Abstract
We report a new class of deubiquitinating enzyme (DUB) probes that resemble the native diubiquitin with a same linkage size and contain a Michael addition acceptor for trapping the DUB active-site cysteine. Both K63- and K48-linked diubiquitin probes were generated using a facile chemical ligation method. The diUb probes were demonstrated to label DUBs from different families and revealed intrinsic linkage specificities of DUBs.
Deubiquitinating enzymes (DUBs) are essential for many cellular pathways and have emerged as promising targets for pharmacological intervention.1, 2 DUBs exert effects by reversing the mono- and polyubiquitination of a large number of proteins in various cellular pathways. Activity-based probes (ABPs) have been widely used in profiling different classes of enzymes.3, 4 In recent years, ABPs were also developed for DUBs.5 The most widely used DUB probe is a monoubiquitin with an electrophilic group introduced at its C-terminus, such as ubiquitin-vinylsulfone (Ub-VS) or ubiquitin-vinylmethyl ester (Ub-VME) 6,7. This approach was further extended by either varying the C-terminal electrophilic group, aiming to improve reactivity or introducing a fluorescent group for easy readout.8–11
Although the monoubiquitin DUB probes have proven to be useful tools for profiling DUBs, they provide no information of the chain linkage- and target-specificity of DUBs because the probes contain no ubiquitin acceptor protein. Recent work described DUB probes in which a peptide derived from the proximal ubiquitin was conjugated to the C-terminus of an intact ubiquitin.12–14 However, high resolution structures of DUBs in complex with diubiquitin showed that in addition to the peptide flanking the ubiquitinated lysine residue, more extensive interactions between DUB and the proximal ubiquitin also contribute to the diubiquitin recognition by DUBs15, 16. In particular residues in the proximal ubiquitin that are distant from the modified lysine residue were found to contribute to the diubiquitin binding and DUB catalysis. Therefore, diubiquitin probes that harbor an intact proximal ubiquitin are essential for probing the ubiquitin chain-linkage specificity of DUBs.
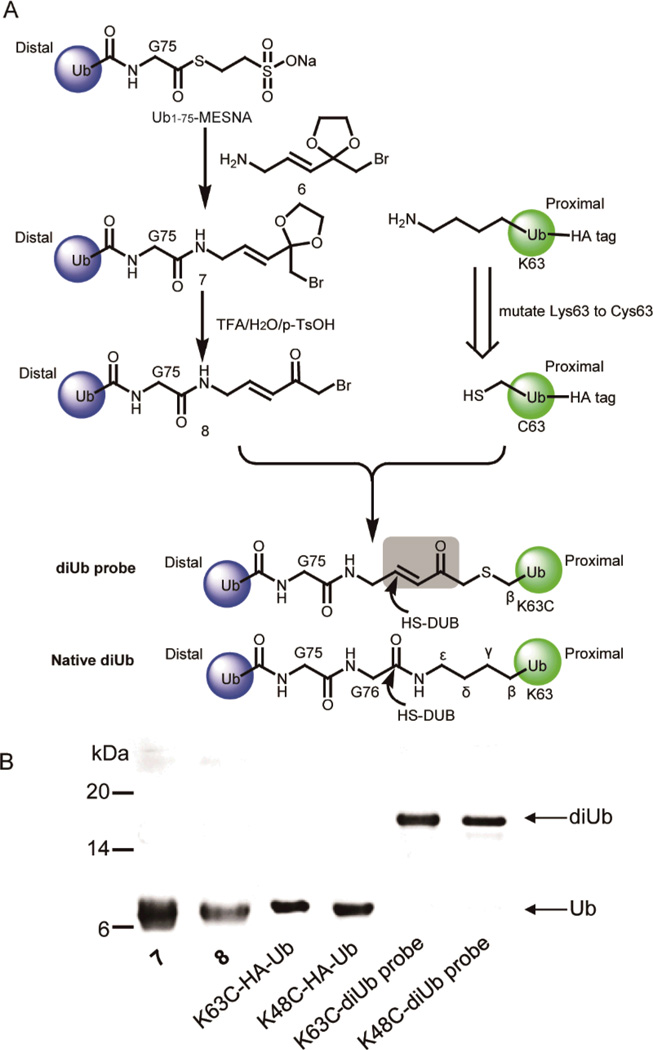
Here we generated a new class of activity-based diubiquitin probes with defined linkages (Fig. 1A). Structurally, they closely mimic the native diubiquitin in the linker size. A Michael acceptor is introduced in the linker between the proximal and distal ubiquitin moieties in order to trap the catalytic cysteine thiol of the DUB active site.
Fig. 1.
