Abstract
Physiological data demonstrates theta frequency oscillations associated with memory function and spatial behavior. Modeling and data from animals provides a perspective on the functional role of theta rhythm, including correlations with behavioral performance and coding by timing of spikes relative to phase of oscillations. Data supports a theorized role of theta rhythm in setting the dynamics for encoding and retrieval within cortical circuits. Recent data also supports models showing how network and cellular theta rhythmicity allows neurons in the entorhinal cortex and hippocampus to code time and space as a possible substrate for encoding events in episodic memory. Here we discuss these models and relate them to current physiological and behavioral data.
Keywords: hippocampus, entorhinal cortex, memory, resonance, grid cells, place cells, electroencephalograph
Theta frequency oscillations appear in electroencephalographic (EEG) recordings from scalp electrodes and depth electrodes in human subjects, but controversy continues over the functional role of theta frequency oscillations (commonly referred to as theta rhythm). Understanding the functional role of theta rhythm will benefit from attention to data on the role of theta rhythm in animals. Here we will review data on theta rhythm in humans and animals and recent theories linking theta rhythm to mechanisms of memory and spatial navigation.
1. Experimental data on theta rhythm
1.1. Theta rhythm in humans and animals
In early EEG studies in humans (Niedermeyer, 1999), Hans Berger used the greek letter alpha to designate 8-12 Hz frequencies observed first in resting participants, then used beta for 12-30 Hz frequencies in more attentive participants. Subsequently, gamma (30 to 100 Hz), and delta (below 4 Hz) were named. The 4 to 7 Hz band was designated theta (Walter & Dovey, 1944) to stand for thalamus (Niedermeyer, 1999) because thalamic lesions in monkeys shifted cortical dynamics from alpha (8-12 Hz) to theta (4-7 Hz). Early studies showed that theta in the cortical EEG correlates with developmental age and pathological conditions, but intracranial electrodes implanted to detect seizure activity also show cortical theta rhythm associated with performance of memory tasks in humans (Kahana et al., 1999; Kahana et al., 2001; Raghavachari et al., 2001; Sederberg et al., 2003; Raghavachari et al., 2006; Rizzuto et al., 2006; Rizzuto et al., 2006; Guderian et al., 2009; Lega et al., 2011). The data in humans includes recordings during virtual navigation tasks showing increases in power of theta and delta frequency oscillations associated with spatial navigation and movement speed (Caplan et al., 2003; Ekstrom et al., 2005; Watrous et al., 2011; Watrous et al., 2013). Studies in humans also show a relationship of memory function to theta rhythm in scalp EEG recordings (Klimesch et al., 1994; Klimesch et al., 1996; Klimesch, 1999; Jacobs et al., 2006), and in magnetoencephalographic (MEG) recordings (Jensen & Tesche, 2002; Osipova et al., 2006) that include effects of virtual movement on theta rhythm in MEG (de Araujo et al., 2002; Cornwell et al., 2008; Kaplan et al., 2012). Note that many of these studies show power changes in low theta and delta ranges, in contrast to higher theta frequencies found in animals. This article will focus on data from animals, as the topic of theta rhythm in humans is addressed in other reviews in this special issue by Ranganath, Ekstrom and Sederberg and Polyn.
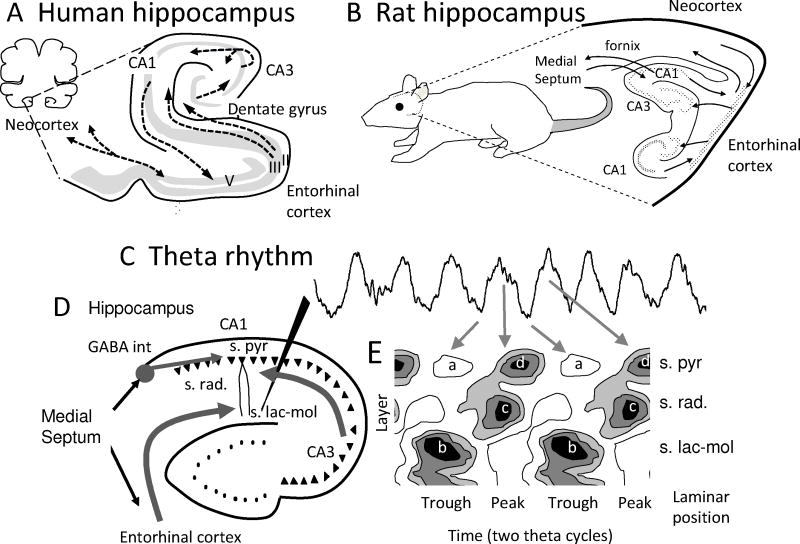
Data from animals suggests functional roles of theta rhythm. Early studies of local field potentials (LFPs) in animals found prominent oscillations in the theta frequency range in the hippocampus (Green & Arduini, 1954). Figure 1 shows theta rhythm recorded from the hippocampus. Theta rhythm refers to frequencies from 3-10 Hz in animals because similar mechanisms appear to underlie this full range of frequencies (Buzsaki, 2002). Theta rhythm LFP oscillations also appear in rat entorhinal cortex (Mitchell & Ranck, 1980; Alonso & Garcia-Austt, 1987; Brandon et al., 2011) and medial prefrontal cortex (Jones & Wilson, 2005; Lee et al., 2005).
FIGURE 1.
Theta rhythm in the hippocampus. A. Location and anatomy of the hippocampus in the human brain. B. Anatomy of the hippocampus in the rat brain showing the input from the medial septum via the fornix. C. Theta rhythm in the EEG recorded in stratum lacunosum-moleculare (s. lac-mol) of hippocampal region CA1 of the rat. D. The medial septum input to GABA cells in the hippocampus paces theta rhythm (Buzsaki, 2002). Gray arrows show synaptic input from entorhinal cortex and CA3 causing synaptic currents in region CA1. D. Schematic based on current source density data (Brankack et al., 1993) during two cycles of theta rhythm shows a source (“a” outward current) in the pyramidal layer (s. pyr) at the same phase as a sink (“b” inward current) appears due to entorhinal input in stratum lacunosum moleculare (s. lac-mol). At the opposite phase of theta rhythm, a current sink (“c”) occurs due to CA3 input in stratum radiatum (s. rad) and a sink (“d”) appears due to spiking in stratum pyramidale (s. pyr).
1.2. Behavioral correlates of theta rhythm
Theta rhythm power increases with a range of behaviors including attention to predators in rabbits (Green & Arduini, 1954; Sainsbury et al., 1987b), and voluntary movement in rats (Vanderwolf, 1969; Whishaw & Vanderwolf, 1973; Bland & Oddie, 2001; Kelemen et al., 2005; Lenck-Santini et al., 2008; Shin, 2011) including running on a track (O’Keefe & Nadel, 1978; Skaggs et al., 1996; Hinman et al., 2011), running wheel (Buzsaki et al., 1983; Hyman et al., 2003b), or treadmill (Fox et al., 1986; Brankack et al., 1993). Frequency and amplitude of rat theta increases with running speed (Whishaw & Vanderwolf, 1973; Rivas et al., 1996; Maurer et al., 2005; Jeewajee et al., 2008; Hinman et al., 2011, 2013) and with jumping (Vanderwolf, 1969; Lenck-Santini et al., 2008), suggesting a role in coding of velocity and location.
Theta rhythm in the hippocampus correlates with learning and memory function (Berry & Thompson, 1978; Winson, 1978; Givens & Olton, 1990; Vertes & Kocsis, 1997; Seager et al., 2002). Conditioning of eye blink responses to air puff or jaw movements to reward occurs more rapidly in animals with greater power of pre-stimulus theta rhythm (Berry & Thompson, 1978), and when training occurs during periods of theta rhythm (Seager et al., 2002; Griffin et al., 2004). Theta rhythm also appears during conditioning of fear responses (Whishaw, 1972; Sainsbury et al., 1987a; Seidenbecher et al., 2003). In contrast, theta rhythm during passive rotation does not seem to increase with cognitive demands (Kelemen et al., 2005).
Lesions of the medial septum and fornix that reduce theta power in the hippocampus (Rawlins et al., 1979) and entorhinal cortex (Mitchell et al., 1982) cause memory impairments in tasks including delayed spatial alternation (Givens & Olton, 1990; Aggleton et al., 1995), delayed non-match to position (Markowska et al., 1989), delayed alternation (Numan & Quaranta, 1990), spatial reversal (M’Harzi et al., 1987), the Morris water maze (Martin et al., 2007) and the 8-arm radial maze (Mitchell et al., 1982). Reduction in hippocampal theta rhythm correlates with impairments in memory (Winson, 1978) that are specific for recently experienced episodes but not for highly familiar memories (M’Harzi et al., 1987; Givens & Olton, 1994). Temporary inactivation of the medial septum impairs spatial memory and reduces theta rhythm in both the hippocampus (Chrobak et al., 1989; Brioni et al., 1990; Mizumori et al., 1990) and entorhinal cortex (Jeffery et al., 1995), and spatial memory performance after septal inactivation can be recovered by stimulation of the fornix at theta rhythm (McNaughton et al., 2006), supporting a link between theta rhythm and behavioral encoding of space.
1.3. Functional coding by phase of theta rhythm
An ongoing controversy concerns whether neurons code information not just by the rate of spiking but also by the time of spiking (temporal coding, also known as phase coding). Support for temporal or phase coding comes from the spike timing relative to theta rhythm. Hippocampal place cells respond selectively when a rat visits a specific location (O’Keefe & Dostrovsky, 1971; O’Keefe, 1976; Skaggs et al., 1996). When a rat runs through a place cell firing field, the place cell initially spikes at late phases of the theta cycle, and then shifts to progressively earlier phases as the rat continues through the place field (O’Keefe & Recce, 1993). This phenomenon, termed theta phase precession, has been replicated in numerous recordings (e.g. Skaggs et al., 1996; Mehta et al., 2002; Huxter et al., 2003; Huxter et al., 2008; Lenck-Santini et al., 2008; Mizuseki et al., 2009) showing that the phase of spikes relative to network oscillations codes space. This code could contribute to a cognitive map allowing association of items with locations (Burgess et al., 1997; Redish & Touretzky, 1998; Jensen & Lisman, 2000; Arleo & Gerstner, 2000; Hasselmo & Eichenbaum, 2005; Erdem & Hasselmo, 2012; Hasselmo, 2012). In humans, hippocampal place cells have been shown with depth electrodes in participants performing virtual navigation tasks (Ekstrom et al., 2003), but theta phase precession has not yet been shown in humans.
