pancreas.
| Genes | Control±SEM | TTendo±SEM | P-Value |
|---|---|---|---|
| MafA cds | 1.0±0.34 | 5.73±0.87 | 2.00E-03 |
| MafA (endogenous) | 1.0±0.29 | 0.06±0.01 | 0.04 |
| Insulin | 1.0±0.22 | 0.02±0.01 | 0.01 |
| Glucagon | 1.0±0.25 | 0.06±0.03 | 0.03 |
| Somatostatin | 1.0±0.10 | 0.02±0.01 | 3.00E-04 |
| Neurog3 | 1.0±0.18 | 0.74±0.14 | 0.32 |
| Insm1 | 1.0±0.14 | 0.44±0.03 | 0.02 |
| NeuroD1 | 1.0±0.14 | 0.17±0.02 | 4.00E-03 |
| MafB | 1.0±0.23 | 0.19±0.04 | 0.03 |
Mistimed MafA expression in endocrine progenitors did not trigger altered fate selection
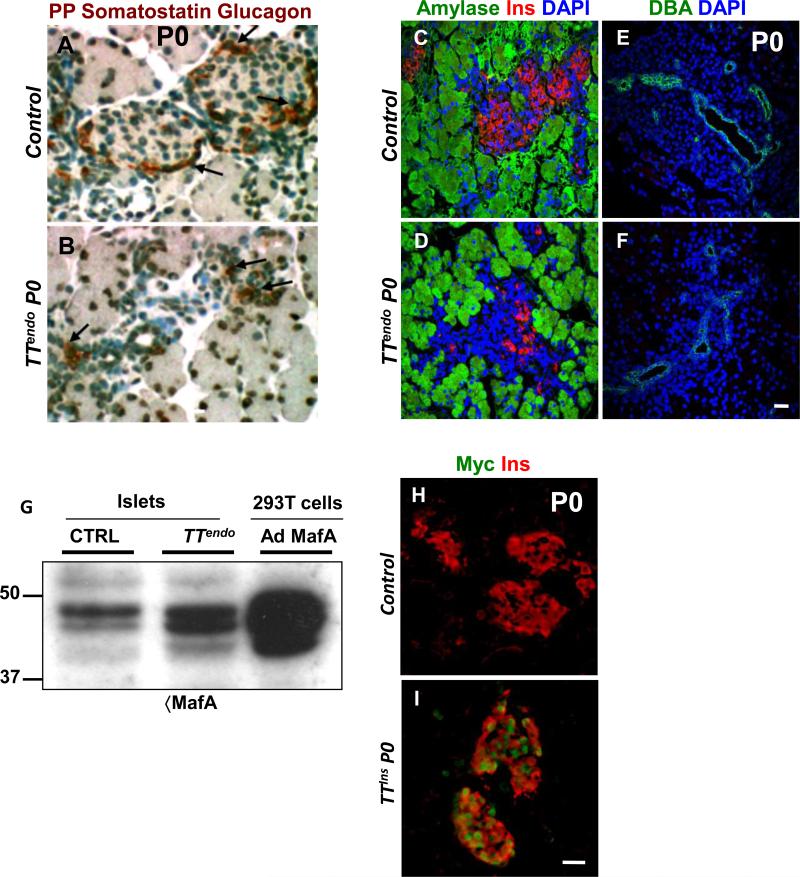
We next examined whether the MafAMyc-mediated inhibition of differentiation altered the fate selection of the endocrine progenitors. Consistent with staining and quantification results (Fig. 1), by qPCR expression of insulin and glucagon mRNA was significantly reduced in P0 TTendo pancreas, and so was the expression of somatostatin mRNA (Table 1). By microarray analysis, TTendo P0 pancreas had 8-12 fold lower expression of genes for hormones insulin, glucagon and somatostatin and 2-5 fold lower Peptide YY, Ghrelin and Pancreatic Polypeptide than control but no significant difference in expression of multiple acinar and duct-enriched genes (Supplemental Table 2). As seen in P0 pancreatic sections stained using a cocktail of antibodies against glucagon, somatostatin and PP, the number of non-β-cells was also reduced (Fig. 2A,B). No differences in ductal (DBA+) and acinar (amylase+) areas were seen in pancreas from control and TTendo littermates (Fig. 2C-F), consistent with no change in the expression of several acinar and ductal genes in TTendo P0 pancreas (Supplemental Table 2). In TTendo pancreas large numbers of non-insulin-expressing cells were seen in “islet–like” areas surrounded by normal exocrine cells (Fig. 2C,D). The proportion of apoptotic cells was comparable to controls (data not shown). These observations suggest that the reduction in hormone+ cells in TTendo pancreas (Fig. 1) did not result from endocrine precursors being diverted into other fates (acinar or ductal) when unable to progress down the endocrine lineage and that the hormone-MafAMyc+ cells can coalesce to form “endocrine” clusters (Figs. 1,2).
Figure 2. Mistimed MafA expression in endocrine progenitors does not alter fate selection.
(A,B) P0 pancreatic sections immunostained with antibody cocktail against glucagon, somatostatin and PP (brown, peroxidase staining) showing an overall reduction in hormone+ cells in TTendoP0 compared to control littermates. Magnification bar = 20 μm. (C-F) Amylase+ and DBA+ duct (green) areas were not affected in TTendoP0 pancreas that has reduced insulin+ (red) cells in islet-like structure. (G-I) Transgene expression level was not detrimental to endocrine cells. (G) Western blot analysis of extracts from TTendo islets isolated from adult mice sacrificed after receiving DOX for 5 days show a 2-4 fold increase in total MafA protein compared to extracts from comparable control islets; 293T cells infected with Adeno-MafA (AdMafA) virus as positive control. (H-I) Induction of MafAMyc (green) expression in insulin+ (red) cells in TTIns (InsCRE;ROSA26rtTA;TetOMafA) animals receiving DOX from E7.5 until sacrifice at P0 did not affect the formation of insulin+ cells. Images of control animals in Fig. 2A,C,E were from double transgenic Ngn3Cre/+;Rosa26rtTA/+, while those for Fig. 2H were from double transgenic InsCre/+;Rosa26rtTA/+. Magnification bar = 20 μm.