Preparation and characterization of K63C- and K48C-diUb probes. (A) Preparation of diUb probes. (B) Denaturing SDS-PAGE gel showing the formation of the K63C- and K48C-diUb probes.
In order to introduce a Michael addition acceptor to diubiquitin (diUb) probe, we designed and synthesized a linker molecule 6 (Scheme S1, ESI†). Compound 6 harbors a Michael acceptor and acts as a linker to conjugate two ubiquitin moieties (Fig. 1A). Considering that an amino group and a carbonyl group in 6 cannot coexist due to a ready Schiff base formation (data not shown), we decided to protect the carbonyl group by forming a ketal. A synthetic route of compound 6 is shown in Scheme S1. We synthesized 6 by 6 steps with an overall yield of 6%. All synthesized compounds were characterized by NMR and mass spectrometry (ESI†).
Next we conjugated 6 to the distal ubiquitin following an intein-based method17. To closely mimic the isopeptide linkage between the proximal and distal ubiquitin moieties, we utilized a distal ubiquitin with its last glycine residue (Gly76) removed (Ub1-75). The Ub1-75-intein construct was expressed in E. coli BL21(DE3) cells. The Ub1-75-intein fusion was purified by a chitin-affinity column and cleaved by sodium 2-mercaptoethanesulfonate (MESNA) to generate Ub1-75-MESNA as previously described.17 Ub1-75-MESNA can be readily ligated with 6 to generate a modified ubiquitin species 7 (Fig. 1A). The modified ubiquitin species was next deprotected by TFA/H2O/p-TsOH to form the distal ubiquitin species 8 conjugated with a reactive α-bromo-vinylketone group. For the proximal ubiquitin, we mutated the targeted lysine, e.g. Lys63 for the K63-linked diubiquitin, to cysteine. The unique cysteine in the proximal ubiquitin reacts with the α-bromo-ketone to form a stable bond between the two ubiquitin moieties. Note that a HA tag was introduced to the N-terminus of the proximal ubiquitin to facilitate its detection by anti-HA antibody. Following the ligation reaction as shown in Figure 1A, we obtained a diUb probe that closely mimics the native K63-linked diUb with the same linker size as its native counterpart (Fig. 1A). Importantly, a Michael acceptor (α,β-unsaturated ketone) was introduced into the linkage so that the activated vinyl group can trap the DUB catalytic cysteine once the probe is bound to the enzyme active site. Following the ligation reaction between the distal and proximal ubiquitin moieties and the subsequent purification, a species corresponding to the diUb probe was detected in the SDS-PAGE analysis of the ligation product (Fig. 1B). The identity of the diUb probe was confirmed by ESI mass spectrometry analysis and the linkage specificity was confirmed by the MS/MS analysis of the ubiquitin tryptic peptide that contains the diubiquitin linkage (Fig. S1–S3, ESI†). Following the same method, we also generated a diUb probe with a K48 linkage by using the proximal ubiquitin that harbors a K48C mutation (Fig. S4–S6, ESI†).
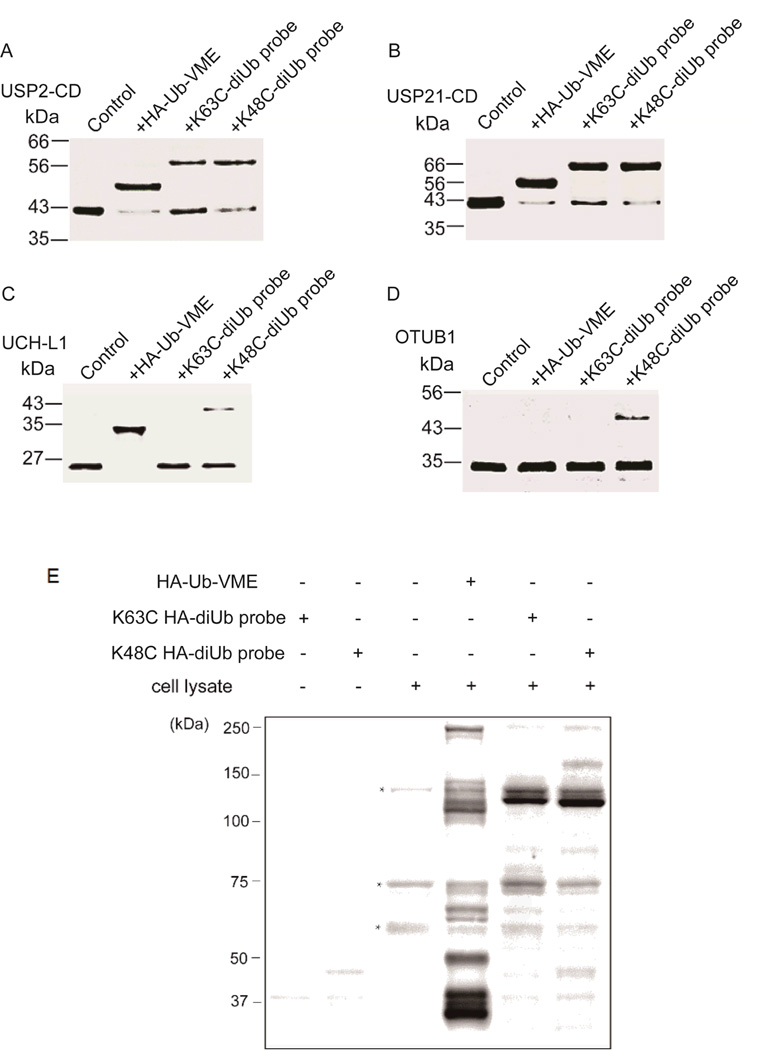