A phase code for space also appears in recordings of grid cells in the entorhinal cortex in rats (Hafting et al., 2008; Moser & Moser, 2008). Grid cells respond as a rat visits a regular array of locations in the environment described as falling on the vertices of tightly packed equilateral triangles (Fyhn et al., 2004; Hafting et al., 2005; Hafting et al., 2008). Each time a rat passes through the firing field of a grid cell, the spiking starts at late phases of the LFP theta cycle, and shifts to earlier phases of the theta cycle (Hafting et al., 2008; Climer et al., 2013). Data supporting grid cells in humans comes from research based on a six-fold rotational symmetry of fMRI activation during virtual navigation (Doeller et al., 2010) and unit recording from depth electrodes in human entorhinal cortex (Kahana, personal communication).
2. Theta rhythm may provide separate phases of encoding and retrieval
The behavioral data indicates a role of theta rhythm in the encoding of new information, but the mechanisms for this role are not known. Modeling shows how specific physiological processes at different phases of theta rhythm could enhance encoding by separating the dynamics of encoding and retrieval on different phases of the theta rhythm (Hasselmo et al., 2002a).
Memory requires separation of information arriving from the external world, to be encoded as new, from the information retrieved from internal circuits, which must be treated as old. While speaking with a friend, you might remember something they said yesterday, but you must respond to what they said to you in the current moment, not your memory of what they said to you yesterday. Physiological data on theta rhythm inspired a model (Hasselmo et al., 2002a) in which the changes in synaptic current at different phases of theta rhythm mediate separate phases of encoding and retrieval that repeat on every cycle (Figure 2), referred to here as the SPEAR model (Separate Phases of Encoding And Retrieval). This model has inspired further research described below.
FIGURE 2.
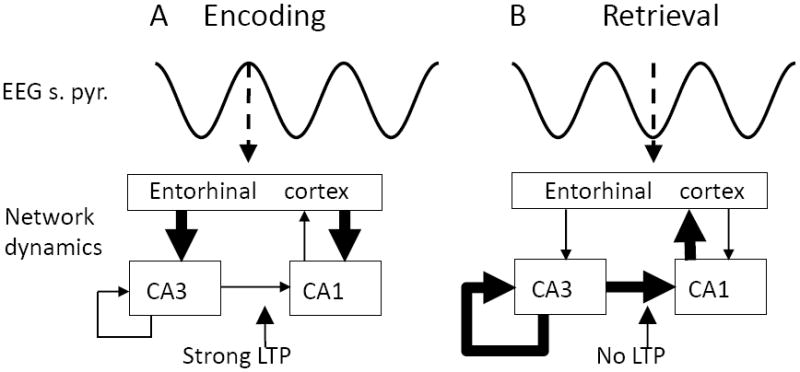
Model of separate phases of encoding and retrieval (SPEAR) during different phases of hippocampal theta rhythm (Hasselmo et al., 2002a). A. Encoding. At the peak of the LFP in region CA1 stratum pyramidale (s. pyr), synaptic input from entorhinal cortex is strong (thick arrows), driving activity in CA3 and CA1. Synapses from CA3 to CA1 have weak transmission (thin arrows), causing less retrieval, but can undergo long-term potentiation (LTP) to encode new associations. B. Retrieval. At the trough of the LFP, synaptic input from entorhinal cortex is weaker (thin arrows), but synaptic transmission from CA3 is strong, allowing retrieval of previously stored associations to drive spiking in region CA1. LTP is weak (No LTP) to prevent encoding of retrieved activity as new.
In the SPEAR model, during the encoding phase of each theta cycle (Figure 2A), external input from entorhinal cortex is strong (Hasselmo et al., 2002a), setting a new pattern of depolarization in the postsynaptic dendrites in region CA1 for encoding. During this encoding phase, synaptic input from region CA3 is weaker so that it does not drive the postsynaptic depolarization. However, synaptic modification at the synapses in region CA1 is strong during this phase, allowing synapses with NMDA receptors to strengthen, encoding associations between the presynaptic activity in CA3 and the postsynaptic activity induced in CA1 neurons by input from entorhinal cortex. Physiological data shows that long-term potentiation is strongest at this phase of the EEG (Huerta and Lisman, 1995; Holscher et al., 1997; Hyman et al., 2003a).
During the retrieval phase of each theta cycle, external input from entorhinal cortex is weaker, but the excitatory input from region CA3 is stronger. The stronger input from CA3 means postsynaptic activity in region CA1 is driven by the spread of activity across previously modified synapses, retrieving previously stored associations. At this time, the cell body receives the least inhibition, allowing retrieval to drive the spiking output of the neurons. Long-term potentiation is reduced during this time, so that the retrieval activity is not stored as a new event.
Simulations show that separate phases of encoding and retrieval allow effective separation of new external input from prior retrieval (Hasselmo et al., 2002a). Encoding and retrieval can overlap, but too much overlap could cause problems. If encoding dynamics happen during the retrieval phase, then you may mistake current sensory input for a retrieved memory (déjà vu), or you might mistake your retrieved memory from yesterday as a new event happening today and new information may distort old memories. If retrieval is allowed during the encoding phase, the spread of retrieval activity causes postsynaptic spiking to occur during induction of long-term potentiation, resulting in formation of incorrect associations between old and new events, causing a breakdown in network function (Hasselmo et al., 2002b).
2.1. Physiological data supporting the SPEAR model
Physiological data on theta rhythm are consistent with the encoding/retrieval model, including membrane potential dynamics, changes of inhibition, changes in synaptic transmission, and changes in LTP.
2.1.1. Phasic membrane potential dynamics
Hippocampal neurons fire at different rates on different phases of theta rhythm (Fox et al., 1986; O’Keefe & Recce, 1993; Skaggs et al., 1996), possibly due to phasic changes in membrane potential (Fujita and Sato, 1964; Fox, 1989; Kamondi et al., 1998). At one phase, the dendrites are depolarized (Kamondi et al., 1998) by entorhinal input, allowing encoding, while the cell body is hyperpolarized, preventing spiking due to interference from retrieval of previous associations (Hasselmo et al., 2002a). At the opposite phase, cell bodies are depolarized during input from region CA3, allowing spiking output for retrieval of previously stored associations. Consistent with this, spiking in region CA1 occurs at a phase following spiking in region CA3 and not during spiking in entorhinal layer III (Mizuseki et al., 2009).
2.1.2. Phasic changes in inhibition
Membrane potential changes could arise due to different morphological classes of inhibitory interneurons that spike at different phases of theta rhythm (Klausberger et al., 2003; Klausberger & Somogyi, 2008). Modeling suggests functional roles for different phases of interneuron firing in separating encoding and retrieval (Kunec et al., 2005; Cutsuridis & Hasselmo, 2012). Inhibitory axo-axonic and basket cells could inhibit the cell bodies and axons of excitatory cells to reduce spiking output during encoding (Cutsuridis & Hasselmo, 2012). At the opposite, retrieval phase, oriens lacunosum-moleculare cell spiking inhibits the layer where entorhinal input contacts the distal dendrites, reducing external input during retrieval of associations at previously modified synapses in stratum radiatum (Hasselmo et al., 2002a; Kunec et al., 2005).
2.1.3. Phasic changes in synaptic input
Current source density analysis (Buzsaki et al., 1986; Brankack et al., 1993) shows systematic changes in synaptic currents during theta. During one phase, strong excitatory currents in stratum lacunosum-moleculare (Buzsaki et al., 1986; Brankack et al., 1993) could allow encoding by driving depolarization throughout the dendritic tree to form associations with coincident synaptic input from region CA3, even though spiking driven by retrieval is reduced by inhibition at the cell body (Kamondi et al., 1998). At the opposite phase, stronger excitatory currents in stratum radiatum (Brankack et al., 1993) could reflect previously strengthened synapses from region CA3 driving retrieval that causes spiking of CA1 pyramidal cells (Fox et al., 1986; Skaggs et al., 1996; Csicsvari et al., 1999) based on the pattern of previously encoded associations (Hasselmo et al., 2002a).
Phasic changes in synaptic input could also arise from differences in presynaptic inhibition of synaptic transmission (Wyble et al., 2000) causing changes in size of synaptic potentials (Wyble et al., 2000; Villarreal et al., 2007) and population spiking (Rudell et al., 1980; Buzsaki et al., 1981; Rudell et al., 1984; Villarreal et al., 2007), possibly due to presynaptic inhibition caused by GABAB receptors (Hasselmo & Fehlau, 2001; Molyneaux & Hasselmo, 2002). These phasic changes allow synaptic transmission in stratum radiatum to be weak when induction of long-term potentiation is strong during encoding (Hasselmo et al., 2002a).
2.1.4. Phasic changes in long-term potentiation
The separation of encoding and retrieval is consistent with phasic changes in long-term potentiation (LTP). LTP is stronger in dentate gyrus when a tetanus is delivered on positive phases of theta (Pavlides et al., 1988; Orr et al., 2001). In intracellular slice preparations of region CA1 in rat hippocampus showing theta rhythm, stimulation on the peak of theta causes LTP, while stimulation on the trough causes long-term depression (Huerta & Lisman, 1995). The phase change in LTP induction also occurs in anesthetized rats (Holscher et al., 1997), and awake, behaving animals (Hyman et al., 2003a) indicating that LTP can be induced at the synapses from region CA3 when synaptic transmission is weak at these CA3-CA1 synapses but postsynaptic dendrites are depolarized by entorhinal input. At this phase, the cell body is hyperpolarized, preventing spiking due to retrieval, but dendritic spikes can underlie LTP even when the soma is hyperpolarized (Golding et al., 2002). Thus, extensive physiological data are consistent with separation of encoding and retrieval on different phases of theta rhythm.