To evaluate whether the inhibition of endocrine differentiation was due to unusually high MafA expression from the transgene, the level of MafAMyc expression was determined. In P0 TTendo pancreas total MafA mRNA levels (MafA cds) were only 5.7-fold greater than control (Table 1). In pancreas, MafA expression is restricted to β-cells. Hence, a 5.7-fold increase in MafA message in P0 TTendo pancreatic RNA was still less than the levels of endogenous MafA in adult islets/β-cells (~10-fold higher than at birth) (Aguayo-Mazzucato et al., 2011). Furthermore, isolated islets from adult TTendo mice after receiving DOX for 5-days had only 2-4 fold greater total MafA protein levels than control (Fig. 2G). When we introduced the same transgene in insulin+ cells (TTins) by breeding Ins2Cre (Postic et al., 1999) with Rosa26rtTA and TetOMafA mice, we found that continued induction of MafAMyc expression [DOX-ON from before specification of insulin+ cells until birth (E7.5 to P0)] did not prevent islet formation nor insulin-expressing β-cells (Fig. 2H,I), showing that MafAMyc was expressed at a level that per se did not inhibit insulin+ cell formation. Thus, the loss of hormone+ cells in TTendo pancreas likely resulted from the mistimed MafA expression inhibiting a specific earlier step(s) in endocrine differentiation.
Expression of MafA in endocrine progenitors does not affect the formation of endocrine progenitors but inhibits completion of endocrine differentiation
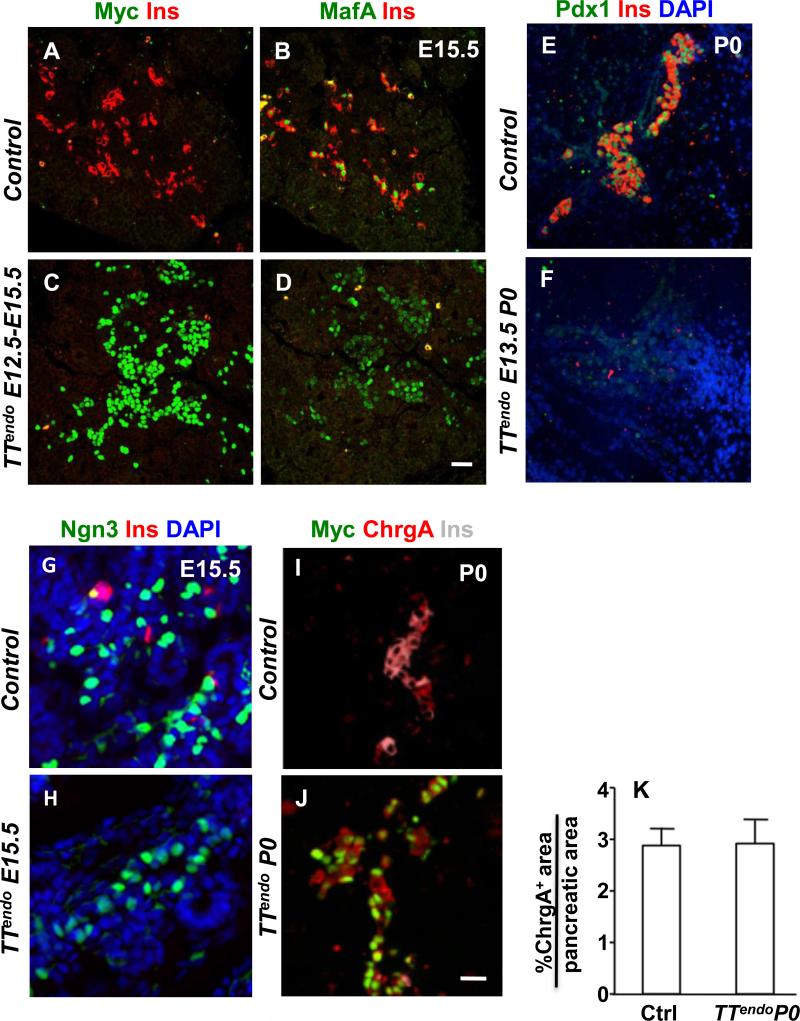
By varying the timing of DOX administration, we could define when MafA expression in Ngn3 expression-enabled endocrine progenitors becomes detrimental to their differentiation. After receiving DOX during secondary transition (from E12.5 to E15.5 with sacrifice at E15.5 or from E13.5 to P0 with sacrifice at P0) TTendo embryos had dramatically reduced number of endocrine cells (Fig. 3A-F) compared to corresponding controls. These observations show that precocious MafA expression in Ngn3+ cells potentially affects the steps regulating their formation and differentiation into endocrine cells.
Figure 3. MafA transgene expression in endocrine progenitors during secondary transition dramatically inhibited endocrine differentiation but did not affect the formation of Ngn3+ or ChrgA+ cells.
(A-D) After DOX exposure from E12.5 to E15.5, TTendo E15.5 pancreas had large numbers of Myc+ and MafA+ cells that did not express insulin, demonstrating inhibition of endocrine differentiation. MafA, Myc (green) and insulin (red) DAPI (blue). (E-F) Similarly, after DOX from E13.5 to P0, TTendo P0 pancreas had dramatically reduced insulin+ and Pdx1+ cells compared to corresponding controls. Pdx1 (green), insulin (red) and nuclei (DAPI, blue). Magnification bar = 20 μm.(G,H) After DOX from 7.5 until sacrifice at E15.5, TTendo E15.5 pancreas shows that the specification of Ngn3+ cells was not inhibited by MafAMyc expression compared to control. Ngn3 (green), Ins (red) and DAPI (blue). (I,J) Transgene MafAMyc expression in endocrine progenitors resulted in endocrine cells expressing chromograninA (ChrgA) but not insulin. In control P0 pancreas (I) cells coexpress (pink) ChrgA (red) and insulin (grey pseudo color) but in TTendoP0 pancreas (J) many ChrgA (red) cells express only the transgene (Myc, green) and no insulin. DOX-ON from E7.5 to P0. (K) Quantification of ChrgA+ cells as % of pancreatic area; n= 3. Images of control animals in Fig. 3A,B,E were from double transgenic Ngn3Cre/+;Rosa26rtTA/+, while those for Fig. 3I were from double transgenic TetOMafAMyc;Rosa26rtTA/+. Magnification bar = 20 μm.