Next, using the K63C- and K48C-diubiquitin probes we assessed the reactivity of the diUb probes towards different DUBs. DUBs can be classified into several families and the ubiquitin-specific proteases (USPs) constitute the largest DUB family. Many USPs are large, multiple domain enzymes that contain a catalytic core. The reactivity of different USPs towards the diUb probes was tested by incubating the USPs with either the K63-diUb probe or the K48-diUb probe. HA-Ub-VME was used as a comparison. As shown in Fig. 2A, the USP2 catalytic core (USP2-CD) can be efficiently labeled by both the K63C- and the K48C-diUb probes. Western blotting using anti-HA antibody showed that these bands are indeed ubiquitin adducts (Fig. S7). The formation of the USP2-diUb adduct was weakened by the thiol-containing agents DTT or β-mercaptoethanol (Fig. S8). Importantly, the reactivity was highly dependent on the tertiary fold of DUBs and diUb, because the addition of SDS or guanidine hydrochloride to the reaction solution abolished the ability of the diUb probe to form adduct with USP2-CD (Fig. S9). Several other USPs including USP21, USP7 and USP8 were also tested. Successful labeling of these USPs by the two diUb probes was observed (Fig. 2B and Fig. S10). We noted that USP8 was labeled by the K63C- and the K48C-diUb probes albeit with lower efficiency compared to other USPs tested. Importantly, no significant difference was observed between the K63C- and K48C-diUb probes in labeling the USPs. Our observation agrees with the notion that many USPs are promiscuous in ubiquitin chain linkage specificity.18, 19
Fig. 2.
The reactivity of diUb probes towards different DUBs and DUB profiling using the diUb probes in cell lysates. (A-D) USP2-catalytic domain (USP2-CD), USP21-catalytic domain (USP21-CD), UCH-L1 and OTUB1 were incubated with different probes for 2 hr, separated by SDS-PAGE, and stained with Coomassie brilliant blue for detection. (E) DUB profiling using the diUb probes in cells in comparison to the monoubiquitin probe HA-Ub-VME. HEK293T cell lysates were labeled with HA-Ub-VME, K63C-linked HA-diUb probe and K48C-linked HA-diUb probe respectively. Samples were separated by SDS-PAGE and immunoblotted using anti-HA antibody. Asterisks indicate non-specific binding by the anti-HA antibody in cell lysate.
We next probed UCH-L1 that belongs to the ubiquitin C-terminal hydrolase (UCH) family with the diUb probes. The UCH family DUBs are known to prefer to cleave small adducts or unfolded polypeptides from the C terminus of ubiquitin2, although earlier studies also reported that some UCHs can also cleave ubiquitin off larger proteins20. We incubated UCH-L1 with both the K48C- and K63C-diUb probes. In marked contrast to USPs, no detectable adduct was observed for the K63C-diUb probe and only a very weak adduct was detected for the K48-linked diUb probe (Fig. 2C). In comparison, the monoUb probe HA-Ub-VME showed stronger reactivity towards UCH-L1 (Fig. 2C).
Different from USPs, the ovarian tumor (OTU) family DUBs display marked chain linkage specificity.21, 22 Otubain 1 (OTUB1) harbors strong Lys48 linkage specificity over Lys63 linkage.23, 24 We investigated whether our diUb probes can recapitulate the ubiquitin linkage specificity of OTUB1. As shown in Fig. 2D, the K48-diUb probe formed a diUb-adduct, while the K63C-diUb probe did not label OTUB1. Consistent with previous report, the HA-Ub-VME did not label OTUB125. Clearly our diUb probes revealed the same diubiquitin linkage specificity of OTUB1 as previously reported.