2.2. Network data relating to encoding and retrieval
The SPEAR models helps in understanding impairments of memory encoding during loss of theta rhythm (Winson, 1978; Givens and Olton, 1994). In rats, fornix lesions that reduce theta rhythm cause an increase in the number of erroneous visits to a previously rewarded arm in a T-maze task (M’Harzi et al., 1987). Without theta to separate encoding and retrieval, strong synaptic transmission may mediate retrieval of the memory for food at the now unrewarded location at the phase when long-term potentiation enhances synaptic strength, thereby slowing the extinction of the old food memory (Hasselmo et al., 2002a). The loss of the encoding phase could also underlie the impairments in memory tasks associated with inactivation of the medial septum (Chrobak et al., 1989).
The SPEAR model is consistent with data showing that the phase of theta rhythm correlates with sniffing (Macrides et al., 1982), that theta rhythm shows phase reset during stimulus encoding (Givens, 1996), and phase resetting enhances induction of long-term potentiation in rats (McCartney et al., 2004). EEG oscillations in human subjects show phase reset to different phases of theta rhythm during behavioral trials requiring item encoding versus response to a retrieval probe (Rizzuto et al., 2006). In rats performing a delayed non-match to sample task, spiking occurs at different phases of theta for match (retrieval) versus non-match (encoding) stimuli (Manns et al., 2007). Studies of gamma frequency oscillations in rats also support the model. Gamma appears on specific phases of theta in hippocampus (Bragin et al., 1995) and entorhinal cortex (Chrobak & Buzsaki, 1998; Tort et al., 2009). Consistent with the encoding phase of the model, high frequency gamma oscillations are coherent between entorhinal cortex and region CA1 at one phase of theta (Colgin et al., 2009). At a different phase of theta, region CA1 shows coherence of low frequency gamma with region CA3 (Colgin et al., 2009) consistent with a retrieval phase.
Because the retrieval phase is associated with long-term depression of synapses, the SPEAR model has the property of retrieval-induced forgetting, similar to another model using oscillations to strengthen target memories and weaken competing memories (Norman et al., 2006; Norman et al., 2007), consistent with EEG data in humans (Newman & Norman, 2010). Behavioral studies show that modulatory separation of encoding and retrieval may also occur over longer time courses, as indicated by data showing enhanced pattern completion after periods of retrieval (Duncan et al., 2012).
3. Theta rhythm and models of the phase coding of space
The extensive data on theta phase precession (O’Keefe & Recce, 1993; Skaggs et al., 1996; Mehta et al., 2002; Huxter et al., 2003; Huxter et al., 2008; Mizuseki et al., 2009) inspired a range of models addressing how the phase of spiking relative to theta rhythm codes the spatial location of an animal (O’Keefe and Recce, 1993; Tsodyks et al., 1996; Jensen and Lisman, 1996; Wallenstein and Hasselmo, 1997; Geisler et al., 2007). The first paper on theta phase precession proposed that it arose from oscillatory interference (O’Keefe & Recce, 1993) between a baseline oscillation (e.g. network theta rhythm) and a velocity-controlled oscillator pushed to higher frequency by running speed (e.g. single cell membrane potential). Consistent with this, data shows that average frequency of place cell spiking (detected by autocorrelation of spike trains) is higher than the frequency of network theta rhythm, which could cause the higher frequency spiking to systematically shift to earlier phases relative to the baseline.
The models of theta phase precession also address mechanisms of place cell generation. The oscillatory interference model (OIM) (O’Keefe & Recce, 1993) generates spatial firing fields because the sum of two oscillations shows a transition from zero amplitude when the oscillations are out of phase to a peak amplitude due to constructive interference when the oscillations are close in phase, followed by a decrease as they go back out of phase. As the oscillations continuously shift in and out of phase, this would produce multiple firing fields. This could be seen as the model of O’Keefe and Recce predicting the multiple firing fields of grid cells that were reported many years later in the entorhinal cortex (Hafting et al., 2005; Moser & Moser, 2008). When grid cells were discovered, Burgess, Barry and O’Keefe showed how the model could be extended to simulate the repeating array of grid cell firing fields as the rat moves through the environment due to interaction of multiple oscillators regulated by velocity relative to preferred directions of movement (Burgess et al., 2005; Burgess et al., 2007; Burgess, 2008). In this model, velocity shifts the frequency of the oscillators, such that the relative phase of oscillations codes the spatial position of the animal, and summation of interfering oscillators generates grid cell firing fields as shown in Figure 3. This model links the circuit dynamics of theta rhythm to the coding of space for behavior (Burgess et al., 2005; 2007; Burgess, 2008; Blair et al., 2007; Hasselmo et al., 2007; Blair et al., 2008; Hasselmo, 2008; Welday et al., 2011; Hasselmo & Brandon, 2012). This model is referred to here as the OIM (Oscillatory Interference Model).
FIGURE 3.
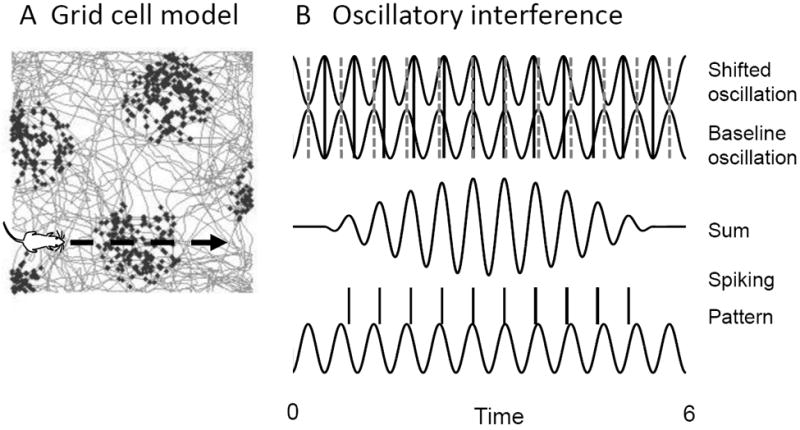
Theta phase precession in the oscillatory interference model (OIM) of grid cells. A. This example shows the model as a rat runs straight through the firing field of a simulated grid cell at constant velocity. B. In the model (Burgess et al., 2007) the velocity alters the frequency of the Shifted oscillation relative to the Baseline oscillation, causing a shift in phase (solid lines) relative to baseline phase (dashed lines) that is proportional to the integral of velocity (location). The relative phase can be read out by spiking due to the Sum of the oscillations. The model replicates experimental data (Harvey et al., 2009) showing that spikes occur at the peak of membrane potential oscillations and spiking precesses (shifts backward in phase) relative to network field potential oscillations.
Despite the wealth of data, some researchers still argue that theta phase precession is an epiphenomenon not central to the cognitive function of cortical circuits, focusing on the role of attractor dynamics and rate coding in representation of space. However, recent models have shown that attractor dynamics and oscillatory interference are not incompatible, merging them to address experimental data on grid cells and place cells. This section will first address experimental data supporting the oscillatory interference model (OIM), and then will address data that presents a problem for this model.
3.1. Support for phase coding involving theta rhythm
3.1.1. Spiking frequency and theta phase precession
The OIM explicitly requires theta phase precession (Burgess et al., 2007) and predicted the experimental data showing theta phase precession of entorhinal grid cell spiking (Hafting et al., 2008), and the difference in slope of theta phase precession at different dorsal to ventral positions in entorhinal cortex and hippocampus (Hafting et al., 2008; Kjelstrup et al., 2008). This contrasts with models of grid cells using attractor dynamics, which do not automatically generate theta phase precession. The OIM also predicted the relationship of the intrinsic spiking frequency of grid cells tested by autocorrelograms (Burgess, 2008) to rat running speed and the spacing of grid cell firing fields (Jeewajee et al., 2008; Stensola et al., 2012). Consistent with the model, modulation of spiking frequency by the cosine of head direction has been shown in theta rhythmic neurons of the hippocampus and medial septum (Blair et al., 2008; Welday et al., 2011).
3.1.2. Dependence on oscillations
The OIM motivated tests of whether grid cells depend upon theta rhythm, using blockade of entorhinal theta rhythm via pharmacological inactivation of the medial septum (Brandon et al., 2011; Koenig et al., 2011). The blockade of theta rhythm was accompanied by loss of spatial periodicity of grid cells, without loss of head direction cell selectivity. Conjunctive grid cells that showed both grid cell spatial periodicity and head direction selectivity lost their grid cell spatial periodicity but not their head direction sensitivity during loss of theta rhythm (Brandon et al., 2011).
3.1.3. Intracellular membrane properties
The OIM predicted (Burgess et al., 2007) that the difference in spacing between firing fields of grid cells at different dorsal to ventral positions in the medial entorhinal cortex (Hafting et al., 2005; Sargolini et al., 2006) should be associated with a difference in intrinsic frequency of neurons (Figure 4). Intracellular recordings in stellate cells showed a corresponding difference in frequency of resonance and subthreshold membrane potential oscillations at different anatomical positions in medial entorhinal cortex (Giocomo et al., 2007) that has been extensively replicated (Giocomo & Hasselmo, 2008b; a; 2009; Boehlen et al., 2010; Heys et al., 2010; Dodson et al., 2011; Pastoll et al., 2012; Shay et al., 2012), building on the initial work showing resonance (Haas and White, 2002; Erchova et al., 2004). The OIM also accounts for the appearance of grid cells in medial but not lateral entorhinal cortex (Hargreaves et al., 2005) in correlation with membrane potential resonance appearing in medial but not entorhinal cortex neurons (Canto & Witter, 2012; Shay et al., 2012). Physiologically, resonance is tested by intracellular injection of oscillatory current that increases in frequency. The frequency that causes a maximal voltage change in the neuron is the resonance frequency, which also reflects the time course of depolarizing rebound from hyperpolarization.
FIGURE 4.
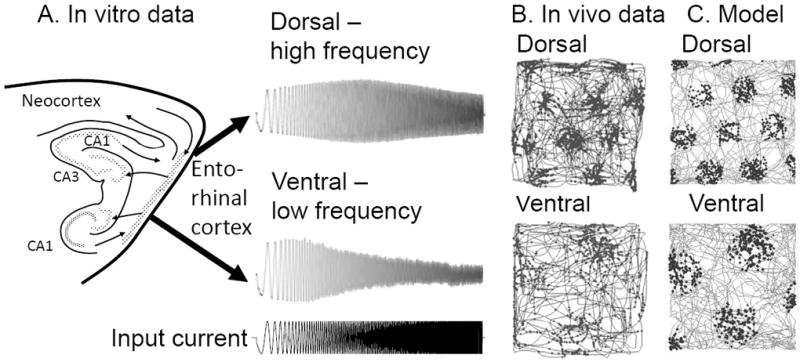
Intrinsic resonance frequency of neurons and spacing of grid cell firing fields. A. Experimental data shows that the intrinsic resonance of layer II stellate cells in response to an input current sweeping through increasing frequencies (bottom trace) shows a higher peak frequency in dorsal cells and lower peak frequency in ventral cells (Giocomo et al., 2007; Giocomo and Hasselmo, 2008b; Shay et al., 2012). B. This decrease in frequency scales with the increase in spacing between grid cell firing fields in data from awake behaving rats (Hafting et al., 2005). C. The oscillatory interference model can link the in vitro data in A to the in vivo data on grid cell firing fields in B.
These resonance properties correlated with grid field spacing can arise from the hyperpolarization-activated cation current (h current) that causes a depolarizing rebound from hyperpolarization (Dickson et al., 2000; Fransén et al., 2004). Mice with knockout of the HCN1 subunit of the h current channel show a decrease in resonance frequency (Giocomo & Hasselmo, 2009) and an increase in spacing of grid cell firing fields (Giocomo et al., 2011), supporting the link to molecular mechanisms. However, grid cell firing patterns were not abolished by HCN1 knockout, indicating the role of other mechanisms (e.g. HCN2 subunits). Activation of muscarinic acetylcholine receptors decreases the magnitude of h current (Heys & Hasselmo, 2012), and the frequency of resonance (Heys et al., 2010). The OIM shows how this could underlie the increase of grid cell spacing in novel environments (Barry et al., 2012b; Barry et al., 2012c), because acetylcholine levels increase in novel environments (Acquas et al., 1996). The h current has also been implicated in generation of theta rhythm in the hippocampus (Rotstein et al., 2005).
3.2. Current issues for phase coding models
Some data do not support the OIM. Aspects of the data support complementary models of grid cells that involve attractor dynamics (Fuhs & Touretzky, 2006; McNaughton et al., 2006; Guanella et al., 2007; Burak & Fiete, 2009), or self-organization of afferent input (Kropff & Treves, 2008; Mhatre et al., 2010; Si et al., 2012). These points of discussion include the following areas of experimental data.
3.2.1. Intracellular membrane potential properties
The OIM correctly predicts differences in intrinsic frequency, but an OIM involving interactions of membrane potential within a single neuron cannot function. Oscillations of different phase within a neuron will synchronize (Remme et al., 2010). An alternate OIM used the influence of velocity on spiking frequency of neurons (Hasselmo, 2008). Single neuron persistent spiking is too variable in phase (Zilli et al., 2009), but this problem was overcome by using populations of spiking neurons (Zilli & Hasselmo, 2010).
The OIM requires a linear change in oscillation frequency with depolarization driven by running speed. Recordings from neurons in slices of entorhinal cortex show that depolarization does not cause a linear shift in membrane potential oscillations (Yoshida et al., 2011), but membrane potential resonance does shows a systematic linear change in resonance frequency with depolarization (Shay et al., 2012). The properties of resonance motivated a newer variant of the oscillatory interference model (Figure 5) in which the rebound depolarization correlated with resonance regulates the movement of grid cell spiking, and can replicae theta cycle skipping properties of entorhinal neurons (Brandon et al., 2013).
FIGURE 5.
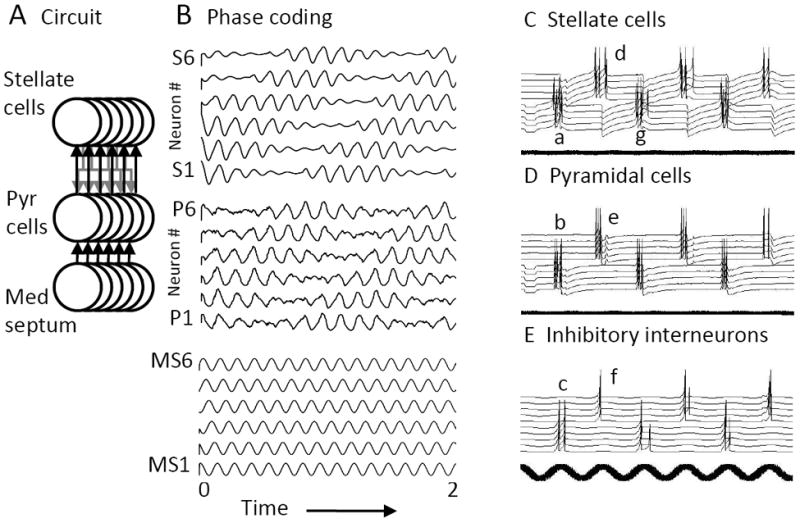
A-B. Neurons with resonance properties can interact in oscillatory attractor dynamics to generate coding of spatial location by relative phase similar to the OIM. A. The circuit involves a population of resonant stellate cells with excitatory connections with pyramidal cells (Pyr Cells) that receive phase-specific oscillatory input from medial septal neurons (Med Septum). B. Stellate cell numbers S1-S3 start out active and then show rebound activity that activates pyramidal cells P1-P4 according to the phase of Medial Septum (MS) input to pyramidal cells, and engages excitatory feedback between the stellates and pyramidals. Stellate cells rebound slightly faster than the frequency of MS input, activating pyramidal cells at earlier phases, causing a progressive shift in attractor dynamics to different populations. C-E. A similar model uses resonance to generate the theta cycle skipping properties of entorhinal neurons. A set of five stellate cells (C) respond to a hyperpolarizing pulse with rebound spikes (Ca) that excites a set of pyramidal cells (D) causing spiking (Db) that activates a set of inhibitory interneurons (E). The spiking of the interneurons (Ec) causes hyperpolarization in another set of stellate cells to cause rebound spiking (Cd) that induces spiking in a different group of pyramidal cells (De) and interneurons (Ef). These interneurons cause inhibition that induces rebound spiking in the first set of stellate cells (Cg) to start the cycle again. The firing on alternate cycles of theta rhythm resembles theta cycle skipping observed in unit recording from entorhinal cortex (Deshmukh et al., 2010; Brandon et al., 2013).
Recently, the use of virtual visual worlds allowed intracellular recordings from grid cells in head fixed mice. Consistent with the OIM, these recordings show oscillations in membrane potential during running that shift in phase relative to network theta rhythm (Schmidt-Hieber & Hausser, 2012). These recordings also show a depolarizing shift in membrane potential that correlates with the firing field of a neuron (Domnisoru et al., 2013; Schmidt-Hieber & Hausser, 2013) that could arise from attractor dynamics that could occur at the same time as OIM mechanisms.
3.2.2. Spiking properties of neurons
Data supports the need for network attractor dynamics to generate modules of grid cells with shared orientation (Hafting et al., 2005; Stensola et al., 2012) and shared size and spacing of firing fields (Barry et al., 2007; Stensola et al., 2012). Consistent with the OIM, the Moser lab showed that grid cells share intrinsic frequency within modules (Stensola et al., 2012), but argued that the predicted correlation of intrinsic frequency with grid cell spacing does not occur in all rats. However, the correlation does appear when all cells are pooled, and the inconsistent examples usually involve smaller numbers of cells.
Attractor models do not require theta phase precession, but precession can be generated in attractor models by adding rhythmic rebound properties (Navratilova et al., 2012). Attractor dynamics and oscillatory dynamics are compatible and can be combined in many ways (Hasselmo & Brandon, 2012). For example, a shift in intrinsic resonance frequency can shift an attractor due to the interaction between neurons with different oscillation phases as shown in Figure 5B.
3.2.3. Oscillations in rats and bats
As noted above, grid cell spatial periodicity disappears during the blockade of theta rhythm in rats (Brandon et al., 2011; Koenig et al., 2011). However, the role of theta rhythm may be species specific, as grid cells have been demonstrated in crawling bats that show only brief bouts of theta rhythmicity in the field potential and do not show theta rhythm in the spiking autocorrelograms (Yartsev et al., 2011). The absence of theta rhythmicity in autocorrelograms could be due to the low firing rate of grid cells recorded in crawling bats (Barry et al., 2012a). Recordings in slices show that bat medial entorhinal cortex neurons do not reveal the same distribution of theta frequency resonance that appears in rodents (Heys et al., 2013), though bat cells do show lower frequency resonance that could still underlie oscillatory interference.
3.2.4. Movement direction input
An important problem for all of these models concerns the consistent use of velocity to shift grid cell firing in both oscillatory interference models and attractor models. These models cite data on changes in firing rate with running speed in hippocampus (O’Keefe et al., 1998; Maurer et al., 2005) and entorhinal cortex (Sargolini et al., 2006; Wills et al., 2012), and neurons that change firing rate with head direction relative to the preferred head direction of a neuron, as if responding to the compass direction of the head (Taube et al., 1990; Sargolini et al., 2006). However, recent data (Raudies, Brandon, Chapman, Hasselmo, unpublished) shows that the spiking response of head direction cells and conjunctive grid by head direction cells does not correlate with the actual movement direction of the animal. As an alternative to current models, head direction may allow tracking of the direction and speed of change in the spherical angle of sensory stimuli for updating of grid cell firing, with grid cells of different spacing responding to features at different vertical positions or distances within the visual field.
4. Theta rhythm and episodic memory
Theta rhythm may contribute to encoding the where and when of episodic memory. In addition to coding spatial location, neurons in the hippocampus and entorhinal cortex also respond selectively at consistent time points within the trials of a behavioral task (Pastalkova et al., 2008; MacDonald et al., 2011; Kraus et al., in press). These responses have been referred to as “time cells” (MacDonald and Eichenbaum, 2011). The firing of time cells could allow events or items to be associated with a specific time point coded by neural activity as well as a specific location coded by place cells. Previous modeling shows that the same framework used for modeling grid cells with theta rhythm could contribute to coding of time intervals (Hasselmo, 2009; 2012) consistent with phase precession relative to jumping time (Lenck-Santini et al., 2008). This use of oscillations to code time intervals resembles previous models of coding of time intervals (Miall, 1989; Brown et al., 2000). Figure 6A shows how neurons rhythmically spiking near theta frequency could generate the response of a time cell.
FIGURE 6.
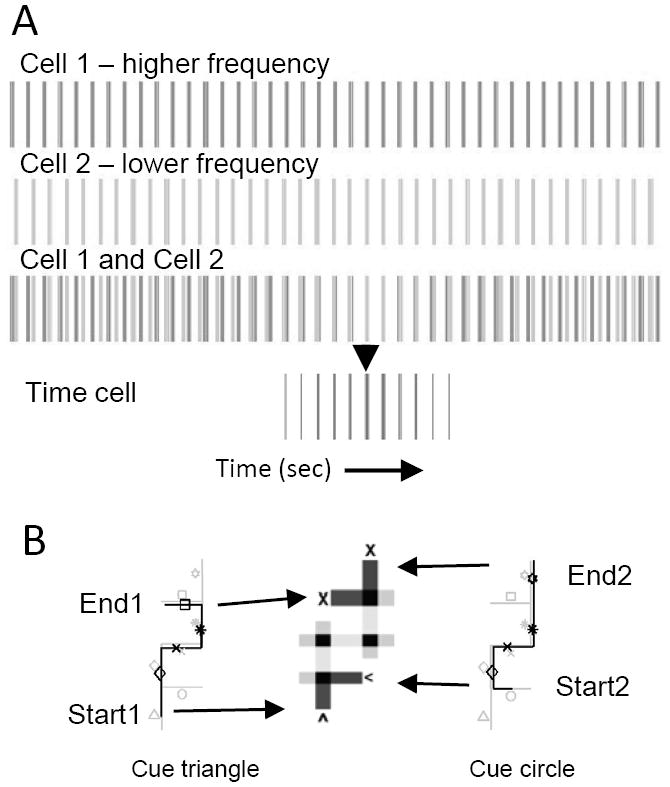
A. Model of generation of time cell firing by interaction of two theta rhythmic neurons. Cell 1 (top) fires at a slightly higher fixed difference in frequency relative to the theta rhythmic firing of Cell 2 (middle). The two cells start out of phase with each other and gradually approach the same phase of firing (Cell 1 and Cell 2). When they fire at the same phase, they drive the spiking of a Time Cell (arrow), that codes the time from the onset of firing. B. Time cells allow disambiguation of different memories involving overlapping spatial trajectories in a simulation of a behavioural task using virtual hallways with items in each hallway (Brown et al., 2010). In a simulation (Hasselmo, 2012), items are represented by different icons. One item (cue triangle) in a start hallway (Start1) can cue retrieval of a full spatiotemporal trajectory that ends with the correct turn into the final hallway (End1). A different item (cue circle) in a different start hallway (Start2) can cue retrieval of a different spatiotemporal trajectory that shares spatial locations, but uses different time cells to code the different trajectory, allowing correct turn into the correct final hallway (End2).
Models of episodic memory show how the coding of time and space by theta rhythm could account for episodic retrieval (Hasselmo, 2009; 2012) that could underlie the hippocampal activity found in functional magnetic resonance imaging studies of navigation (Brown et al., 2010; Brown and Stern, 2013). In this framework, an episodic memory involves continuous movement through time and space along a spatiotemporal trajectory that induces a continuous shift in relative phase in different populations of neurons in the entorhinal cortex (Hasselmo, 2009; 2012). Neurons that shift frequency based on velocity can generate grid cell firing responses that code location, whereas neurons that maintain a fixed difference in frequency that starts at a specific phase at the start of a trial can code time intervals (Fig. 6A). The phase coding of space and time in entorhinal cortex could drive hippocampal place cells and time cells that allow the spatiotemporal trajectory to be associated with specific items and events occurring at specific positions along the spatiotemporal trajectory (Hasselmo, 2012).
Figure 6B shows an example of how the coding of a spatiotemporal trajectory allows bidirectional associations of positions along the trajectory with items in the environment. This allows individual items to cue episodic retrieval of trajectories through the environment, with coding by time cells allowing disambiguation of spatially overlapping trajectories (Hasselmo, 2009; 2012). In this framework, the theta rhythm observed in depth electrodes in humans could provide the phase coding of space and time for encoding of episodic memories (Kahana et al., 1999; Kahana et al., 2001; Raghavachari et al., 2001; Sederberg et al., 2003; Raghavachari et al., 2006; Rizzuto et al., 2006; Lega et al., 2011). The simulation of spatiotemporal trajectories using phase coding provides an explicit model linking the cellular mechanisms for coding space and time to the elements of episodic memory.
Acknowledgments
Research supported by R01 MH60013, R01 MH61492, Silvio O. Conte Center P50 MH094263 and the Office of Naval Research MURI grant N00014-10-1-0936.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acquas E, Wilson C, Fibiger HC. Conditioned and unconditioned stimuli increase frontal cortical and hippocampal acetylcholine release: effects of novelty, habituation, and fear. J Neurosci. 1996;16:3089–3096. doi: 10.1523/JNEUROSCI.16-09-03089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Neave N, Nagle S, Hunt PR. A comparison of the effects of anterior thalamic, mamillary body and fornix lesions on reinforced spatial alternation. Behav Brain Res. 1995;68:91–101. doi: 10.1016/0166-4328(94)00163-a. [DOI] [PubMed] [Google Scholar]
- Alonso A, Garcia-Austt E. Neuronal sources of theta rhythm in the entorhinal cortex of the rat. I. Laminar distribution of theta field potentials. Experimental Brain Research. 1987;67:493–501. doi: 10.1007/BF00247282. [DOI] [PubMed] [Google Scholar]
- Arleo A, Gerstner W. Spatial cognition and neuro-mimetic navigation: a model of hippocampal place cell activity. Biol Cybern. 2000;83:287–299. doi: 10.1007/s004220000171. [DOI] [PubMed] [Google Scholar]
- Barry C, Bush D, O’Keefe J, Burgess N. Models of grid cells and theta oscillations. Nature. 2012a;488:E1–2. doi: 10.1038/nature11276. discussion E2-3. [DOI] [PubMed] [Google Scholar]
- Barry C, Ginzberg LL, O’Keefe J, Burgess N. Grid cell firing patterns signal environmental novelty by expansion. Proc Natl Acad Sci USA. 2012b;109:17687–17692. doi: 10.1073/pnas.1209918109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry C, Hayman R, Burgess N, Jeffery KJ. Experience-dependent rescaling of entorhinal grids. Nat Neurosci. 2007;10:682–684. doi: 10.1038/nn1905. [DOI] [PubMed] [Google Scholar]
- Barry C, Heys JG, Hasselmo ME. Possible role of acetylcholine in regulating spatial novelty effects on theta rhythm and grid cells. Front Neural Circuits. 2012c;6:5. doi: 10.3389/fncir.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry SD, Thompson RF. Prediction of learning rate from the hippocampal electroencephalogram. Science. 1978;200:1298–1300. doi: 10.1126/science.663612. [DOI] [PubMed] [Google Scholar]
- Blair HT, Gupta K, Zhang K. Conversion of a phase- to a rate-coded position signal by a three-stage model of theta cells, grid cells, and place cells. Hippocampus. 2008;18:1239–1255. doi: 10.1002/hipo.20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Welday AC, Zhang K. Scale-invariant memory representations emerge from moire interference between grid fields that produce theta oscillations: a computational model. J Neurosci. 2007;27:3211–3229. doi: 10.1523/JNEUROSCI.4724-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behav Brain Res. 2001;127:119–136. doi: 10.1016/s0166-4328(01)00358-8. [DOI] [PubMed] [Google Scholar]
- Boehlen A, Heinemann U, Erchova I. The range of intrinsic frequencies represented by medial entorhinal cortex stellate cells extends with age. J Neurosci. 2010;30:4585–4589. doi: 10.1523/JNEUROSCI.4939-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40-100 Hz) oscillation in the hippocampus of the behaving rat. Journal of Neuroscience. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon MP, Bogaard AR, Libby CP, Connerney MA, Gupta K, Hasselmo ME. Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science. 2011;332:595–599. doi: 10.1126/science.1201652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon MP, Bogaard AR, Schultheiss NW, Hasselmo ME. Segregation of cortical head direction cell assemblies on alternating theta cycles. Nature Neuroscience. 2013;16:739–748. doi: 10.1038/nn.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brankack J, Stewart M, Fox SE. Current source density analysis of the hippocampal theta rhythm: associated sustained potentials and candidate synaptic generators. Brain Research. 1993;615:310–327. doi: 10.1016/0006-8993(93)90043-m. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Decker MW, Gamboa LP, Izquierdo I, McGaugh JL. Muscimol injections in the medial septum impair spatial learning. Brain Res. 1990;522:227–234. doi: 10.1016/0006-8993(90)91465-s. [DOI] [PubMed] [Google Scholar]
- Brown TI, Stern CE. Contributions of medial temporal lobe and striatal memory systems to learning and retrieving overlapping spatial memories. Cereb Cortex. 2013 doi: 10.1093/cercor/bht041. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TI, Ross RS, Keller JB, Hasselmo ME, Stern CE. Which way was I going? Contextual retrieval supports the disambiguation of well learned overlapping navigational routes. J Neurosci. 2010;30:7414–7422. doi: 10.1523/JNEUROSCI.6021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Preece T, Hulme C. Oscillator-based memory for serial order. Psychol Rev. 2000;107:127–181. doi: 10.1037/0033-295x.107.1.127. [DOI] [PubMed] [Google Scholar]
- Burak Y, Fiete IR. Accurate path integration in continuous attractor network models of grid cells. PLoS Comput Biol. 2009;5:e1000291. doi: 10.1371/journal.pcbi.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N. Grid cells and theta as oscillatory interference: theory and predictions. Hippocampus. 2008;18:1157–1174. doi: 10.1002/hipo.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Barry C, Jeffery KJ, O’Keefe J. A grid and place cell model of path integration utilizing phase precession versus theta. Computational Cognitive Neuroscience Meeting.2005. [Google Scholar]
- Burgess N, Barry C, O’Keefe J. An oscillatory interference model of grid cell firing. Hippocampus. 2007;17:801–812. doi: 10.1002/hipo.20327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Donnett JG, Jeffery KJ, O’Keefe J. Robotic and neuronal simulation of the hippocampus and rat navigation. Philos Trans R Soc Lond B Biol Sci. 1997;352:1535–1543. doi: 10.1098/rstb.1997.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Czopf J, Kondakor I, Kellenyi L. Laminar distribution of hippocampal rhythmic slow activity (RSA) in the behaving rat: current-source density analysis, effects of urethane and atropine. Brain Res. 1986;365:125–137. doi: 10.1016/0006-8993(86)90729-8. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287:139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- Canto CB, Witter MP. Cellular properties of principal neurons in the rat entorhinal cortex. II. The medial entorhinal cortex. Hippocampus. 2012;22:1277–1299. doi: 10.1002/hipo.20993. [DOI] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Schulze-Bonhage A, Aschenbrenner-Schiebe R, Newman EL, Kahana MJ. Human theta oscillations related to sensorimotor integration and spatial learning. J Neurosci. 2003;23:4725–2736. doi: 10.1523/JNEUROSCI.23-11-04726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsaki G. Gamma oscillations in the entorhinal cortex of the freely behaving rat. J Neurosci. 1998;18:388–398. doi: 10.1523/JNEUROSCI.18-01-00388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Stackman RW, Walsh TJ. Intraseptal administration of muscimol produces dose-dependent memory impairments in the rat. Behav Neural Biol. 1989;52:357–369. doi: 10.1016/s0163-1047(89)90472-x. [DOI] [PubMed] [Google Scholar]
- Climer JR, Newman EL, Hasselmo ME. Phase coding by grid cells in unconstrained environments: two-dimensional phase precession. Eur J Neurosci. 2013 doi: 10.1111/ejn.12256. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Johnson LL, Holroyd T, Carver FW, Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J Neurosci. 2008;28:5983–5990. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving Rat. J Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutsuridis V, Hasselmo M. GABAergic contributions to gating, timing, and phase precession of hippocampal neuronal activity during theta oscillations. Hippocampus. 2012;22:1597–1621. doi: 10.1002/hipo.21002. [DOI] [PubMed] [Google Scholar]
- De Araujo DB, Baffa O, Wakai RT. Theta oscillations and human navigation: a magnetoencephalography study. J Cogn Neurosci. 2002;14:70–78. doi: 10.1162/089892902317205339. [DOI] [PubMed] [Google Scholar]
- Deshmukh SS, Yoganarasimha D, Voicu H, Knierim JJ. Theta modulation in the medial and the lateral entorhinal cortices. J Neurophysiol. 2010;104:994–1006. doi: 10.1152/jn.01141.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson CT, Magistretti J, Shalinsky MH, Fransen E, Hasselmo ME, Alonso A. Properties and role of I(h) in the pacing of subthreshold oscillations in entorhinal cortex layer II neurons. J Neurophysiol. 2000;83:2562–2579. doi: 10.1152/jn.2000.83.5.2562. [DOI] [PubMed] [Google Scholar]
- Dodson PD, Pastoll H, Nolan MF. Dorsal-ventral organization of theta-like activity intrinsic to entorhinal stellate neurons is mediated by differences in stochastic current fluctuations. J Physiol. 2011;589:2993–3008. doi: 10.1113/jphysiol.2011.205021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeller CF, Barry C, Burgess N. Evidence for grid cells in a human memory network. Nature. 2010;463:657–661. doi: 10.1038/nature08704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domnisoru C, Kinkhabwala AA, Tank DW. Membrane potential dynamics of grid cells. Nature. 2013;495:199–204. doi: 10.1038/nature11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K, Sadanand A, Davachi L. Memory’s penumbra: episodic memory decisions induce lingering mnemonic biases. Science. 2012;337:485–487. doi: 10.1126/science.1221936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, Fried I. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Caplan JB, Ho E, Shattuck K, Fried I, Kahana MJ. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15:881–889. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- Erdem UM, Hasselmo M. A goal-directed spatial navigation model using forward trajectory planning based on grid cells. Eur J Neurosci. 2012;35:916–931. doi: 10.1111/j.1460-9568.2012.08015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SE, Wolfson S, Ranck JB., Jr Hippocampal theta rhythm and the firing of neurons in walking and urethane anesthetized rats. Brain Research. 1986;62:495–508. doi: 10.1007/BF00236028. [DOI] [PubMed] [Google Scholar]
- Fox SE. Membrane potential and impedance changes in hippocampal pyramidal cells during theta rhythm. Exp Brain Res. 1989;77:283–294. doi: 10.1007/BF00274985. [DOI] [PubMed] [Google Scholar]
- Fransén E, Alonso AA, Dickson CT, Magistretti J, Hasselmo ME. Ionic mechanisms in the generation of subthreshold oscillations and action potential clustering in entorhinal layer II stellate neurons. Hippocampus. 2004;14:368–384. doi: 10.1002/hipo.10198. [DOI] [PubMed] [Google Scholar]
- Fuhs MC, Touretzky DS. A spin glass model of path integration in rat medial entorhinal cortex. J Neurosci. 2006;26:4266–4276. doi: 10.1523/JNEUROSCI.4353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Sato T. Intracellular records from hippocampal pyramidal cells in rabbit during theta rhythm activity. J Neurophysiol. 1964;27:1012–1025. doi: 10.1152/jn.1964.27.6.1011. [DOI] [PubMed] [Google Scholar]
- Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science. 2004;305:1258–1264. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- Geisler C, Robbe D, Zugaro M, Sirota A, Buzsaki G. Hippocampal place cell assemblies are speed-controlled oscillators. Proc Natl Acad Sci USA. 2007;104:8149–8154. doi: 10.1073/pnas.0610121104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giocomo LM, Hasselmo ME. Computation by oscillations: implications of experimental data for theoretical models of grid cells. Hippocampus. 2008a;18:1186–1199. doi: 10.1002/hipo.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giocomo LM, Hasselmo ME. Time constants of h current in layer II stellate cells differ along the dorsal to ventral axis of medial entorhinal cortex. J Neurosci. 2008b;28:9414–9425. doi: 10.1523/JNEUROSCI.3196-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giocomo LM, Hasselmo ME. Knock-out of HCN1 subunit flattens dorsal-ventral frequency gradient of medial entorhinal neurons in adult mice. J Neurosci. 2009;29:7625–7630. doi: 10.1523/JNEUROSCI.0609-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giocomo LM, Hussaini SA, Zheng F, Kandel ER, Moser MB, Moser EI. Grid cells use HCN1 channels for spatial scaling. Cell. 2011;147:1159–1170. doi: 10.1016/j.cell.2011.08.051. [DOI] [PubMed] [Google Scholar]
- Giocomo LM, Zilli EA, Fransen E, Hasselmo ME. Temporal frequency of subthreshold oscillations scales with entorhinal grid cell field spacing. Science. 2007;315:1719–1722. doi: 10.1126/science.1139207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens B. Stimulus-evoked resetting of the dentate theta rhythm: relation to working memory. Neuroreport. 1996;8:159–163. doi: 10.1097/00001756-199612200-00032. [DOI] [PubMed] [Google Scholar]
- Givens B, Olton DS. Local modulation of basal forebrain: effects on working and reference memory. J Neurosci. 1994;14:3578–3587. doi: 10.1523/JNEUROSCI.14-06-03578.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens BS, Olton DS. Cholinergic and GABAergic modulation of the medial septal area: Effect on working memory. Behav Neurosci. 1990;104:849–855. doi: 10.1037//0735-7044.104.6.849. [DOI] [PubMed] [Google Scholar]
- Golding N, Staff N, Spruston N. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature. 2002;418:326–331. doi: 10.1038/nature00854. [DOI] [PubMed] [Google Scholar]
- Green JD, Arduini AA. Hippocampal electrical activity and arousal. J Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Griffin AL, Asaka Y, Darling RD, Berry SD. Theta-contingent trial presentation accelerates learning rate and enhances hippocampal plasticity during trace eyeblink conditioning. Behav Neurosci. 2004;118:403–411. doi: 10.1037/0735-7044.118.2.403. [DOI] [PubMed] [Google Scholar]
- Guanella A, Kiper D, Verschure P. A model of grid cells based on a twisted torus topology. Int J Neural Syst. 2007;17:231–240. doi: 10.1142/S0129065707001093. [DOI] [PubMed] [Google Scholar]
- Guderian S, Schott BH, Richardson-Klavehn A, Duzel E. Medial temporal theta state before an event predicts episodic encoding success in humans. Proc Natl Acad Sci USA. 2009;106:5365–5370. doi: 10.1073/pnas.0900289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas JS, White JA. Frequency selectivity of layer II stellate cells in the medial entorhinal cortex. J Neurophysiol. 2002;88:2422–2429. doi: 10.1152/jn.00598.2002. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Bonnevie T, Moser MB, Moser EI. Hippocampus-independent phase precession in entorhinal grid cells. Nature. 2008;453:1248–1252. doi: 10.1038/nature06957. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308:1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Harvey CD, Collman F, Dombeck DA, Tank DW. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature. 2009;461:941–946. doi: 10.1038/nature08499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. Grid cell mechanisms and function: contributions of entorhinal persistent spiking and phase resetting. Hippocampus. 2008;18:1213–1229. doi: 10.1002/hipo.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. A model of episodic memory: Mental time travel along encoded trajectories using grid cells. Neurobiol Learn Mem. 2009;92:559–573. doi: 10.1016/j.nlm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. How we remember: Brain mechanisms of episodic memory. MIT Press; Cambridge, MA: 2012. [Google Scholar]
- Hasselmo ME, Bodelon C, Wyble BP. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 2002a;14:793–817. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Brandon MP. Linking cellular mechanisms to behavior: entorhinal persistent spiking and membrane potential oscillations may underlie path integration, grid cell firing, and episodic memory. Neural Plast. 2008;2008:658323. doi: 10.1155/2008/658323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Brandon MP. A model combining oscillations and attractor dynamics for generation of grid cell firing. Front Neural Circuits. 2012;6:30. doi: 10.3389/fncir.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Eichenbaum H. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Netw. 2005;18:1172–1190. doi: 10.1016/j.neunet.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Fehlau BP. Differences in time course of ACh and GABA modulation of excitatory synaptic potentials in slices of rat hippocampus. J Neurophysiol. 2001;86:1792–1802. doi: 10.1152/jn.2001.86.4.1792. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Giocomo LM, Zilli EA. Grid cell firing may arise from interference of theta frequency membrane potential oscillations in single neurons. Hippocampus. 2007;17:1252–1271. doi: 10.1002/hipo.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Wyble BP, Cannon RC. From spike frequency to free recall; How neural circuits perform encoding and retrieval. In: Parker A, Bussey TJ, Wilding E, editors. The Cognitive Neuroscience of Memory: Encoding and Retrieval. Psychology Press; London: 2002b. [Google Scholar]
- Heys JG, Giocomo LM, Hasselmo ME. Cholinergic modulation of the resonance properties of stellate cells in layer II of medial entorhinal cortex. J Neurophysiol. 2010;104:258–270. doi: 10.1152/jn.00492.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heys JG, Hasselmo ME. Neuromodulation of I(h) in layer II medial entorhinal cortex stellate cells: a voltage-clamp study. J Neurosci. 2012;32:9066–9072. doi: 10.1523/JNEUROSCI.0868-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heys JG, MacLeod KM, Moss CF, Hasselmo ME. Bat and rat neurons differ in theta frequency resonance despite similar coding of space. Science. 2013;340:363–367. doi: 10.1126/science.1233831. [DOI] [PubMed] [Google Scholar]
- Hinman JR, Penley SC, Long LL, Escabi MA, Chrobak JJ. Septotemporal variation in dynamics of theta: speed and habituation. J Neurophysiol. 2011;105:2675–2686. doi: 10.1152/jn.00837.2010. [DOI] [PubMed] [Google Scholar]
- Hinman JR, Penley SC, Escabi MA, Chrobak JJ. Ketamine disrupts theta synchrony across the septotemporal axis of the CA1 region of hippocampus. J Neurophysiol. 2013;109:570–579. doi: 10.1152/jn.00561.2012. [DOI] [PubMed] [Google Scholar]
- Holscher C, Anwyl R, Rowan MJ. Stimulation on the positive phase of hippocampal theta rhythm induces long-term potentiation that can Be depotentiated by stimulation on the negative phase in area CA1 in vivo. J Neurosci. 1997;17:6470–6477. doi: 10.1523/JNEUROSCI.17-16-06470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron. 1995;15:1053–1063. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- Huxter J, Burgess N, O’Keefe J. Independent rate and temporal coding in hippocampal pyramidal cells. Nature. 2003;425:828–832. doi: 10.1038/nature02058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxter JR, Senior TJ, Allen K, Csicsvari J. Theta phase-specific codes for two-dimensional position, trajectory and heading in the hippocampus. Nat Neurosci. 2008;11:587–594. doi: 10.1038/nn.2106. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Wyble BP, Goyal V, Rossi CA, Hasselmo M. Stimulation in hippocampal region CA1 in behaving rats yields LTP when delivered to the peak of theta and LTD when delivered to the trough. Journal of Neuroscience. 2003a;23:11725–11731. doi: 10.1523/JNEUROSCI.23-37-11725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Wyble BP, Goyal V, Rossi CA, Hasselmo ME. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J Neurosci. 2003b;23:11725–11731. doi: 10.1523/JNEUROSCI.23-37-11725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Hwang G, Curran T, Kahana MJ. EEG oscillations and recognition memory: theta correlates of memory retrieval and decision making. Neuroimage. 2006;32:978–987. doi: 10.1016/j.neuroimage.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Jeewajee A, Barry C, O’Keefe J, Burgess N. Grid cells and theta as oscillatory interference: electrophysiological data from freely moving rats. Hippocampus. 2008;18:1175–1185. doi: 10.1002/hipo.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery KJ, Donnett JG, O’Keefe J. Medial septal control of theta-correlated unit firing in the entorhinal cortex of awake rats. Neuroreport. 1995;6:2166–2170. doi: 10.1097/00001756-199511000-00017. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Hippocampal CA3 region predicts memory sequences: accounting for the phase precession of place cells. Learn Mem. 1996;3:279–287. doi: 10.1101/lm.3.2-3.279. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Position reconstruction from an ensemble of hippocampal place cells: Contribution of theta phase coding. J Neurophysiol. 2000;83:2602–2609. doi: 10.1152/jn.2000.83.5.2602. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol. 2001;11:739–744. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, Kirschen M, Madsen JR. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature. 1999;399:781–784. doi: 10.1038/21645. [DOI] [PubMed] [Google Scholar]
- Kamondi A, Acsady L, Wang XJ, Buzsaki G. Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: activity-dependent phase-precession of action potentials. Hippocampus. 1998;8:244–261. doi: 10.1002/(SICI)1098-1063(1998)8:3<244::AID-HIPO7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kaplan R, Doeller CF, Barnes GR, Litvak V, Duzel E, Bandettini PA, Burgess N. Movement-related theta rhythm in humans: coordinating self-directed hippocampal learning. PLoS Biology. 2012;10:e1001267. doi: 10.1371/journal.pbio.1001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser MB. Finite scale of spatial representation in the hippocampus. Science. 2008;321:140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T. Theta band power in the human scalp EEG and the encoding of new information. Neuroreport. 1996;7:1235–1240. doi: 10.1097/00001756-199605170-00002. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalogr Clin Neurophysiol. 1994;91:428–441. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Koenig J, Linder AN, Leutgeb JK, Leutgeb S. The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science. 2011;332:592–595. doi: 10.1126/science.1201685. [DOI] [PubMed] [Google Scholar]
- Kraus BJ, Robinson RJ, White JA, Eichenbaum H, Hasselmo ME. Hippocampal “Time Cells”: Time versus path integration. Neuron. 2013;78:1090–1101. doi: 10.1016/j.neuron.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropff E, Treves A. The emergence of grid cells: Intelligent design or just adaptation? Hippocampus. 2008;18:1256–1269. doi: 10.1002/hipo.20520. [DOI] [PubMed] [Google Scholar]
- Kunec S, Hasselmo ME, Kopell N. Encoding and retrieval in the CA3 region of the hippocampus: a model of theta-phase separation. J Neurophysiol. 2005;94:70–82. doi: 10.1152/jn.00731.2004. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Alonso A, Jones BE. Cholinergic basal forebrain neurons burst with theta during waking and paradoxical sleep. J Neurosci. 2005;25:4365–4369. doi: 10.1523/JNEUROSCI.0178-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lega BC, Jacobs J, Kahana M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2011;22:748–761. doi: 10.1002/hipo.20937. [DOI] [PubMed] [Google Scholar]
- Lenck-Santini PP, Fenton AA, Muller RU. Discharge properties of hippocampal neurons during performance of a jump avoidance task. J Neurosci. 2008;28:6773–6786. doi: 10.1523/JNEUROSCI.5329-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M’Harzi M, Palacios A, Monmaur P, Willig F, Houcine O, Delacour J. Effects of selective lesions of fimbria-fornix on learning set in the rat. Physiol Behav. 1987;40:181–188. doi: 10.1016/0031-9384(87)90205-8. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, LePage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71:737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrides FH, Eichenbaum H, Forbes WB. Temporal relationship between sniffing and limbic theta rhythm during odor discrimination reversal learning. Journal of Neuroscience. 1982;2:1705. doi: 10.1523/JNEUROSCI.02-12-01705.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Zilli EA, Ong KC, Hasselmo ME, Eichenbaum H. Hippocampal CA1 spiking during encoding and retrieval: relation to theta phase. Neurobiol Learn Mem. 2007;87:9–20. doi: 10.1016/j.nlm.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Olton DS, Murray EA, Gaffan D. A comparative analysis of the role of fornix and cingulate cortex in memory: rats. Exp Brain Res. 1989;74:187–201. doi: 10.1007/BF00248292. [DOI] [PubMed] [Google Scholar]
- Martin MM, Horn KL, Kusman KJ, Wallace DG. Medial septum lesions disrupt exploratory trip organization: evidence for septohippocampal involvement in dead reckoning. Physiol Behav. 2007;90:412–424. doi: 10.1016/j.physbeh.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Maurer AP, Vanrhoads SR, Sutherland GR, Lipa P, McNaughton BL. Self-motion and the origin of differential spatial scaling along the septo-temporal axis of the hippocampus. Hippocampus. 2005;15:841–852. doi: 10.1002/hipo.20114. [DOI] [PubMed] [Google Scholar]
- McCartney H, Johnson AD, Weil ZM, Givens B. Theta reset produces optimal conditions for long-term potentiation. Hippocampus. 2004;14:684–687. doi: 10.1002/hipo.20019. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Ruan M, Woodnorth M-A. Restoring theta-like rhythmicity in rats restores initial learning in the Morris water maze. Hippocampus. 2006;16:1102–1110. doi: 10.1002/hipo.20235. [DOI] [PubMed] [Google Scholar]
- Mehta MR, Lee AK, Wilson MA. Role of experience and oscillations in transforming a rate code into a temporal code. Nature. 2002;417:741–746. doi: 10.1038/nature00807. [DOI] [PubMed] [Google Scholar]
- Mhatre H, Gorchetchnikov A, Grossberg S. Grid cell hexagonal patterns formed by fast self-organized learning within entorhinal cortex. Hippocampus. 2010;22:320–334. doi: 10.1002/hipo.20901. [DOI] [PubMed] [Google Scholar]
- Miall R. The storage of time intervals using oscillating neurons. Neural Comput. 1989;1:359–371. [Google Scholar]
- Mizuseki K, Sirota A, Pastalkova E, Buzsaki G. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron. 2009;64:267–280. doi: 10.1016/j.neuron.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Ranck JB., Jr Generation of theta rhythm in medial entorhinal cortex of freely moving rats. Brain Res. 1980;189:49–66. doi: 10.1016/0006-8993(80)90006-2. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Rawlins JN, Steward O, Olton DS. Medial septal area lesions disrupt theta rhythm and cholinergic staining in medial entorhinal cortex and produce impaired radial arm maze behavior in rats. J Neurosci. 1982;2:292–302. doi: 10.1523/JNEUROSCI.02-03-00292.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori SJY, Perez GM, Alvarado MC, Barnes CA, Mcnaughton BL. Reversible inactivation of the medial septum differentially affects 2 forms of learning in rats. Brain Research. 1990;528:12–20. doi: 10.1016/0006-8993(90)90188-h. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Hasselmo ME. GABA(B) presynaptic inhibition has an in vivo time constant sufficiently rapid to allow modulation at theta frequency. J Neurophysiol. 2002;87:1196–1205. doi: 10.1152/jn.00077.2001. [DOI] [PubMed] [Google Scholar]
- Moser EI, Moser MB. A metric for space. Hippocampus. 2008;18:1142–1156. doi: 10.1002/hipo.20483. [DOI] [PubMed] [Google Scholar]
- Navratilova Z, Giocomo LM, Fellous JM, Hasselmo ME, McNaughton BL. Phase precession and variable spatial scaling in a periodic attractor map model of medial entorhinal grid cells with realistic after-spike dynamics. Hippocampus. 2012;22:772–789. doi: 10.1002/hipo.20939. [DOI] [PubMed] [Google Scholar]
- Newman EL, Norman KA. Moderate excitation leads to weakening of perceptual representations. Cereb Cortex. 2010;20:2760–2770. doi: 10.1093/cercor/bhq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer E. The normal EEG of the waking adult. In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalography: Basic Principles, Clinical Applications and Related Fields. Lippincott Williams & Wilkins; Baltimore, MD: 1999. pp. 149–173. [Google Scholar]
- Numan R, Quaranta JR., Jr Effects of medial septal lesions on operant delayed alternation in rats. Brain Res. 1990;531:232–241. doi: 10.1016/0006-8993(90)90779-b. [DOI] [PubMed] [Google Scholar]
- O’Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Burgess N, Donnett JG, Jeffery KJ, Maguire EA. Place cells, navigational accuracy, and the human hippocampus. Philos Trans R Soc Lond B Biol Sci. 1998;353:1333–1340. doi: 10.1098/rstb.1998.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford University Press; Oxford, UK: 1978. [Google Scholar]
- O’Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Orr G, Rao G, Houston FP, McNaughton BL, Barnes CA. Hippocampal synaptic plasticity is modulated by theta rhythm in the fascia dentata of adult and aged freely behaving rats. Hippocampus. 2001;11:647–654. doi: 10.1002/hipo.1079. [DOI] [PubMed] [Google Scholar]
- Osipova D, Takashima A, Oostenveld R, Fernandez G, Maris E, Jensen O. Theta and gamma oscillations predict encoding and retrieval of declarative memory. J Neurosci. 2006;26:7523–7531. doi: 10.1523/JNEUROSCI.1948-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastoll H, Ramsden HL, Nolan MF. Intrinsic electrophysiological properties of entorhinal cortex stellate cells and their contribution to grid cell firing fields. Front Neural Circuits. 2012;6:17. doi: 10.3389/fncir.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Greenstein YJ, Grudman M, Winson J. Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm. Brain Res. 1988;439:383–387. doi: 10.1016/0006-8993(88)91499-0. [DOI] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21:3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, Kahana MJ. Theta oscillations in human cortex during a working-memory task: evidence for local generators. J Neurophysiol. 2006;95:1630–1638. doi: 10.1152/jn.00409.2005. [DOI] [PubMed] [Google Scholar]
- Rawlins JN, Feldon J, Gray JA. Septo-hippocampal connections and the hippocampal theta rhythm. Exp Brain Res. 1979;37:49–63. doi: 10.1007/BF01474253. [DOI] [PubMed] [Google Scholar]
- Redish AD, Touretzky DS. The role of the hippocampus in solving the Morris water maze. Neural Comput. 1998;10:73–111. doi: 10.1162/089976698300017908. [DOI] [PubMed] [Google Scholar]
- Remme MW, Lengyel M, Gutkin BS. Democracy-independence trade-off in oscillating dendrites and its implications for grid cells. Neuron. 2010;66:429–437. doi: 10.1016/j.neuron.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas J, Gaztelu JM, Garcia-Austt E. Changes in hippocampal cell discharge patterns and theta rhythm spectral properties as a function of walking velocity in the guinea pig. Exp Brain Res. 1996;108:113–118. doi: 10.1007/BF00242908. [DOI] [PubMed] [Google Scholar]
- Rizzuto DS, Madsen JR, Bromfield EB, Schulze-Bonhage A, Kahana MJ. Human neocortical oscillations exhibit theta phase differences between encoding and retrieval. Neuroimage. 2006;31:1352–1358. doi: 10.1016/j.neuroimage.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Rotstein HG, Pervouchine DD, Acker CD, Gillies MJ, White JA, Buhl EH, Whittington MA, Kopell N. Slow and fast inhibition and an H-current interact to create a theta rhythm in a model of CA1 interneuron network. J Neurophysiol. 2005;94:1509–1518. doi: 10.1152/jn.00957.2004. [DOI] [PubMed] [Google Scholar]
- Sainsbury RS, Harris JL, Rowland GL. Sensitization and hippocampal type 2 theta in the rat. Physiol Behav. 1987a;41:489–493. doi: 10.1016/0031-9384(87)90085-0. [DOI] [PubMed] [Google Scholar]
- Sainsbury RS, Heynen A, Montoya CP. Behavioral correlates of hippocampal type 2 theta in the rat. Physiol Behav. 1987b;39:513–519. doi: 10.1016/0031-9384(87)90382-9. [DOI] [PubMed] [Google Scholar]
- Samsonovich A, McNaughton BL. Path integration and cognitive mapping in a continuous attractor neural network model. J Neurosci. 1997;17:5900–5920. doi: 10.1523/JNEUROSCI.17-15-05900.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Häusser M. Cellular mechanisms of spatial navigation in the medial entorhinal cortex. Nature Neuroscience. 2013;16:325–31. doi: 10.1038/nn.3340. [DOI] [PubMed] [Google Scholar]
- Seager MA, Johnson LD, Chabot ES, Asaka Y, Berry SD. Oscillatory brain states and learning: Impact of hippocampal theta-contingent training. Proc Natl Acad Sci U S A. 2002;99:1616–1620. doi: 10.1073/pnas.032662099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- Shay CF, Boardman IS, James NM, Hasselmo ME. Voltage dependence of subthreshold resonance frequency in layer II of medial entorhinal cortex. Hippocampus. 2012;22:1733–1749. doi: 10.1002/hipo.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J. The interrelationship between movement and cognition: Theta rhythm and the P300 event-related potential. Hippocampus. 2011;21:744–752. doi: 10.1002/hipo.20792. [DOI] [PubMed] [Google Scholar]
- Si B, Kropff E, Treves A. Grid alignment in entorhinal cortex. Biol Cybern. 2012;106:483–506. doi: 10.1007/s00422-012-0513-7. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Stensola H, Stensola T, Solstad T, Froland K, Moser MB, Moser EI. The entorhinal grid map is discretized. Nature. 2012;492:72–78. doi: 10.1038/nature11649. [DOI] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci. 1990;10:420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci U S A. 2009;106:20942–20947. doi: 10.1073/pnas.0911331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Wallenstein GV, Hasselmo ME. GABAergic modulation of hippocampal population activity: sequence learning, place field development, and the phase precession effect. J Neurophysiol. 1997;78:393–408. doi: 10.1152/jn.1997.78.1.393. [DOI] [PubMed] [Google Scholar]
- Walter W, Dovey V. Electro-encephalography in cases of sub-cortical tumour. The journal of Neurology, Neurosurgery and Psychiatry. 1944;7:57–65. doi: 10.1136/jnnp.7.3-4.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watrous AJ, Fried I, Ekstrom AD. Behavioral correlates of human hippocampal delta and theta oscillations during navigation. J Neurophysiol. 2011;105:1747–1755. doi: 10.1152/jn.00921.2010. [DOI] [PubMed] [Google Scholar]
- Watrous AJ, Lee DJ, Izadi A, Gurkoff GG, Shahlaie K, Ekstrom AD. A comparative study of human and rat hippocampal low-frequency oscillations during spatial navigation. Hippocampus. 2013 doi: 10.1002/hipo.22124. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welday AC, Shlifer IG, Bloom ML, Zhang K, Blair HT. Cosine directional tuning of theta cell burst frequencies: evidence for spatial coding by oscillatory interference. J Neurosci. 2011;31:16157–16176. doi: 10.1523/JNEUROSCI.0712-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ. Hippocampal electroencephalographic activity in the Mongolian gerbil during natural behaviours and wheel running and in the rat during wheel running and conditioned immobility. Can J Psychol. 1972;26:219–239. doi: 10.1037/h0082431. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Vanderwolf CH. Hippocampal EEG and behavior: changes in amplitude and frequency of RSA (theta rhythm) associated with spontaneous and learned movement patterns in rats and cats. Behav Biol. 1973;8:461–484. doi: 10.1016/s0091-6773(73)80041-0. [DOI] [PubMed] [Google Scholar]
- Wills TJ, Barry C, Cacucci F. The abrupt development of adult-like grid cell firing in the medial entorhinal cortex. Front Neural Circuits. 2012;6:21. doi: 10.3389/fncir.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Wyble BP, Linster C, Hasselmo ME. Size of CA1-evoked synaptic potentials is related to theta rhythm phase in rat hippocampus. J Neurophysiol. 2000;83:2138–2144. doi: 10.1152/jn.2000.83.4.2138. [DOI] [PubMed] [Google Scholar]
- Yartsev MM, Witter MP, Ulanovsky N. Grid cells without theta oscillations in the entorhinal cortex of bats. Nature. 2011;479:103–107. doi: 10.1038/nature10583. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Giocomo LM, Boardman I, Hasselmo ME. Frequency of subthreshold oscillations at different membrane potential voltages in neurons at different anatomical positions on the dorsoventral axis in the rat medial entorhinal cortex. J Neurosci. 2011;31:12683–12694. doi: 10.1523/JNEUROSCI.1654-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilli EA, Hasselmo ME. Coupled noisy spiking neurons as velocity-controlled oscillators in a model of grid cell spatial firing. J Neurosci. 2010;30:13850–13860. doi: 10.1523/JNEUROSCI.0547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilli EA, Yoshida M, Tahvildari B, Giocomo LM, Hasselmo ME. Evaluation of the oscillatory interference model of grid cell firing through analysis and measured period variance of some biological oscillators. PLoS Comput Biol. 2009;5:e1000573. doi: 10.1371/journal.pcbi.1000573. [DOI] [PMC free article] [PubMed] [Google Scholar]