We next examined whether MafAMyc expression in endocrine progenitors altered formation of endocrine progenitors by affecting lateral inhibition. The E15.5 pancreases from controls and TTendo mice had comparable number of Ngn3+ cells (Fig. 3G,H). Quantification of Ngn3+ and MafAMyc+ cells from E15.5 controls and TTendo pancreases showed comparable number of Ngn3+ and Myc+ cells in TTendo and control E15.5 pancreases. Furthermore, similar number of Myc- and of Ngn3-expressing cells in TTendo pancreas suggest that most, if not all, Ngn3+ cells are also Myc+ (Supplemental Figure 1). The presence of many MafAMyc+ cells in TTendo embryos at E15.5 (Fig. 3C) indicated that the Ngn3 promoter was sufficiently active to induce Cre-mediated excision of the Stop cassette and that the MafAMyc+ progeny of Ngn3+ cells (GFP+, Myc+ cells) survived at least until birth (Figs. 1I,M,N & 3J). Thus although forced MafAMyc expression inhibited endocrine differentiation, it did not affect the formation of additional endocrine progenitors nor inhibit the survival of endocrine progeny.
To determine if the transgene expression completely blocked or altered the endocrine differentiation program, we examined the expression of chromogranin-A (ChrgA) as a marker of all endocrine cell-types (Fig. 3I,J). At birth, control pancreas had numerous insulin+ChrgA+ cells and a few insulin-ChrgA+ that represent the other hormone+ cells. As predicted from the loss of hormone+ cells in P0 TTendo pancreas (Fig. 1), many ChrgA+ cells were MafAMyc+insulin- and only a few were MafAMyc-insulin+ cells (Fig. 3J). Images showing expression of Myc, insulin and chromogranin-A in separate channels (Supplemental Figure 2) clearly demonstrate the presence of ChrgA+Ins-Myc+ cells in P0 TTendo pancreas. Quantification of relative pancreatic area of ChrgA+ cells showed comparable proportions of ChrgA+ cells in both control and TTendo littermates (Fig. 3K). Microarray results (Supplemental Table 2) from P0 TTendo and control pancreases showed a reduction in expression of some secretory granule genes like Synaptotagmin-like 4, Secretogranin III and chromograninB in TTendo pancreas, but the expression of ChrgA and Synaptophysin were not altered. Furthermore, qRT-PCR and microarray data (Table 1, Supplemental Table 2) showed a slight reduction in Ngn3 expression but significant reductions in expression of endocrine hormone genes, downstream targets of Ngn3, Glut 2 (2.34-fold; Mean values 579 vs 247 p= 4.30E-04), and key endocrine transcription factors. We interpret these results to suggest that all lineage-marked endocrine progenitors expressing MafAMyc maintain their endocrine phenotype, progress down the endocrine lineage and turn-on some endocrine specific genes but cannot complete the differentiation process. A consequence of such block in endocrine differentiation was that at birth TTendo pups with milk in their stomach were hyperglycemic compared to similar wild type control littermates [blood glucose 111±18 (n=3) vs 64±2 (n=7) mg/dl, p=0.003].
Forcing MafAMyc expression blocks differentiation of endocrine progenitors after their commitment to a specific hormone-expressing fate
Since transgene expression was regulated by DOX, we could examine whether stopping MafAMyc expression in hormone-MafAMyc+ cells will result in these cells resuming their endocrine differentiation program. Two groups of pregnant females were given DOX from E7.5; in one, DOX was stopped at E15.5 to reverse the differentiation block (TTendoE15.5Rev animals), while in the other, DOX was continued until birth (TTendoP0 animals); pups were sacrificed at birth. As expected, continued expression of transgene from E7.5 to P0 dramatically reduced the insulin+ cells but had many MafAMyc+insulin- cells in the pancreas (Fig. 4E-H). MafB and Pdx1-expressing cells, normally expressed early in the differentiation of insulin+ cells, were also reduced in number. Other key endocrine transcription factors (Supplemental Table 2) as well as the expression of cell adhesion molecules E-Cadherin and N-Cadherin (Supplemental Fig. 3) were down regulated in P0 TTendo pancreas. These observations suggest that TTendo animals can form endocrine cell aggregates that are similar but not identical to the endocrine cell aggregates seen in control pancreas. Stopping the MafAMyc expression by removing DOX at E15.5 (TTendoE15.5Rev) resulted in a remarkable restoration of insulin+ cells by P0 (Fig. 4I-L). Some insulin+ cells expressed MafA, many expressed MafB and Pdx1, and the expression of E-Cadherin and N-Cadherin was recovered. We interpret these findings as evidence of resumption of the normal pancreatic development and β-cell differentiation program.
Figure 4. Reversible inhibition of endocrine differentiation by transgene MafAMyc expression in endocrine progenitors.
