Abstract
Objective:
We examined whether depression and anxiety disorders in early childhood were associated with changes in resting state functional connectivity (RSFC) of the ventral attention network (VAN), and whether RSFC in the VAN was associated with alterations in attention specific to these disorders. Important clinical features of these illnesses, including changes in attention toward novel stimuli and changes in attention to stimuli of negative valence (threat/sad bias), indirectly implicate the VAN.
Method:
We collected resting state functional magnetic resonance imaging data in children aged 8 to 12 years. Data were volume censored to reduce artifact from submillimeter movement, resulting in analyzable data from 30 children with a history of depression and/or anxiety and 42 children with no psychiatric history. We compared pairwise RSFC among the following VAN regions: right ventro-lateral prefrontal cortex (VLPFC), right posterior superior temporal gyrus (pSTG), and right ventral supramarginal gyrus (vSMG). We also collected measures of threat bias and current clinical symptoms.
Results:
Children with a history of depression and/or anxiety had reduced RSFC among the regions of the VAN compared to children with no psychiatric history. The magnitude of VAN RSFC was correlated with measures of attention bias toward threat but not with current depressive, internalizing, or externalizing symptoms. No RSFC changes were detected between groups among homotopic left hemisphere regions.
Conclusions:
Disruption in the VAN may be an early feature of depression and anxiety disorders. VAN changes were associated with attention bias and clinical history but not with current symptoms of depression and anxiety.
Keywords: attention bias, anxiety, depression, functional connectivity, ventral attention network
Depression and anxiety disorders are associated with several changes in cognition.1 Two cognitive changes that could explain a wide range of problematic symptoms in these illnesses are alterations in attention to novel, unexpected stimuli,2-7 and changes in attention to stimuli of negative valence.8,9 The brain systems supporting altered attention in depression and anxiety, however, are not well understood.
Adults with depression and/or anxiety have changes in attention to novel, suddenly appearing stimuli. Many studies have demonstrated that adults with anxiety disorders have an increased magnitude of specific early electroencephalographic (EEG) responses associated with attention, including the P300, following the onset of nonemotional novel stimuli relative to adults without anxiety disorders.2-4 Adults with high trait anxiety, furthermore, may attend more quickly to suddenly appearing nonemotional stimuli and may be more easily distracted by irrelevant stimuli.7 Accordingly, Eysenck’s attentional control theory posits that anxiety is associated with increased influence of the stimulus-driven attention system.9A Interestingly, adults with depression may have the opposite response to novel stimuli, with a few studies reporting decreased early EEG responses.2,3,5 Changes in attention to suddenly appearing, novel stimuli could be related to key signs and symptoms, such as hypervigilance in anxiety and psychomotor retardation in depression.
Adults with depression and anxiety also exhibit changes in attention to stimuli with negative valence. Many studies suggest that individuals with anxiety disorders have an attention bias toward threatening stimuli,8 although individuals with anxiety disorders can also exhibit a bias away from threat, depending on experimental conditions.10 Individuals with depression, similarly, preferentially attend to sad stimuli.9 Attention bias may be related to the etiology of depression and anxiety, because experimentally inducing an attention bias toward stimuli of negative valence causes measureable changes in mood in healthy individuals,11 and treatments that reduce disorder-specific attention biases alleviated symptoms in several small randomized controlled trials.12,13
Children and adolescents with depression or anxiety also demonstrate changes in attention similar to adults. As in adults, children with anxiety have increased early EEG responses to novel stimuli,14 although less is known about children with depression. Children with depression or anxiety disorders, and even very young children at risk for these disorders, also tend to demonstrate attention biases toward stimuli of negative valence15; as in adults, however, a number of studies in children with clinically significant anxiety report attention biases away from negative stimuli.10,16-18 Intriguingly, infants with high behavioral inhibition (BI), a well-studied and operationally defined temperament, have increased physiological but avoidant behavioral responses to novel stimuli and are at elevated risk for later development of depression and anxiety.19
Although the above evidence supports changes in attention in adults and children with depression and anxiety, the brain networks supporting these changes are not well understood. The ventral attention network (VAN), a collection of brain regions in the right cerebral hemisphere including the right ventrolateral prefrontal cortex (VLPFC) and right temporal-parietal junction (TPJ), is associated with orienting and responding to novel stimuli.20 Indirect evidence supports the hypothesis that some of the changes in attention in depression and anxiety are associated with the VAN. Activity in the VLFPC, a component of the VAN, is increased in adults21 and adolescents16,22 with high levels of anxiety after the onset of emotional and nonemotional stimuli. The EEG responses to novel stimuli discussed above, including the P300, may localize to portions of the VAN.20 Infants with high BI demonstrate increased EEG activity specifically in the right cerebral hemisphere,19 consistent with rightward lateralization of the VAN. Finally, attention bias occurs as early as 150 milliseconds after stimulus onset,8 and orienting responses occurring at this time scale generally rely on stimulus-driven (as opposed to voluntary) attention, a function associated with the VAN.
Although these studies are suggestive of VAN alterations in individuals with depression and anxiety, the evidence is indirect. The right VLPFC is an anatomically defined brain region with subcomponents in multiple different functional brain networks, and it is not clear whether the part of right VLPFC studied to date is part of the VAN. The studies reviewed above, further-more, are task-based and primarily use general linear models (GLMs) to estimate activity evoked in the VLPFC after the onset of stimuli; less is known about the integrity of the VAN as a whole in individuals with depression or anxiety. Resting state functional connectivity (RSFC) studies may provide a measure of integrity of networks such as the VAN, because RSFC is increased between brain regions with a history of correlated activity changes during goal-directed activity.23 RSFC studies may be of particular interest, therefore, in clinical populations in which aberrant function of a particular network is suspected.
Given that changes in attention may be an important component of the psychopathology of mood and anxiety disorders, investigations of the integrity of the VAN in children with these illnesses are of high interest. To address this issue, we examined resting state functional magnetic resonance imaging data in children ages 8 to 12 collected as part of an ongoing longitudinal study of childhood-onset depression. We hypothesized that children with a history of depression and/or an anxiety disorder would have differences in resting state functional connectivity in the VAN relative to subjects without any history of a psychiatric disorder. We predicted that any group differences would not be attributable solely to the presence of comorbid disorders of attention such as attention-deficit/hyperactivity disorder (ADHD; common in childhood mood and anxiety disorders). To begin to address the behavioral relevance of findings, we also tested whether VAN connectivity was related to measures of attention bias to threatening stimuli.
METHOD
Participants
This investigation used data from the ongoing Validation of Preschool Depression Study.24,25 Beginning in 2002, children aged 3 to 6 years were screened from primary care sites and oversampled for symptoms of depression (psychiatric and healthy control subjects were also ascertained). A neuroimaging study was added when the children were 8 to 12 years of age, and data in this investigation are from the first of 3 planned waves of structural and functional data.26,27
For this study, subjects were initially divided into 3 groups based on the following: history of an anxiety disorder but not depression (ANX); history of depression but not an anxiety disorder (DEP); and no history of any psychiatric disorder (healthy control subjects [HC]). When no significant difference in VAN functional connectivity between ANX and DEP were found, these groups were combined (ANX/DEP). Children with a history of both depression and an anxiety disorder, initially excluded on the basis of comorbidity, were added to the ANX/DEP group, now comprising 30 subjects (12 ANX, 8 DEP, 10 with both). Anxiety disorders included separation anxiety disorder (SAD), generalized anxiety disorder (GAD), social phobia (SOC), agoraphobia, and specific phobia (SP). Subjects with dysthymia and/or mania were excluded. ADHD, conduct disorder (CD), and oppositional defiant disorder (ODD) were permitted in all diagnostic groups, and these comorbidities were taken into account in the imaging analysis as described below. Because we had no specific hypothesis about VAN changes in obsessive-compulsive disorder (OCD) and posttraumatic stress disorder (PTSD), these illnesses were neither inclusion nor exclusion criteria for diagnostic groups and were present in small numbers in each diagnostic group other than HC (Table 1).
TABLE 1.
Comorbidity Characteristics of the Study Sample
| History of Anxiety (n = 12) |
History of Depression (n = 8) |
History of Anxiety and Depression (n = 10) |
History of Anxiety and/or Depression (n = 30) |
|
|---|---|---|---|---|
| Depression | 0 | 100.0 | 100.0 | 60.0 |
| Any Anxiety Disorder | 100.0 | 12.5a | 100.0 | 100.0 |
| GAD | 41.7 | 0 | 60.0 | 36.7 |
| PTSD | 0 | 0 | 20.0 | 6.7 |
| SAD | 75.0 | 0 | 70.0 | 50.0 |
| OCD | 25.0 | 12.5 | 20.0 | 20.0 |
| Agoraphobia | 8.3 | 0 | 0 | 3.3 |
| SOC | 33.0 | 0 | 50.0 | 30.0 |
| SP | 16.7 | 0 | 20.0 | 13.3 |
| Any Externalizing Disorder | 33.3 | 50.0 | 70.0 | 50.0 |
| ADHD | 16.7 | 25.0 | 60.0 | 33.3 |
| ODD | 25.0 | 50.0 | 40.0 | 36.7 |
| CD | 8.3 | 0 | 20.0 | 10.0 |
Note: ADHD = attention-deficit/hyperactivity disorder; CD = conduct disorder; GAD = generalized anxiety disorder; OCD = obsessive compulsive disorder; ODD = oppositional defiant disorder; PTSD = posttraumatic stress disorder; SAD = separation anxiety disorder; SOC = social phobia; SP = specific phobia.
One child with depression had OCD.
Diagnosis and Demographic Factors
Diagnostic status was assessed annually and was determined by parent report on the Preschool-Age Psychiatric Assessment (PAPA)28 for children aged 8.0 years and younger and by combined parent and child report29 (from separate interviews) on the Child and Adolescent Psychiatric Assessment (CAPA)30 for children older than 8.0 years. Interviews were conducted by trained staff, and established procedures to maintain interrater reliability were ongoing throughout data collection. Criteria were modified for depression arising during the preschool period as previously described (e.g., duration criterion set aside).31 Group status was based on any history of an Axis I disorder before the time of scan. Maternal affective disorder history was obtained using the Family Interview for Genetic Studies (FIGS). Depression sum scores, internalizing scores, and externalizing scores were derived using the CAPA or PAPA from the annual assessment that occurred closest to the day of scanning. Depression sum score was the sum of core depressive symptoms endorsed; internalizing score was the sum of core symptoms for GAD, PTSD, and SAD; and externalizing score was the sum of core symptoms for ADHD, CD, and ODD. Parent and child versions of the Child Depression Inventory (CDI)32 were administered on the day of scanning.
Functional Magnetic Resonance Imaging
Two resting state functional magnetic resonance imaging (fMRI) scans (164 frames, ~6.8 minutes each) were collected in each subject using a 3T TIM TRIO Scanner at Washington University School of Medicine. Subjects were instructed to lie awake quietly with their eyes closed. Pads were inserted around all sides of the head to minimize head motion. Data were acquired using an asymmetric spin-echo, echo-planar sequence, which was maximally sensitive to blood-oxygenation-level–dependent (BOLD) contrast (T2*) (repetition time [TR] = 2,500 ms, echo time [TE] = 27 ms, field of view [FOV] = 256 mm, flip angle = 90°, voxel size = 4×4×4 mm, slices = 36). A T1 structural image was acquired for alignment purposes using a sagittal magnetization-prepared gradient echo (MP-RAGE) 3-dimensional sequence (TR = 2,400 ms, TE = 3.16 ms, flip angle = 8°, voxel size 1×1×1 mm). A T2 image was acquired in the same space as the functional scans to facilitate registration of the T1 image (TE = 96 ms, TR = 5 s, 189 × 256 acquisition matrix, 36 slices, voxel size = 1.0×1×3 mm).
fMRI Data Preprocessing
All data were subjected to standard fMRI preprocessing including the following: removal of the first 5 frames of data from each run to allow for stabilization of the BOLD signal; temporal realignment using sinc interpolation to correct odd versus even slice intensity differences attributable to interleaved acquisition; realignment of data within and across runs to compensate for rigid body motion33; intensity normalization to a whole-brain mode (across all TRs and voxels) of 1,000; registration of the T1 to the atlas representative template in the Talairach coordinate system34 using a 12-parameter affine transform; coregistration of the 3-dimensional fMRI volume to the T1 via the T2; and transformation of the fMRI volumes to atlas space using a single affine 12-parameter transform that included resampling to a 3-mm cubic representation.33,35
fcMRI Preprocessing
The initial stage of functional connectivity pre-processing36 included the following: multiple regression of nuisance variables from the BOLD data; a temporal band-pass filter (0.009 Hz < f < 0.08 Hz); and spatial smoothing (6 mm full width at half maximum). Nuisance regressors were calculated using regions of interest (ROIs) including the average signals from the ventricles, white matter, whole brain, 6 head realignment parameters obtained by rigid body head motion correction, and the derivatives of each of these signals.
After the initial fcMRI preprocessing, 2 motion parameters were calculated for each volume acquisition, framewise displacement (FD) and root mean squared signal change compared to the previously acquired volume (DVARS).36 FD reflects instantaneous head motion and is calculated by adding up the absolute values of 6 displacement values comparing brain position to the previously acquired volume (the 3 rotational values are first converted to millimeters by calculating displacement on the surface of a sphere of radius 50 mm). DVARS is the root mean square signal change over voxels from the previous compared to the current volume. Volumes with FD greater than 0.25 or DVARS greater than 2.5 (equivalent to 0.25% BOLD signal; based on output from fcMRI pre-processing step 1) were considered contaminated by motion, and were censored in subsequent analyses. Volumes acquired 2 before or 2 after contaminated volumes were also excluded because of possible spin history effects or spread of motion-contaminated data by frequency filtering. Only subjects with at least 120 remaining frames of data were included in further analyses, reducing the sample size from a potential 150 subjects to 72 subjects. We next parsed each subject’s dataset to 122 frames so that each subject contributed an equal amount of data to the analyses.
Finally, the initial fcMRI preprocessing was redone (on the output of the initial generic preprocessing) using only the 122 frames in each subject who had passed the strict motion criteria to ensure that nuisance regressors were not unduly influenced by frames contaminated by motion artifact. In these steps, multiple regression b-values were calculated using only retained volumes but were applied to all volumes to produce a continuous set of residuals for the purposes of frequency filtering.
ROI Definition
ROIs were 10-mm diameter spheres taken from an empirical study of functional networks38 that consistently participate in the VAN. Regions included the frontal operculum near the VLPFC (+50 +27 +6 in Talairach space) and 2 regions near the TPJ: the posterior superior temporal gyrus (+49 −35 +9) and the ventral supramarginal gyrus (+53 −48 +12). These regions were defined in adults, but large-scale network properties are similar in children aged 8 to 12 years (J. Power, B. Schlaggar, S. Petersen, unpublished data). As a control, we examined functional connectivity of homotopic regions in the left hemisphere. These regions were identical to the VAN regions but reflected into the left hemisphere (−50 +27 +6), (−49 −35 +9), and (−53 −48 +12).
Functional Connectivity Data Analysis
We extracted the volume-censored time series from each ROI.39 Fisher z–transformed Pearson correlation coefficients were computed between the time series for each of the 3 possible (right hemisphere) ROI pairs in the VAN in each individual. For graphical purposes and for examining correlations with other continuous measures, we computed average VAN RSFC strength for each subject as the average of the 3 Fisher-z– transformed Pearson’s correlations comprising the VAN. A repeated-measures analysis of variance (ANOVA) was used to compare functional connectivity between groups, with ROI pair as a within-subject factor and diagnostic status as a between-subject factor. We used propensity scoring40 to control for socio-demographic factors. First, we performed a logistic regression with each of the sociodemographic factors (listed below) as main effects and diagnostic group as the dependent variable. Next, we computed the logit for each subject on the basis of the continuous output of the logistic regression, and this logit served as the propensity score to be used as a covariate in the ANOVA of interest.
Measurement of Attention Bias
We collected measures of attention bias to threatening stimuli in a subset of the subjects (19 ANX-DEP and 13 HC; 1 outlier removed from ANX-DEP group, leaving 18 for analysis) at an average age of 12.9 years (SD 0.95 years), on average 2.1 years after imaging. Threat bias was measured using a standard dot-probe task (details are provided in Supplement 1, available online).
RESULTS
Demographic Characteristics of Sample
Specific diagnostic characteristics and demographic information for each group are displayed in Table 1 and Table 2, respectively. Because initial group comparisons of functional connectivity data (Figure 1) did not show any differences between children with depression only (DEP) compared to children with an anxiety disorder only (ANX), children with a history of anxiety and/or depression were combined into 1 group (ANX/DEP) for subsequent analyses. Children in the ANX/DEP group and healthy control subjects (HC) did not differ significantly by gender, ethnicity, age, pubertal status, family income, parental education, dominant hand, IQ, or history of maternal psychiatric illness. As expected, there were significant group differences in internalizing and externalizing symptoms, exposure to stressful and traumatic life events, and use of psychotropic medications.
TABLE 2.
Demographic Characteristics by Diagnostic Category
| ANX/DEP (n = 30) | HC (n = 42) | p Value | |
|---|---|---|---|
| Male, % | 40.0 | 47.6 | 0.52 |
| Ethnicity, % | |||
| White | 53.3 | 52.4 | 0.99 |
| Black | 40.0 | 40.5 | |
| Other | 6.7 | 7.1 | |
| