Abstract
Importance
Older adults commonly report disturbed sleep, and recent studies in humans and animals suggest links between sleep and Alzheimer disease biomarkers. Studies are needed that evaluate whether sleep variables are associated with neuroimaging evidence of β-amyloid deposition.
Objective
To determine the association between self-reported sleep parameters and β-amyloid deposition in community-dwelling older adults.
Design
cross-sectional
Setting
Baltimore Longitudinal Study of Aging, a prospective study of normative aging
Participants
70 adults (mean age = 76; range 53 - 91) in the BLSA neuroimaging study
Main Outcome Measure
β-amyloid burden, measured by [11C] Pittsburgh compound B (PiB) positron emission tomography (PET) distribution volume ratios (DVR)
Results
After adjustment for potential confounders, reports of shorter sleep duration were associated with greater β-amyloid burden, measured by mean cortical DVR (cDVR; B = 0.08, 95% confidence interval (CI) 0.03, 0.14, p = 0.005) and precuneus DVR (B = 0.11, 95% CI 0.03, 0.18, p = 0.007). Reports of lower sleep quality were associated with greater β-amyloid burden measured by precuneus DVR (B = 0.08, 95% CI 0.01, 0.15, p = 0.025).
Conclusions
Among community-dwelling older adults, reports of shorter sleep duration and lower sleep quality are associated with greater β-amyloid burden. Further studies with objective sleep measures are needed to determine whether sleep disturbance causes or accelerates Alzheimer disease.
Introduction
Numerous studies have linked disturbed sleep to cognitive impairment in older adults. Individuals with Alzheimer disease (AD) have been shown to spend more time in bed awake1, 2 and have more fragmented sleep than those without AD,1-3 and studies of healthier older adults document associations between worse self-reported sleep and lower cognitive performance.4, 5 In addition, recent research demonstrates that poor sleep, measured using wrist actigraphy, is associated with lower cognitive performance in community-dwelling elders.6 While these findings indicate that sleep disturbance is associated with poor cognitive outcomes, it remains unclear whether poor sleep contributes to the neuropathology underlying cognitive decline.
β-amyloid plaques are one of the hallmarks of AD, and fluctuations in amyloid-β (Aβ) peptide may be regulated by sleep/wake patterns. Kang et al. demonstrated, in wild-type mice and a mouse model of AD, that levels of Aβ in brain interstitial fluid increased with time awake and decreased during sleep; they demonstrated similar fluctuations in cerebrospinal fluid (CSF) Aβ levels in young humans.7 Intriguingly, sleep deprivation in the AD mouse model produced a substantial increase in β-amyloid plaque burden.7 We are unaware of any published studies that have investigated whether sleep disturbance is associated with neuroimaging evidence of β-amyloid in the brains of older living humans.
We used data from community-dwelling participants in the Baltimore Longitudinal Study of Aging (BLSA) to investigate whether self-reported sleep parameters were associated with fibrillar β-amyloid burden, measured in vivo with [11C] Pittsburgh compound B (PiB) positron emission tomography (PET). We hypothesized that reports of more fragmented sleep, shorter sleep duration, and lower sleep quality would be associated with greater amyloid burden.
Methods
Participants
We studied participants in the BLSA neuroimaging study (BLSA-NI),8 a substudy of the larger BLSA study of normative aging.9 Upon enrollment, BLSA participants must be free of cognitive impairment, mobility limitations and physical disability, major diseases (other than controlled hypertension) and conditions that can negatively affect functioning or life expectancy, or require ongoing antibiotic, immunosuppressant, corticosteroid, chronic pain medication or H2 blockers. At study visits, participants spend >48 consecutive hours at the BLSA Clinical Laboratory, where they have their height and weight measured, undergo a medical exam, complete multiple questionnaires and measures of cognition and physical function, and provide blood and urine for assays.
BLSA participants were eligible for the BLSA-NI (1994-present) if they were free of neurological disease, significant cardiovascular and pulmonary disease, and metastatic cancer at the BLSA-NI baseline. We studied 70 individuals in the BLSA-NI with sleep data from a BLSA visit and a [11C]PiB PET scan <5 years after that visit.
BLSA participants provided informed consent upon enrollment and at subsequent visits. Study protocols were approved by IRBs affiliated with the National Institute on Aging Intramural Research Program and the Johns Hopkins Medical Institutions.
[11C]PiB PET Acquisition
Prior to [11C]PiB PET studies, participants were fitted with a thermoplastic face mask to decrease head motion. Scans were conducted on a GE Advance scanner in 3-dimensional mode immediately following an intravenous bolus injection of 14.6 ±0.90 mCi of [11C]PiB. PET data were acquired per the following protocol for the duration of the frames: 4×0.25, 8×0.5, 9×1, 2×3, and 10×5 min (70 min total, 33 frames).
MRI Acquisition
Depending on scan year, participants were imaged with a spoiled gradient-recalled (SPGR) acquisition sequence (N=5; GE Signa 1.5T, TR=35ms, TE=5ms, α=45°, 256×256 image matrix, 124 slices, pixel size=0.94×0.94 mm, slice thickness=1.5 mm) or a magnetization prepared rapid acquisition with gradient echo (MPRAGE) sequence (N=65 total; for N=42 subjects a Philips 1.5T scanner was used with TR=6.8ms, TE=3.3ms, α=8°, 256×256 matrix, 124 slices, pixel size=0.94×0.94 mm, slice thickness=1.5 mm; and for the remaining N=23 subjects a Philips Intera 3T scanner was used with TR=6.8ms, TE=3.2ms, α=8°, 256×256 matrix, 170 slices, pixel size = 1×1 mm, slice thickness=1.2 mm). MR images were obtained at the same study visit as PiB images.
Image Processing
Dynamic [11C]PiB PET images (70 minutes) were processed using an in-house pipeline with the Java Image Science Toolkit (JIST)10 that was developed for the Medical Image Processing, Analysis and Visualization program (MIPAV).11 The pipeline involved: RF coil inhomogeneity correction12 and segmentation13, 14 of the MRI to define the cerebellar gray matter reference region which was subsequently registered to the time-aligned PET; a multi-atlas approach using four templates15 with cortical region delineations to define regions of interest using non-linear deformation16 for label registration with subsequent label fusion17 on each individual's MRI; model fitting of the PET image to generate voxel-based DVR and R1 parametric images18; and transformation of the MRI-based segmentation and labels onto the PET images for calculation of regional and mean cortical DVR.
We studied two PiB indices: mean cortical DVR (cDVR) and precuneus DVR. cDVR is the weighted average of values for the superior, middle and inferior frontal and orbitofrontal, superior parietal, supramarginal and angular gyrus regions, precuneus, superior, middle and inferior occipital, superior, middle and inferior temporal, anterior, middle and posterior cingulate regions. cDVR provides a global index of cortical β-amyloid burden. Precuneus DVR was examined separately because it is likely a region affected early in the AD course.19
Sleep Assessment
Participants reported in a standardized interview the average number of hours of sleep obtained each night over the prior month using the following response options: “more than 7”; “more than 6, up to 7”; “more than 5, up to 6”; or “5 or fewer.” Responses were coded 0 to 3; each one-unit increase indicated at least a one-hour decrease in sleep duration. Participants also completed a modified version of the five-item Women's Health Initiative Insomnia Rating Scale (WHIIRS).20 This version queried about sleep over the past month, rather than the prior four weeks, and was administered by interview, rather than questionnaire. The first four WHIIRS items query about how often respondents “have trouble falling asleep,” “wake up several times at night,” “wake up earlier than you planned to,” and “have trouble getting back to sleep” after early waking.20 Participants indicated the frequency of these problems on a five-point scale (0 to ≥5 times per week). On the fifth WHIIRS item, respondents rate their sleep quality on a five-point scale (“very sound or restful” to “very restless”). Responses are summed, yielding a total score; higher scores on items or the total score indicate more frequent sleep disturbance or worse sleep quality.20
Other Measures
Participants provided demographic data upon enrollment. At imaging study visits, participants completed a neuropsychological test battery, including Clinical Dementia Rating Scale (CDR) administration.21 Data were reviewed at a case conference for all autopsy participants and to non-autopsy participants with ≥4 errors on the Blessed-Information-Memory-Concentration Test.22 Mild cognitive impairment (MCI) was diagnosed using Petersen criteria23 and dementia diagnoses followed Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM-III-R) criteria.24 Depressive symptomatology was assessed with the 20-item Center for Epidemiological Studies-Depression Scale (CES-D).25 At each BLSA visit, a nurse practitioner conducts an interview covering signs, symptoms, and diagnosed health conditions, and reviews medical records to obtain a medical history. Participants also reported frequency of sleep medication use over the prior month on a five-point scale (0 to ≥5 times per week). Apolipoprotein E (APOE) genotype (e4 carrier vs. non-carrier) was measured in BLSA participants because the e4 allele is a potent risk factor for AD26 and APOE is associated with brain amyloid burden.27
Statistical Analysis
First, we explored distributions of, and correlations among responses to WHIIRS items, and the distribution of sleep duration data. Responses to two WHIIRS items (i.e., early waking, difficulty falling asleep after early waking) were highly correlated with other items or had a limited distribution. Thus, we did not consider these items as individual predictors, but they were included in the WHIIRS total score calculation. Next, we fit unadjusted and multivariable (MV)-adjusted linear regression models with either continuous cDVR or precuneus DVR as the outcome. The primary predictors included sleep duration, difficulty falling asleep, waking several times, sleep quality ratings, or the WHIIRS total, with each predictor analyzed in a separate model. To account for cardiovascular or pulmonary disease, we created a dichotomous variable indicating history of heart attack or myocardial infarction, heart failure or congestive heart failure, angina, coronary bypass surgery or angioplasty, chronic bronchitis, emphysema, or COPD. MV-adjusted models included age, sex, race, CES-D score, body mass index (BMI; kg/m2), APOE e4 status, history of cardiovascular or pulmonary disease, and current sleep medication use (any vs. none) as covariates. Partial correlations were calculated to quantify associations between sleep variables and continuous outcomes, after controlling for covariates. We conducted two sets of sensitivity analyses. In the first, we re-ran analyses after excluding participants with an MCI or dementia diagnosis. In the second, to assess how robust our results were to the assumption of the data distribution, we fit logistic regression models with dichotomous (i.e., elevated vs. low PiB binding) versions of the cDVR (≥1.125) and precuneus DVR (≥1.38). Cutpoints for these variables were selected based on the clustering of DVRs in our sample (eFigure 1). Predictors and covariates were from the study visit closest to PiB PET scans.
Results
Participants were aged (mean ±standard deviation) 78.2 ±7.9 years when they completed PiB PET, and 76.4 ±8.0 (range 53 – 91) when they completed sleep measures; sleep assessment occurred concurrently with or prior to PiB PET. There were 1.7 ±1.6 years (range 0.0-4.9) between PiB and the most proximal sleep measure (Table 1). Thirty-three participants (47.1 %) were women, and 13 (18.6%) were Black. Participants had 16.8 ±2.3 years of education (range 12-20), and at sleep assessment, their mean MMSE score was 28.9 ±1.6, and mean CES-D score was 5.2 ±5.7. Three participants had MCI and 1 had dementia. Two met MCI criteria at the time of sleep assessment, and three met MCI criteria and one met dementia criteria at PiB assessment. Seven (10.0%) took sleep medication over the prior month.
Table 1.
Participant characteristics.
| Mean ±SD or n (%) | |
|---|---|
| Female sex | 33 (47.1) |
| Black race | 13 (18.6) |
| Education (years) | 16.8 ±2.3 |
| Age at sleep assessment (years) | 76.4 ±8.0 |
| Age at PiB PET (years) | 78.2 ±7.9 |
| Interval between sleep and PiB PET (years) | 1.7 ±1.6 |
| CES-D (0 to 60) | 5.2 ±5.7 |
| PiB cDVR | 1.12 ±0.19 |
| PiB precuneus DVR | 1.25 ±0.26 |
| Elevated cDVR | 24 (34.3) |
| Elevated precuneus DVR | 16 (22.9) |
| Body mass index (kg/m2) | 27.2 ±4.1 |
| APOE e4-positive | 20 (28.6) |
| MMSE (0 to 30) | 28.9 ±1.6 |
| CVD or pulmonary disease | 9 (12.9) |
| Takes any sleep medication | 7 (10.0) |
Note: N = 70. APOE = apolipoprotein E; CES-D = Center for Epidemiological Studies Depression Scale; CVD = cardiovascular disease; MMSE = Mini-Mental State Examination.
Seventy participants had both PiB PET data and sleep data from BLSA interviews occurring at or after 2004; 62 participants had data on sleep duration. Overall, 24 participants (34.3%) had elevated PiB according to cDVR and 16 (22.9%) had elevated PiB in precuneus. Forty-two percent reported >6 to ≤7 hours of sleep per night and 31% reported >7 hours; however, 21% reported >5 to ≤6 hours and 7% reported ≤5 hrs (Table 2). Their mean WHIIRS total score was 7.1 ±4.3 (range 0-19); the distribution of responses to individual items is presented in Table 2.
Table 2.
Distribution of sleep characteristics over the past month.
| Average hours of sleep | n (%) |
|---|---|
| >7 | 19 (30.6) |
| >6 to ≤7 | 26 (41.9) |
| >5 to ≤6 | 13 (21.0) |
| ≤5 | 4 (6.5) |
| Trouble falling asleepa | n (%) |
|---|---|
| none | 39 (55.7) |
| <1 time/week | 9 (12.9) |
| 1−2 times/week | 15 (21.4) |
| 3−4 times/week | 4 (5.7) |
| 5+ times/week | 3 (4.3) |
| Wake several timesa | n (%) |
|---|---|
| none | 10 (14.3) |
| <1 time/week | 10 (14.3) |
| 1−2 times/week | 6 (8.6) |
| 3−4 times/week | 5 (7.1) |
| 5+ times/week | 39 (55.7) |
| Sleep qualitya | n (%) |
|---|---|
| Very sound/restful | 15 (21.4) |
| Sound/restful | 20 (28.6) |
| Average quality | 28 (40.0) |
| Restless | 7 (10.0) |
| Very restless | 0 |
N = 70 for all variables except average hours of sleep (n = 62).
From the Women's Health Initiative Insomnia Rating Scale (WHIIRS).
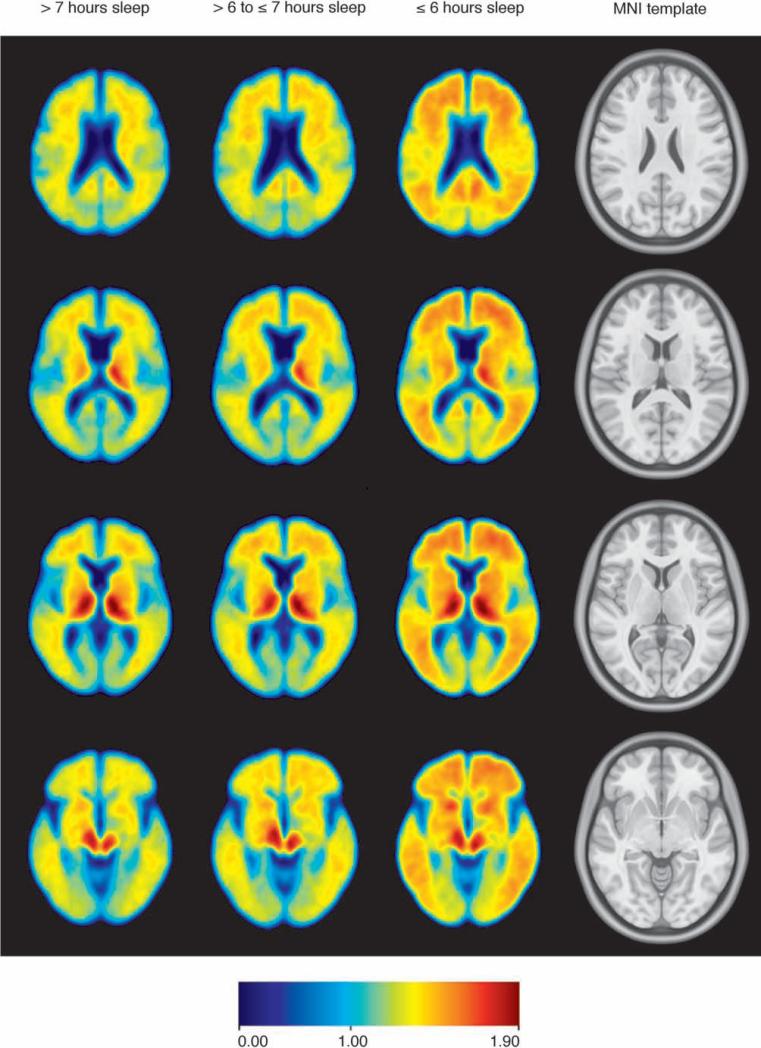
In adjusted analyses, each one-unit decrease in sleep duration was associated with a 0.08-point increase in cDVR (B = 0.08, 95% confidence interval (CI) 0.03, 0.14, partial r = 0.38, p = 0.005) (Table 3). This association is evident when unadjusted mean cDVR images are compared as a function of sleep duration (Figure 1). There was a comparable association between shorter sleep duration and precuneus DVR. In addition, each one-unit increase in sleep quality rating (i.e., worse sleep quality) was associated with a 0.06-point increase in cDVR in unadjusted analyses (B = 0.06, 95% CI 0.01, 0.10, p = 0.019); this became non-significant after adjustment. Worse sleep quality was associated with greater precuneus DVR, however, in unadjusted and adjusted analyses (adjusted B = 0.08, 95% CI 0.01, 0.15; partial r = 0.29, p = 0.025). There was no association between waking several times and either cDVR or precuneus DVR, but there was a trend toward an association between greater frequency of difficulty falling asleep and both cDVR and precuneus DVR in unadjusted and adjusted analyses, and between WHIIRS total scores and both cDVR and precuneus DVR in unadjusted analyses.
Table 3.
Association between sleep variables and continuous DVRs.
| Unadjusted | MV-adjusted | |||||
|---|---|---|---|---|---|---|
| B (95% CI) | Partial r | p-value a | B (95% CI) | Partial r | p-value a | |
| Shorter sleep duration | ||||||
| cDVR | 0.06 (0.004, 0.11) | 0.27 | 0.035 | 0.08 (0.03, 0.14) | 0.38 | 0.005 |
| Precuneus DVR | 0.07 (0.0001, 0.14) | 0.25 | 0.0498 | 0.11 (0.03, 0.18) | 0.36 | 0.007 |
| Trouble falling asleepb | ||||||
| cDVR | 0.04 (−0.002, 0.07) | 0.22 | 0.065 | 0.03 (−0.003, 0.07) | 0.23 | 0.071 |
| Precuneus DVR | 0.04 (−0.007, 0.10) | 0.20 | 0.091 | 0.05 (−0.005, 0.10) | 0.23 | 0.076 |
| Wake several timesb | ||||||
| cDVR | 0.01 (−0.02, 0.04) | 0.08 | 0.487 | 0.01 (−0.02, 0.04) | 0.05 | 0.715 |
| Precuneus DVR | 0.01 (-0.03, 0.05) | 0.07 | 0.561 | 0.01 (-0.03, 0.05) | 0.05 | 0.690 |
| Worse sleep qualityb | ||||||
| cDVR | 0.06 (0.01, 0.10) | 0.28 | 0.019 | 0.04 (−0.01, 0.09) | 0.19 | 0.130 |
| Precuneus DVR | 0.09 (0.03, 0.16) | 0.34 | 0.004 | 0.08 (0.01, 0.15) | 0.29 | 0.025 |
| WHIIRS total | ||||||
| cDVR | 0.01 (−0.0001, 0.02) | 0.23 | 0.052 | 0.01 (−0.004, 0.02) | 0.16 | 0.227 |
| Precuneus DVR | 0.01 (−0.0002, 0.03) | 0.23 | 0.054 | 0.01 (−0.004, 0.02) | 0.18 | 0.161 |
Note: N = 70 for all analyses except sleep duration (N = 62). MV-adjusted for age, sex, race, CES-D score, body mass index, APOE e4 status, cardiovascular or pulmonary disease, and use of sleep medication (any vs. none). Partial r's are correlations between sleep variables and imaging outcomes after controlling for the same covariates included in MV analyses.
p-values apply to regression coefficients and partial correlations.
From the Women's Health Initiative Insomnia Rating Scale (WHIIRS; higher scores indicate worse sleep). B = unstandardized regression coefficient; cDVR = cortical distribution volume ratio; CI = confidence interval; MV = multivariable.
Figure 1.
Unadjusted mean PiB DVR images from four axial slices demonstrate that shorter self-reported sleep duration is associated with greater β-amyloid burden. Participants reporting >7 hours sleep (n = 19) have the least amyloid burden, those reporting ≤6 hours sleep duration (n = 17) have the most, and those reporting >6 to ≤7 hours (n = 26) have an intermediate level of burden. Rightmost column contains structural images from the Montreal Neurological Institute space template.42, 43
After removing the four participants with MCI or dementia, associations remained in adjusted models between shorter sleep duration and both cDVR (B = 0.07, 95% CI 0.01, 0.14, partial r = 0.32, p = 0.019) and precuneus DVR (B = 0.10, 95% CI 0.02, 0.18, partial r = 0.32, p = 0.021). The association between worse sleep quality and amyloid burden remained for precuneus DVR in both unadjusted analyses (B = 0.08, 95% CI 0.01, 0.14, partial r = 0.27, p = 0.020), and after adjustment (B = 0.08, 95% CI 0.01, 0.15, partial r = 0.29, p = 0.028).
In our sensitivity analysis examining the association between sleep variables and elevated PiB, results indicated that reports of shorter sleep duration and more frequent difficulty falling asleep each were associated with an increased odds of elevated PiB according to both cDVR and precuneus DVR; lower sleep quality was associated with a greater odds of elevated PiB as measured by precuneus DVR (eTable 1). These associations remained after removing the four participants with MCI or dementia (data not shown).
Comment
We examined the association between self-report indices of sleep and β-amyloid deposition, measured by [11C]PiB PET, in community-dwelling older adults. After adjustment for potential confounders, shorter sleep duration was associated with greater amyloid burden on continuous measures of cortical DVR (cDVR) and precuneus DVR, and worse sleep quality was associated with greater β-amyloid burden according to continuous precuneus DVR. Further, these associations remained after excluding participants with either MCI or dementia, and we observed a similar pattern of associations when using a dichotomous outcome (elevated vs. low PiB). Neither participant-reported frequency of multiple awakenings nor a global index of disturbed sleep was associated with β-amyloid burden. To our knowledge, this is the first published study of self-reported sleep and PiB PET-measured β-amyloid deposition in community-dwelling older adults.
Our results are consistent with those from animal research in which sleep deprivation increased ISF β-amyloid.7 These studies raise the possibility that poor sleep may promote amyloid deposition, but they also raise questions about mechanisms linking sleep/wake and amyloid burden. It has been suggested that wake-related increases in neuronal activity may mediate the association between sleep and β-amyloid levels.7 Indeed, in AD animal models and cultured hippocampal slices, increased neuronal activity promotes generation of Aβ peptide,28-30 and the sleep state is correlated with decreases, and the wake state with increases in synaptic strength.31, 32 Recent functional neuroimaging findings also suggest that excess neuronal excitability may contribute to AD pathogenesis.33
Our results may have significant public health implications. AD is the most common form of dementing illness, and almost half of older adults report insomnia symptoms.34 Because late-life sleep disturbance can be treated, to the extent that poor sleep promotes AD onset and progression, interventions to improve sleep or maintain healthy sleep among older adults may help prevent or slow AD. This would have a substantial impact on independence and quality of life of older adults and their families, and on the significant healthcare costs associated with AD.
The present study has several strengths, including a well-characterized community-dwelling sample, assessment of multiple sleep variables, and [11C]PiB PET imaging. However, it also has limitations. First, because it is cross-sectional in design, we cannot tell whether sleep disturbance preceded β-amyloid deposition, limiting our ability to evaluate the direction of a potential causal association between poor sleep and AD. Indeed, a recent study in an AD mouse model showed that Aβ aggregation is accompanied by increased sleep/wake disruption and alterations in diurnal fluctuation of Aβ in ISF, and that immunization with Aβ1-42 decreases Aβ aggregation and preserves sleep/wake patterns and diurnal ISF fluctuation.35 Another recent study that measured sleep using actigraphy demonstrated that, compared to those without preclinical AD, humans with preclinical AD (measured by CSF Aβ42) spend a smaller proportion of time in bed asleep (i.e., they have lower sleep efficiency).36 It has been suggested that, while poor sleep may promote initial Aβ aggregation, amyloid deposition may promote derangements of sleep/wake that feed forward to promote amyloid deposition, and that prospective studies are needed to characterize the association between sleep/wake disruption and amyloid deposition.35 A second limitation of our study is that our sleep measures were based on self-report and did not include objective measures (e.g., wrist actigraphy, polysomnography). Self-report sleep measures can be influenced by lower cognitive function,37 and in some cases are only modestly correlated or even uncorrelated with objective sleep measures.38 Replication of findings using objective sleep measures would clarify whether perceptions of poor sleep and objective sleep indices are differentially associated with AD pathology. Third, the prevalence of sleep-disordered breathing (SDB) increases with age,39 and SDB has been linked to mild cognitive impairment and dementia.40 Studies using polysomnography are needed to investigate whether SDB contributes to β-amyloid deposition,41 and whether SDB drives the association we observed between poor sleep quality and β-amyloid burden. Finally, in our sleep duration measure, the response option for those with the longest sleep duration was “>7 hours,” placing those obtaining 8 hours of sleep in the same category as those obtaining 11 hours. Consequently, we could not test hypotheses about very long sleepers compared to those with more intermediate sleep duration (e.g., 7 to 8 hours), with respect to β-amyloid burden.
Conclusions
Our findings in a sample of community-dwelling older adults indicate that reports of shorter sleep and lower sleep quality are associated with greater β-amyloid burden. As evidence for this association accumulates, intervention trials will be needed to determine whether optimizing sleep can prevent or slow AD progression.
Supplementary Material
Acknowledgment
Results from this study were presented at the 2013 Annual Meetings of the American Association for Geriatric Psychiatry and the Associated Professional Sleep Societies. Funding/Support and Role of Sponsors: This study was supported in part by the Intramural Research Program (IRP), National Institute on Aging (NIA), National Institutes of Health (NIH) and by Research and Development Contract HHSN-260-2004-00012C. Support also came from a Synapses, Circuits and Cognitive Disorders Award from the Johns Hopkins School of Medicine Brain Science Institute. Dr. Spira is supported by a Mentored Research Scientist Development Award (1K01AG033195) from the National Institute on Aging. Dr. Wong was partly supported during part of this time by NIH NIDA career award (K24 DA000412; 2000-2011).
This study was funded in part by the NIA IRP NIH and NIA IRP NIH Investigators were involved in all aspects of this manuscript, including the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The NIA Extramural Research Program and the Johns Hopkins School of Medicine Brain Science Institute played no role in the study design or conduct, or in data collection, management, analysis, or interpretation, or in manuscript preparation, review or approval, or decision to submit the manuscript for publication.
Dr. Susan Resnick had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Yang An, M.S. (Intramural Research Program, National Institute on Aging, National Institutes of Health) performed the statistical analysis, Murat Bilgel, B.S. (Intramural Research Program, National Institute on Aging, National Institutes of Health and Department of Biomedical Engineering, Johns Hopkins University) performed the image analysis, Adam P. Spira, Ph.D. (Department of Mental Health, Johns Hopkins Bloomberg School of Public Health), and Susan M. Resnick, Ph.D. (Intramural Research Program, National Institute on Aging, National Institutes of Health) supervised the statistical and image analysis and assume responsibility for the overall analysis.
Footnotes
Conflicts of interest: Dr. Dean Wong has received contracts from Avid through Johns Hopkins University. The other authors report no conflicts of interest.
References
- 1.Prinz PN, Peskind ER, Vitaliano PP, Raskind MA, Eisdorfer C, Zemcuznikov N, Gerber CJ. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am Geriatr Soc. 1982;30(2):86–93. doi: 10.1111/j.1532-5415.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 2.Prinz PN, Vitaliano PP, Vitiello MV, Bokan J, Raskind M, Peskind E, Gerber C. Sleep, EEG and mental function changes in senile dementia of the Alzheimer's type. Neurobiol Aging. 1982;3(4):361–370. doi: 10.1016/0197-4580(82)90024-0. [DOI] [PubMed] [Google Scholar]
- 3.Vitiello MV, Prinz PN, Williams DE, Frommlet MS, Ries RK. Sleep disturbances in patients with mild-stage Alzheimer's disease. J Gerontol. 1990;45(4):M131–138. doi: 10.1093/geronj/45.4.m131. [DOI] [PubMed] [Google Scholar]
- 4.Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64(2):180–187. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006;20(1):41–48. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- 6.Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, Laffan A, Stone KL. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011;34(10):1347–1356. doi: 10.5665/SLEEP.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, Bryan RN, Zonderman AB. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10(5):464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- 9.Shock N, Greulich R, Andres R, Arenberg D, Costa P, Lakatta E, Tobin J. Normal human aging: The Baltimore Longitudinal Study of Aging. NIH; Washington, DC: 1984. [Google Scholar]
- 10.Lucas BC, Bogovic JA, Carass A, Bazin PL, Prince JL, Pham DL, Landman BA. The Java Image Science Toolkit (JIST) for rapid prototyping and publishing of neuroimaging software. Neuroinformatics. 2010;8(1):5–17. doi: 10.1007/s12021-009-9061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAuliffe MJ, Lalonde FM, McGarry D, Gandler W, Csaky K, Trus BL. Medical Image Processing, Analysis and Visualization in clinical research.. Paper presented at: Computer-Based Medical Systems, 2001. CBMS 2001. Proceedings. 14th IEEE Symposium on; 2001; 2001. [Google Scholar]
- 12.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 13.Bazin PL, Pham DL. Topology-preserving tissue classification of magnetic resonance brain images. IEEE Trans Med Imaging. 2007;26(4):487–496. doi: 10.1109/TMI.2007.893283. [DOI] [PubMed] [Google Scholar]
- 14.Carass A, Cuzzocreo J, Wheeler MB, Bazin PL, Resnick SM, Prince JL. Simple paradigm for extra-cerebral tissue removal: algorithm and analysis. Neuroimage. 2011;56(4):1982–1992. doi: 10.1016/j.neuroimage.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcus DS, Wang TH, Parker J, Csernansky JG, Morris JC, Buckner RL. Open Access Series of Imaging Studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J Cogn Neurosci. 2007;19(9):1498–1507. doi: 10.1162/jocn.2007.19.9.1498. [DOI] [PubMed] [Google Scholar]
- 16.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (STAPLE): an algorithm for the validation of image segmentation. IEEE Trans Med Imaging. 2004;23(7):903–921. doi: 10.1109/TMI.2004.828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, Endres CJ, Brasic JR, Huang SC, Wong DF. Linear regression with spatial constraint to generate parametric images of ligand-receptor dynamic PET studies with a simplified reference tissue model. Neuroimage. 2003;18(4):975–989. doi: 10.1016/s1053-8119(03)00017-x. [DOI] [PubMed] [Google Scholar]
- 19.Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 20.Levine DW, Kripke DF, Kaplan RM, Lewis MA, Naughton MJ, Bowen DJ, Shumaker SA. Reliability and validity of the Women's Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15(2):137–148. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 21.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 22.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114(512):797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 23.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association . American Psychiatric Association. Work Group to Revise DSM-III. Diagnostic and statistical manual of mental disorders : DSM-III-R. 3rd ed. American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- 25.Radloff L. The CES-D Scale: a self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 26.Corder E, Saunders A, Strittmatter W, Schmechel D, Gaskell P, Small G, Roses A, Haines J, Pericak-Vance M. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 27.Thambisetty M, Tripaldi R, Riddoch-Contreras J, Hye A, An Y, Campbell J, Sojkova J, Kinsey A, Lynham S, Zhou Y, Ferrucci L, Wong DF, Lovestone S, Resnick SM. Proteome-based plasma markers of brain amyloid-beta deposition in non-demented older individuals. J Alzheimers Dis. 2010;22(4):1099–1109. doi: 10.3233/JAD-2010-101350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37(6):925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 29.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48(6):913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 30.Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011;14(6):750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63(6):865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74(3):467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep medicine reviews. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 35.Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, Holtzman DM. Disruption of the Sleep-Wake Cycle and Diurnal Fluctuation of beta-Amyloid in Mice with Alzheimer's Disease Pathology. Sci Transl Med. 2012;4(150):150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ju YE, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, Morris JC, Holtzman DM. Sleep Quality and Preclinical Alzheimer Disease. JAMA Neurol. 2013:1–7. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Den Berg JF, Van Rooij FJ, Vos H, Tulen JH, Hofman A, Miedema HM, Neven AK, Tiemeier H. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008;17(3):295–302. doi: 10.1111/j.1365-2869.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 38.Unruh ML, Redline S, An MW, Buysse DJ, Nieto FJ, Yeh JL, Newman AB. Subjective and objective sleep quality and aging in the sleep heart health study. J Am Geriatr Soc. 2008;56(7):1218–1227. doi: 10.1111/j.1532-5415.2008.01755.x. [DOI] [PubMed] [Google Scholar]
- 39.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proceedings of the American Thoracic Society. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA : the journal of the American Medical Association. 2011;306(6):613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sperling R, Johnson K. To sleep, perchance to delay dementia. Arch Neurol. 2012;69(1):118–120. doi: 10.1001/archneurol.2011.1901. [DOI] [PubMed] [Google Scholar]
- 42.Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL, Brain Development Cooperative G. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54(1):313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fonov VS, Evans AC, McKinstry RC, Almli CR, Collins DL. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage. 2009;47(Supplement 1):S102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.