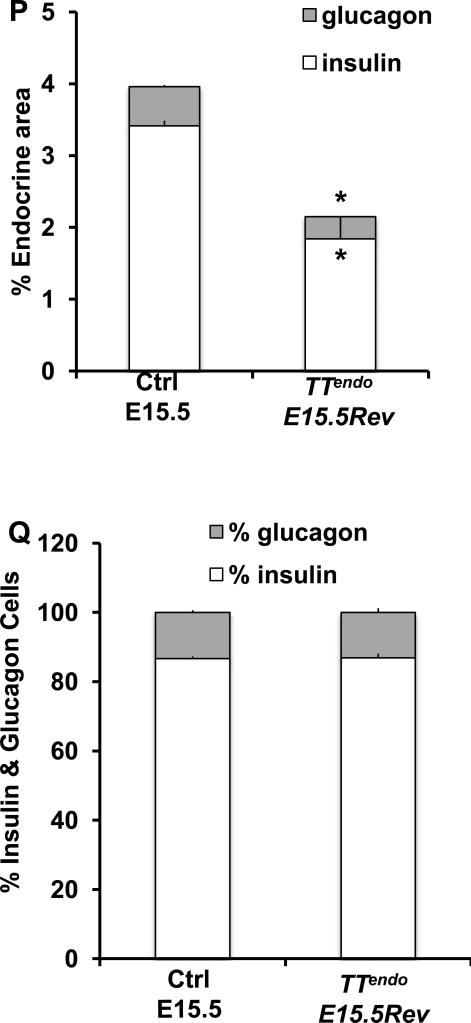
P0 pancreases from control, TTendo P0 (DOX-ON from E7.5 to P0) and TTendoE15.5Rev (DOX-ON from E7.5 to E15.5) were stained for Myc, MafA, MafB, Pdx1, and glucagon (green); insulin (red) and DAPI (blue)(A-O). In TTendoP0 pancreas loss of hormone- and transcription factor-expressing cells was seen, but after stopping DOX at E15.5 (TTendoE15.5Rev) such cells recovered by P0. (P) At P0 TTendoE15.5Rev had significantly increased insulin or glucagon areas compared toTTendoP0 (Fig. 1P) but were still reduced compared to control littermates. (Q) Relative proportions (%) of insulin and glucagon cells in P0 islets were comparable in all groups. Mean ± s.e.m., n=3 animals per group. *p<0.05, **p<0.001. Images of control animals in Fig. 4A-D, M were from double transgenic TetOMafAMyc;Rosa26rtTA/+. Magnification bar = 20 μm.
The competence of pancreatic epithelium to form α-cells is primarily restricted to before E14.5 (Johansson et al., 2007). Hence, if the transgene expression blocked endocrine differentiation before commitment to a specific hormonal fate, releasing the block after E15.5 should lead to a relatively selective, large-scale reduction in α-cells. However, stopping MafA transgene expression from E15.5 (TTendoE15.5Rev) resulted in restoration of both insulin and glucagon cells (Fig. 4M-O). At P0, insulin+ and glucagon+ cells in TTendoE15.5Rev were more numerous than in TTendoP0. Forced MafAMyc expression in endocrine progenitors reduced both α- and β-cell volumes to 20% of control in TTendoP0 (80% inhibition) (Fig. 1P) but only to 60% of control in TTendo E15.5 Rev animals (40% inhibition) (Fig. 4P). However, this inhibition did not alter the relative proportion of α- and β-cells (~20% and 80%, respectively) in either group (Figs. 1Q, 4Q). Thus, the initiation of endocrine differentiation after E15.5 did not result in the expected preferential reduction in α-cells.
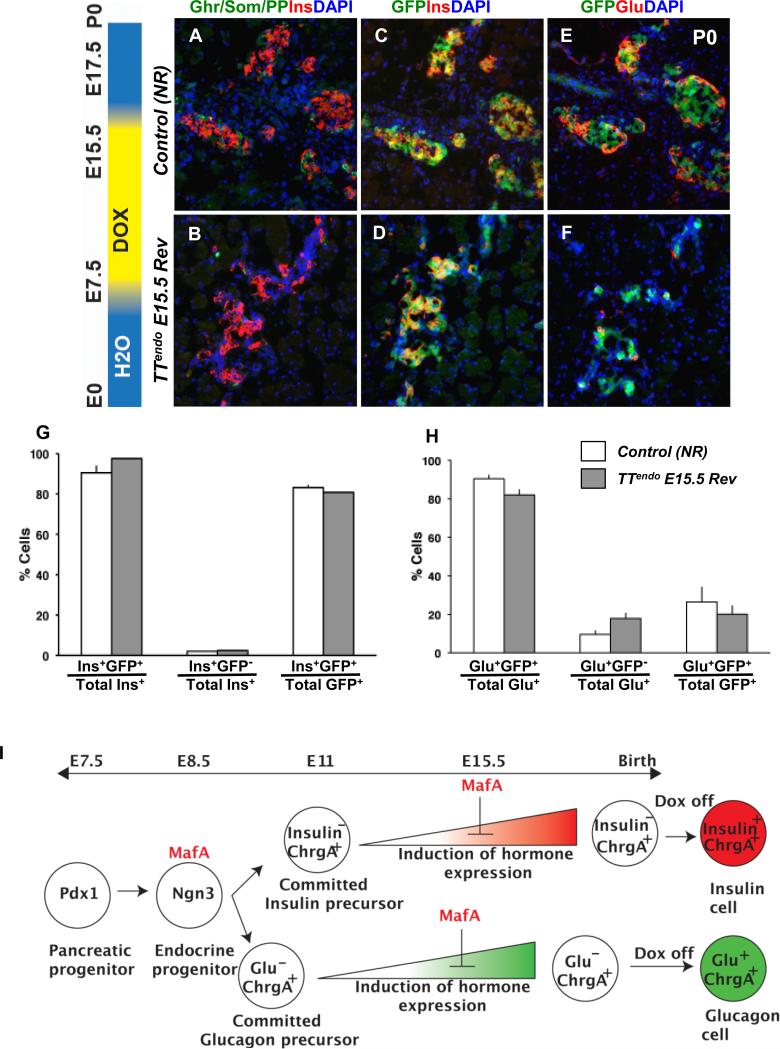
To confirm that stopping DOX in TTendo E15.5 Rev triggered a resumption of the endocrine differentiation of MafAMyc+hormone- cells, we examined the proportion of lineage-marked cells expressing hormones. In addition to Myc, GFP expression in TTendo pancreas marks the progeny of endocrine progenitors. However, unlike DOX dependent MafAMyc expression, GFP expression from Rosa26 promoter-driven rtTA-IRES-EGFP transcript should be detected in all progeny of Ngn3+ cells upon the excision of the stop cassette regardless of presence of DOX. In TTendo pancreas at E15.5 and P0 (Fig. 1, 3), MafAMyc+hormone- cells express lineage marker GFP (Fig. 1 and data not shown); we expect that even after stopping DOX at E15.5 (TTendo E15.5 Rev), all progeny of Ngn3+ cells would retain GFP expression. In adjacent pancreatic sections from TTendoE15.5Rev or control littermates bearing Ngn3Cre and Rosa26rtTA transgenes (Control NR) immunostained for different hormones and GFP (Fig. 5), the majority of GFP+ cells expressed either insulin or glucagon (Fig. 5C-F). Ngn3Cre BAC-based lineage marking was highly efficient with ~90 % of insulin+ and glucagon+ cells in P0 pancreases from TTendoE15.5Rev and NR control littermates expressing GFP (Fig. 5G,H).
Figure 5. MafA transgene inhibited endocrine differentiation after hormonal fate commitment.
(A-F) In P0 TTendoE15.5Rev pancreas resumption of endocrine differentiation after stopping DOX at E15.5 resulted in formation of all hormonal cell types. Adjacent sections stained with cocktail of Ghrelin, Somatostatin, PP antibodies (green), insulin (red) and nuclei (DAPI, blue), or GFP (green) with insulin (red) or glucagon (red). Magnification bar = 20 μm. (G) Quantification of Ins+GFP+ cells (lineage-marked cells) and Ins+GFP- (cells that escaped Ngn3-Cre-mediated excision) as proportion of total insulin cells. Ins+GFP+ cells were ~80% of total GFP+ cells in both control and TTendoE15.5Rev. (H) Similar quantification for glucagon+ cells showed ~20% GFP+ cells expressed glucagon. Control NR and TTendoE15.5Rev pancreas had similar proportions of GFP-marked insulin and glucagon cells. GFP+hormone- cells were not detected in TTendoE15.5Rev animals. Ctrl NR= double transgenic Ngn3Cre/+;Rosa26rtTA/+ animals. Mean ± SEM, n= 3 animals per group. (I) Schema summarizing the inhibition of endocrine differentiation by mistimed MafA expression and the consequences of removing DOX on hormone expression in TTendo animals.
Many GFP+Ins- and Myc+hormone- cells were seen in TTendo P0 pancreas in the presence of DOX (Fig. 1), and similar Myc+Ins- cells were seen at E15.5 (Fig. 3). If the transgene expression irreversibly blocked endocrine differentiation of these cells, the removal of DOX at E15.5 should prevent these lineage marked cells to express either hormone. The absence of a large pool of GFP+hormone- cells in TTendoE15.5Rev (Fig. 5D,F-H) is evidence of the resumption of differentiation in MafAMyc+hormone- cells present at E15.5. Furthermore, this observation rules out the possibility that Ngn3+ cells specified after stopping DOX were the sole source of new endocrine cells in TTendoE15.5Rev pancreas. We interpret the unchanged proportion of glucagon+ cells (Figs. 4,5) and the absence of significant numbers of hormone-GFP+ cells (Fig. 5) as suggesting that forced MafA expression in endocrine progenitors inhibits their differentiation at a stage after the progenitor commits to a specific endocrine fate but before induction of hormone expression (Fig. 5I). We propose that during embryonic development as the Ngn3+ endocrine progenitors became specified, they turned-on the expression of MafAMyc but the endocrine progenitor continued their differentiation program, initiated expression of some endocrine genes and committed to one of the hormonal fates, but at this stage the transgene expression blocked their further progression to hormone+ endocrine cells (Fig. 5I).
Cell-autonomous mechanisms are integral to maturation of insulin-producing cells
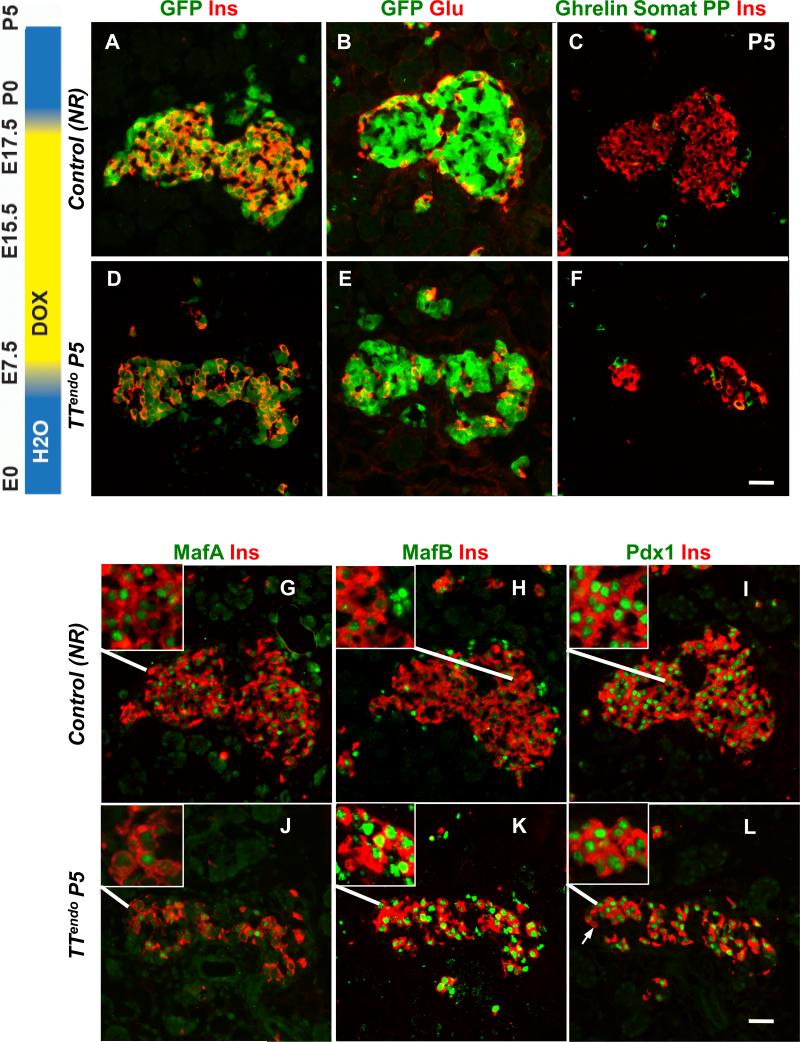
During a maturation stage of development, insulin+ cells progress from mostly expressing MafB (~90%) at end of gestation to solely expressing MafA by adult (Artner et al., 2010; Artner et al., 2006; Nishimura et al., 2006). This switch after birth suggests the loss of MafB expression is signaled by parturition and/or the altered nutritional state. Since MafAMyc reversibly blocked endocrine differentiation (Figs. 4,5), delaying the resumption of endocrine differentiation may be used to dissect the relative contributions of cell-autonomous and postnatal extrinsic signals to the loss of MafB expression. DOX-dependent regulation of TetOPdx1 expression by Pdx1tTA requires ~24h to turn on/off transgene expression (Hale et al., 2005). Robustly insulin+ cells are first detected at E12.5, so assuming that effective changes in MafAMyc expression also requires 24h, providing DOX until E17.5 would delay differentiation of committed insulin precursors between 1 (cells specified late at E17.5) and 6 days (cells specified early at E12.5). A cohort of pregnant dams received DOX from E7.5 until E17.5, and their pups were sacrificed at P5 (TTendoP5 and corresponding NR controls). DOX removal in late gestation relieved the block in differentiation, and the endocrine progeny (GFP+) differentiated into insulin, glucagon and other hormones-expressing cells (Figs. 6A-F). However, in contrast to NR controls, TTendoP5 pancreas had higher proportion of insulin+ cells expressing MafBhigh (10.7±1.2% NR controls vs. 28.2±2.1% TTendoP5 p=0.002)(Fig. 6H,K), occasional Pdx1-insulin+ cells (Fig. 6I,L), few MafA+insulin+ cells, and lower staining intensity of endogenous MafA (Fig. 6G,J). These results are consistent with the delayed endocrine differentiation resulting in more immature insulin+ cells. Thus, the presence of higher proportion of MafB+insulin+ cells in TTendoP5 pancreas reveals that during postnatal maturation, the loss of MafB expression in insulin+ cells is primarily controlled by cell-autonomous mechanisms and not by cell-extrinsic mechanisms that depend on parturition and/or nutritional state of the pup.
Figure 6. Delaying endocrine differentiation resulted in more immature β-cells.
(AF) Delaying endocrine differentiation until E17.5 (DOX-ON from E7.5 to E17.5) resulted in all endocrine cells at P5 being GFP+ in Ngn3Cre; ROSA26rtTA control (NR) and TTendo littermates. Adjacent sections immunostained for GFP, Pdx1, MafA, MafB or a cocktail of ghrelin, somatostatin and PP antibodies (green); insulin or glucagon (red). Insets show higher magnification. Delayed differentiation resulted in higher proportion of insulin+ cells expressing MafB (H,K), reduced endogenous MafA expression (G,J) and occasional cells (arrow) lacking Pdx1 expression (I,L). Magnification bar = 20 μm.
DISCUSSION
Our results demonstrate that precocious MafA expression in Ngn3-expressing cells is detrimental to endocrine differentiation. The point of impact of such MafA expression on the ontogenic program is most likely after the endocrine progenitor commits to a specific hormone-expressing fate. Importantly, these cells are in stasis: when the MafA block is released, cells move forward, apparently towards full differentiation. Delaying the initiation of endocrine differentiation by forced expression of MafA also showed that the transition from MafA+MafB+insulin+ to MafA+MafB-insulin+ cells is regulated by cell-intrinsic mechanisms. Our study thus demonstrates the importance of precise timing of transcription factor expression on the normal progression of cell differentiation, as well as a novel approach to explore the timing of MafA expression as a driver of β-cell differentiation and maturation.
Unlike other endocrine transcription factors, MafA expression in endocrine progenitors inhibits endocrine differentiation. Ngn3 promoter-driven Nkx6.1 expression in endocrine progenitors altered neither the proportion nor number of α- and β-cells (Nelson et al., 2007). Similar to MafA, initiation of Pdx1 expression in the β-cell lineage occurs after insulin expression (Artner et al., 2006; Nishimura et al., 2006). Yet, precocious Pdx1 expression in endocrine progenitors did not halt their differentiation, rather it resulted at birth in Pdx1highglucagon+ cells that by P12 differentiated into insulin+ cells (Yang et al., 2011). Furthermore, the inhibition of endocrine differentiation by precocious MafA expression may not be due to incompatibility between Maf factors and differentiation of Ngn3+ cells, as MafB+Ngn3+hormone- cells are seen during endocrine differentiation (Artner et al., 2006). These observations suggest that precocious/enhanced expression of endocrine transcription factors in Ngn3+ cells does not itself inhibit endocrine differentiation.
Mice with loss of function mutations in some transcription factor genes, including MafB, Nkx6.1 and NeuroD1, show a reduction in endocrine cells, while the loss of function of genes for Nkx2.2, Pax6, Pax4 and Arx alters the fate of endocrine cells (Gittes, 2009; Pan and Wright, 2011). Thus, the phenotype of TTendo mice cannot simply be explained by MafA inhibiting expression of a single endocrine transcription factor. The precise identification of the mechanism underlying this action of MafA will require detailed analyses of embryonic TTendo pancreas, including assessment of expression of key endocrine transcription factors.
Together these results suggest that similar to the unique characteristics of precocious Pdx1 expression in endocrine progenitors that permits conversion of α-cells into β-cells (Yang et al., 2011), MafAMyc in endocrine progenitors uniquely blocks endocrine differentiation. Furthermore, our results support the possibility that the MafA/MafB pair is logically interconnected to regulate specification and maturation of β-cells in order to minimize potential risks of MafA-mediated inhibition of endocrine differentiation during embryonic development.
Multiple lines of evidence support that MafAMyc expression in endocrine progenitors blocked their differentiation after commitment to hormone-expressing state rather than inhibiting the initial stages of endocrine differentiation. At E15.5 we detected both Ngn3+ cells and MafAMyc+ cells (Fig. 3). Unlike in the Ngn3-/- pancreas (Beucher et al., 2012; Magenheim et al., 2011), in TTendo pancreas tubular epithelium appeared normal, and lineage-marked (GFP+, MafAMyc+) hormone- cells were found in small islet-like clusters and were not restricted to the tubules (Fig. 2). These findings suggest that MafAMyc expression in endocrine progenitors did not affect lateral inhibition or impede Ngn3 expression in progenitors nor prevented their movement from tubular epithelium to form islet clusters (Gouzi et al., 2011; Pan and Wright, 2011). Additionally, the comparable proportion of Ngn3+ cells (Supplemental Figure 1) and ChrgA+ endocrine cells in control and TTendo pancreas (Fig. 3) supports the idea that MafAMyc misexpression blocked endocrine differentiation after its initiation but before induction of hormone expression. Consistent with this, removing the MafAMyc influence by DOX withdrawal at E15.5 allowed ChrgA+hormone- cells to resume their differentiation into hormone+ cells (Figs. 4,5).
The restriction of competence of pancreatic epithelium to differentiate into α-cells primarily before E14.5 (Johansson et al., 2007) would predict that releasing the block on MafAMyc+ cells after E15.5 should yield fewer α-cells. However, this release had no preferential reduction of α-cells, and the pancreas showed comparable proportion of lineage-marked α- and β- cells as controls (Figs. 4-6), indicating that the formation of hormone+ cells, including α-cells, resulted from these cells resuming their specified differentiation. These data support the conclusion that precocious MafA expression blocks endocrine differentiation after the cells acquired a specific hormonal fate but before induction of hormone expression. Additional analyses, including demonstration that single ChrgA+hormone- cell give rise to a cluster of cells expressing the same hormone, will be required to confirm this conclusion.
The steps involved in the conversion of Ngn3+ endocrine progenitors to MafBhighinsulin+ cells and their subsequent maturation to MafB-MafA+insulin+ cells, are presently still poorly understood. We suggest that MafA pause/release experiments will address a critical scientific gap in our understanding of differentiation and maturation of β-cells. DOX-regulated Pdx1 expression in Pdx1-/- pancreas showed distinct roles for Pdx1 at morphologically distinct stages of development (Hale et al., 2005). Our MafA-induced pause/release manipulation similarly provides a novel way to evaluate terminal steps in endocrine differentiation including transition from MafB to MafA expression, as well as what triggers the induction and inhibition of these factors during development. Its key advantages are that: 1) the resumption of differentiation can be delayed to discriminate between regulation by cell-intrinsic mechanisms and environmental cues. 2) Ngn3+ cells rapidly induce hormone expression (24h-34h, Beucher et al., 2012), so delaying re-initiation of differentiation by several days will potentially result in a synchronized population of cells, providing a novel means to identify the steps involved in terminal differentiation of endocrine progenitors and their conversion into mature β-cells. Using this approach we showed that the loss of MafB expression in insulin+ cells after birth is under the control of cell intrinsic mechanisms (Figs. 6). This observation raises the possibility that mechanisms underlying the loss of MafB expression during transition of rodent β-cells from insulin+MafB+MafA+ to insulin+MafB-MafA+ might be impaired in human β-cells, which will result in adult human β-cells expressing both MafB and MafA.
Forced MafA expression has important implications for generating functional β-cells. Protocols for differentiating ES/iPS cells to insulin+ cells in vitro have resulted in cells with impaired glucose-stimulated insulin secretion and low MafA expression. Yet the detrimental effects of precocious MafA expression in pancreatic (Nishimura et al., 2009) and endocrine progenitors (this study) caution the use of forced MafA expression to drive maturation of these insulin+ cells. Surprisingly, MafA was required for reprogramming adult liver and acinar cells into insulin+ cells (Kaneto et al., 2005; Zhou et al., 2008), but these reprogrammed insulin+ cells had limited glucose-responsiveness, suggesting that out of normal embryonic context, forced expression of MafA impairs terminal differentiation/maturation of transdifferentiated β-cells or that such cells require a different mechanism for maturation than embryonic β-cells.
In summary, our results provide a rationale for the sequence of transcription factor activation during normal pancreas organogenesis with MafA expression initiated only after that of insulin. They further suggest why approaches inducing MafA expression at or before the endocrine progenitor stage in stem/progenitor cell differentiation would be detrimental to glucose-responsive β-cell formation. We provided evidence of a novel MafA pause/release approach that can be used to study terminal steps of endocrine differentiation. Using this approach we showed the loss of MafB expression in maturing insulin+ cells depends on cell-autonomous mechanisms. Future use of this approach can characterize the endocrine differentiation of a potentially synchronized population of committed insulin precursors, defining the steps these precursors take to become mature insulin+MafA+MafB- cells and identifying intracellular signal transduction pathways regulating MafB and MafA expression during β-cell maturation.
Supplementary Material
Highlights.
Enforced expression of MafA in endocrine progenitors inhibits their differentiation.
MafA blocks differentiation after progenitors commit to a specific hormonal fate.
Stopping MafA expression relieves the block and cells resume their differentiation.
Cell-autonomous mechanisms regulate the loss of MafB in maturing insulin+ cells.
ACKNOWLEDGEMENTS
We thank Drs. Chris Wright and Roland Stein (Vanderbilt University) for their constructive criticisms of the manuscript. We thank Taliesin Lenhart (Harvard Stem Cell Institute, Summer Internship program) for assistance with immunostaining and quantification of images; Anthony Hill, IDDRC Imaging Core Children's Hospital Boston, for assistance with Volocity software.
This study was supported by research grants from NIH (RO1 DK060127), Juvenile Diabetes Research Foundation, American Diabetes Association grant 1-12-BS-187, the Herbert Graetz Fund, Shirley and William Fleisher Family Foundation, and the Alexander and Margaret Stewart Trust to AS; post-doctoral fellowships from Swiss National Foundation (PBGEP3-121298) to KH HH and from Lundbeckfonden (#R7-A714-B576) to KJ; and the Media, Advanced Microscopy, Advanced Genomics/Genetics, and Bioinformatics Cores of Joslin Diabetes Endocrinology Research Center (NIH DK-36836).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aguayo-Mazzucato C, Koh A, El Khattabi I, Li WC, Toschi E, Jermendy A, Juhl K, Mao K, Weir GC, Sharma A, Bonner-Weir S. Mafa expression enhances glucose-responsive insulin secretion in neonatal rat beta cells. Diabetologia. 2011;54:583–593. doi: 10.1007/s00125-010-2026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artner I, Blanchi B, Raum JC, Guo M, Kaneko T, Cordes S, Sieweke M, Stein R. MafB is required for islet beta cell maturation. Proc Natl Acad Sci U S A. 2007;104:3853–3858. doi: 10.1073/pnas.0700013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artner I, Hang Y, Mazur M, Yamamoto T, Guo M, Lindner J, Magnuson MA, Stein R. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artner I, Le Lay J, Hang Y, Elghazi L, Schisler JC, Henderson E, Sosa-Pineda B, Stein R. MafB: an activator of the glucagon gene expressed in developing islet alpha- and beta-cells. Diabetes. 2006;55:297–304. doi: 10.2337/diabetes.55.02.06.db05-0946. [DOI] [PubMed] [Google Scholar]
- Basford CL, Prentice KJ, Hardy AB, Sarangi F, Micallef SJ, Li X, Guo Q, Elefanty AG, Stanley EG, Keller G, Allister EM, Nostro MC, Wheeler MB. The functional and molecular characterisation of human embryonic stem cell-derived insulin-positive cells compared with adult pancreatic beta cells. Diabetologia. 2012;55:358–371. doi: 10.1007/s00125-011-2335-x. [DOI] [PubMed] [Google Scholar]
- Belteki G, Haigh J, Kabacs N, Haigh K, Sison K, Costantini F, Whitsett J, Quaggin SE, Nagy A. Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res. 2005;33:e51. doi: 10.1093/nar/gni051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beucher A, Martin M, Spenle C, Poulet M, Collin C, Gradwohl G. Competence of failed endocrine progenitors to give rise to acinar but not ductal cells is restricted to early pancreas development. Dev Biol. 2012;361:277–285. doi: 10.1016/j.ydbio.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Gittes GK. Developmental biology of the pancreas: A comprehensive review. Developmental Biology. 2009;326:4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Gouzi M, Kim YH, Katsumoto K, Johansson K, Grapin-Botton A. Neurogenin3 initiates stepwise delamination of differentiating endocrine cells during pancreas development. Dev Dyn. 2011;240:589–604. doi: 10.1002/dvdy.22544. [DOI] [PubMed] [Google Scholar]
- Hale MA, Kagami H, Shi L, Holland AM, Elsasser HP, Hammer RE, MacDonald RJ. The homeodomain protein PDX1 is required at mid-pancreatic development for the formation of the exocrine pancreas. Dev Biol. 2005;286:225–237. doi: 10.1016/j.ydbio.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Johansson KA, Dursun U, Jordan N, Gu G, Beermann F, Gradwohl G, Grapin-Botton A. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell. 2007;12:457–465. doi: 10.1016/j.devcel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Matsuoka TA, Nakatani Y, Miyatsuka T, Matsuhisa M, Hori M, Yamasaki Y. A crucial role of MafA as a novel therapeutic target for diabetes. J Biol Chem. 2005;280:15047–15052. doi: 10.1074/jbc.M412013200. [DOI] [PubMed] [Google Scholar]
- Kondo T, El Khattabi I, Nishimura W, Laybutt DR, Geraldes P, Shah S, King G, Bonner-Weir S, Weir G, Sharma A. p38 MAPK is a major regulator of MafA protein stability under oxidative stress. Mol Endocrinol. 2009;23:1281–1290. doi: 10.1210/me.2008-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. Epub 2008 Feb 2020. [DOI] [PubMed] [Google Scholar]
- Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci U S A. 2009;106:15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magenheim J, Klein AM, Stanger BZ, Ashery-Padan R, Sosa-Pineda B, Gu G, Dor Y. Ngn3(+) endocrine progenitor cells control the fate and morphogenesis of pancreatic ductal epithelium. Dev Biol. 2011;359:26–36. doi: 10.1016/j.ydbio.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight KD, Wang P, Kim SK. Deconstructing pancreas development to reconstruct human islets from pluripotent stem cells. Cell Stem Cell. 2010;6:300–308. doi: 10.1016/j.stem.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mfopou JK, Chen B, Sui L, Sermon K, Bouwens L. Recent advances and prospects in the differentiation of pancreatic cells from human embryonic stem cells. Diabetes. 2010;59:2094–2101. doi: 10.2337/db10-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Schaffer AE, Sander M. The transcription factors Nkx6.1 and Nkx6.2 possess equivalent activities in promoting beta-cell fate specification in Pdx1+ pancreatic progenitor cells. Development. 2007;134:2491–2500. doi: 10.1242/dev.002691. [DOI] [PubMed] [Google Scholar]
- Nishimura W, Bonner-Weir S, Sharma A. Expression of MafA in pancreatic progenitors is detrimental for pancreatic development. Dev Biol. 2009;333:108–120. doi: 10.1016/j.ydbio.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura W, Kondo T, Salameh T, El Khattabi I, Dodge R, Bonner-Weir S, Sharma A. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev Biol. 2006;293:526–539. doi: 10.1016/j.ydbio.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura W, Rowan S, Salameh T, Maas RL, Bonner-Weir S, Sell SM, Sharma A. Preferential reduction of [beta] cells derived from Pax6-MafB pathway in MafB deficient mice. Developmental Biology. 2008;314:443–456. doi: 10.1016/j.ydbio.2007.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn. 2011;240:530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J.Biol.Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, Gauvin R, Narayan K, Karanu F, O'Neil JJ, Ao Z, Warnock GL, Kieffer TJ. Maturation of Human Embryonic Stem Cell-Derived Pancreatic Progenitors Into Functional Islets Capable of Treating Pre-existing Diabetes in Mice. Diabetes. 2012;61:2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non endocrine cell types. Dev Biol. 2004;270:443–454. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Yang YP, Thorel F, Boyer DF, Herrera PL, Wright CV. Context-specific alpha- to-beta-cell reprogramming by forced Pdx1 expression. Genes Dev. 2011;25:1680–1685. doi: 10.1101/gad.16875711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, Kudo T, Engel JD, Yamamoto M, Takahashi S. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25:4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.