Further, we carried out activity-based profiling against cellular DUBs using the HA-tagged K63C-diUb and K48C-diUb probes. The HA-tagged ubiquitin vinyl methyl ester (HA-Ub-VME) was used as a comparison. The HEK293T cell lysates were incubated with each of the diUb probes (HA-K63C-diUb probe and HA-K48C-diUb probe) or the monoUb probe HA-Ub-VME. The cell lysates were then separated on a denaturing SDS-PAGE gel, and detected with an anti-HA antibody (Fig. 2E). The labeling of cellular DUBs with HA-Ub-VME is in accordance with previous DUB profiling studies.25 In marked contrast, a much smaller number of DUBs were detected by the HA-K63C-diUb and the HA-K48C-diUb probes. Strong labeling of DUBs by the two diUb probes was observed in the molecular weight range of 100–150 kDa. Weaker labeling of low-molecule weight DUBs was observed for both diUb probes as compared to the labeling by monoubiquitin probe HA-Ub-VME. This is understandable given that the low-molecular weight DUB adducts are mainly due to UCHs.25 The difference further supports the notion that UCHs prefer to act on ubiquitin with small adducts instead of an acceptor protein. Furthermore, the labeling patterns of the cellular DUBs by the two diUb probes are different in cell lysates. For examples, a DUB adduct at molecular weight slightly higher than 150 kDa was observed for the K48C-diUb probe, but not the K63C-diUb probe.
In conclusion, we have generated a new class of diubiquitin probes and demonstrated that they can be used to probe the diubiquitin chain linkage specificity in purified DUBs and in cell lysates. For the USP family DUBs, no obvious difference was detected between the K48C- and K63C-diUb probes for the tested USPs. In contrast, OTUB1 showed strong K48 linkage specificity and little to no labeling was observed for UCH-L1. Importantly, activity-based profiling against cellular DUBs showed that the diUb probes can label DUBs in cell lysates with distinct profiles and improved labeling specificity compared to the monoUb probe. The presence of an intact proximal ubiquitin moiety allows the potential interaction between DUBs and the proximal ubiquitin to be recapitulated using the diubiquitin probes. This represents a major advantage over the monoubiquitin-based probes. The linkage specific diUb probes will help to attain a better understanding of the DUB linkage specificity and cellular function in a cellular environment. Moreover, availability of this class of DUB probes will also facilitate the development of more specific inhibitors against the large number of DUBs that are expected to possess chain- or target-specificities. Furthermore, this approach is not limited to diUb probe and can be applied to other proteins that are ubiquitinated.
Supplementary Material
Acknowledgements
We thank Wade Harper for the USP2 plasmid, Bert Vogelstein for the USP7 plasmid, Cheryl Arrowsmith for the USP21 plasmid, and Sirano Dhe-Paganon for the USP8 plasmid. This work was supported, in part, by U.S. NIH grant R01GM097468 to Z. Zhuang.
Footnotes
Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c000000x/
Notes and references
- 1.Reyes-Turcu FE, Ventii KH, Wilkinson KD. Annu Rev Biochem. 2009;78:363–397. doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amerik AY, Hochstrasser M. Biochimica et biophysica acta. 2004;1695:189–207. doi: 10.1016/j.bbamcr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Cravatt BF, Wright AT, Kozarich JW. Annu Rev Biochem. 2008;77:383–414. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- 4.Sadaghiani AM, Verhelst SH, Bogyo M. Current opinion in chemical biology. 2007;11:20–28. doi: 10.1016/j.cbpa.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 5.Ovaa H. Nature reviews. Cancer. 2007;7:613–620. doi: 10.1038/nrc2128. [DOI] [PubMed] [Google Scholar]
- 6.Borodovsky A, Kessler BM, Casagrande R, Overkleeft HS, Wilkinson KD, Ploegh HL. Embo J. 2001;20:5187–5196. doi: 10.1093/emboj/20.18.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borodovsky A, Ovaa H, Kolli N, Gan-Erdene T, Wilkinson KD, Ploegh HL, Kessler BM. Chemistry & biology. 2002;9:1149–1159. doi: 10.1016/s1074-5521(02)00248-x. [DOI] [PubMed] [Google Scholar]
- 8.Ekkebus R, van Kasteren SI, Kulathu Y, Scholten A, Berlin I, Geurink PP, de Jong A, Goerdayal S, Neefjes J, Heck AJR, Komander D, Ovaa H. Journal of the American Chemical Society. 2013;135:2867–2870. doi: 10.1021/ja309802n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Love KR, Pandya RK, Spooner E, Ploegh HL. Acs Chem Biol. 2009;4:275–287. doi: 10.1021/cb9000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGouran JF, Kramer HB, Mackeen MM, di Gleria K, Altun M, Kessler BM. Organic & Biomolecular Chemistry. 2012;10:3379. doi: 10.1039/c2ob25258a. [DOI] [PubMed] [Google Scholar]
- 11.de Jong A, Merkx R, Berlin I, Rodenko B, Wijdeven RH, El Atmioui D, Yalcin Z, Robson CN, Neefjes JJ, Ovaa H. ChemBioChem. 2012;13:2251–2258. doi: 10.1002/cbic.201200497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanmugham A, Fish A, Luna-Vargas MPA, Faesen AC, Oualid FE, Sixma TK, Ovaa H. Journal of the American Chemical Society. 2010;132:8834–8835. doi: 10.1021/ja101803s. [DOI] [PubMed] [Google Scholar]
- 13.Iphöfer A, Kummer A, Nimtz M, Ritter A, Arnold T, Frank R, van den Heuvel J, Kessler BM, Jänsch L, Franke R. ChemBioChem. 2012;13:1416–1420. doi: 10.1002/cbic.201200261. [DOI] [PubMed] [Google Scholar]
- 14.Bavikar SN, Spasser L, Haj-Yahya M, Karthikeyan SV, Moyal T, Kumar KS, Brik A. Angew Chem Int Ed Engl. 2012;51:758–763. doi: 10.1002/anie.201106430. [DOI] [PubMed] [Google Scholar]
- 15.Keusekotten K, Elliott PR, Glockner L, Fiil BK, Damgaard RB, Kulathu Y, Wauer T, Hospenthal MK, Gyrd-Hansen M, Krappmann D, Hofmann K, Komander D. Cell. 2013;153:1312–1326. doi: 10.1016/j.cell.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato Y, Yoshikawa A, Yamagata A, Mimura H, Yamashita M, Ookata K, Nureki O, Iwai K, Komada M, Fukai S. Nature. 2008;455:358–362. doi: 10.1038/nature07254. [DOI] [PubMed] [Google Scholar]
- 17.Chen JJ, Ai YX, Wang JL, Haracska L, Zhuang ZH. Nat Chem Biol. 2010;6:270–272. doi: 10.1038/nchembio.316. [DOI] [PubMed] [Google Scholar]
- 18.Faesen AC, Luna-Vargas MP, Geurink PP, Clerici M, Merkx R, van Dijk WJ, Hameed DS, El Oualid F, Ovaa H, Sixma TK. Chemistry & biology. 2011;18:1550–1561. doi: 10.1016/j.chembiol.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Komander D, Clague MJ, Urbé S. Nature Reviews Molecular Cell Biology. 2009;10:550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 20.Misaghi S, Galardy PJ, Meester WJ, Ovaa H, Ploegh HL, Gaudet R. J Biol Chem. 2005;280:1512–1520. doi: 10.1074/jbc.M410770200. [DOI] [PubMed] [Google Scholar]
- 21.Mevissen TE, Hospenthal MK, Geurink PP, Elliott PR, Akutsu M, Arnaudo N, Ekkebus R, Kulathu Y, Wauer T, El Oualid F, Freund SM, Ovaa H, Komander D. Cell. 2013;154:169–184. doi: 10.1016/j.cell.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Virdee S, Ye Y, Nguyen DP, Komander D, Chin JW. Nat Chem Biol. 2010;6:750–757. doi: 10.1038/nchembio.426. [DOI] [PubMed] [Google Scholar]
- 23.Edelmann Mariola J, Iphöfer A, Akutsu M, Altun M, di Gleria K, Kramer Holger B, Fiebiger E, Dhe-Paganon S, Kessler Benedikt M. Biochemical Journal. 2009;418:379. doi: 10.1042/BJ20081318. [DOI] [PubMed] [Google Scholar]
- 24.Wang T, Yin L, Cooper EM, Lai M-Y, Dickey S, Pickart CM, Fushman D, Wilkinson KD, Cohen RE, Wolberger C. Journal of Molecular Biology. 2009;386:1011–1023. doi: 10.1016/j.jmb.2008.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altun M, Kramer HB, Willems LI, McDermott JL, Leach CA, Goldenberg SJ, Kumar KG, Konietzny R, Fischer R, Kogan E, Mackeen MM, McGouran J, Khoronenkova SV, Parsons JL, Dianov GL, Nicholson B, Kessler BM. Chemistry & biology. 2011;18:1401–1412. doi: 10.1016/j.chembiol.2011.08.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.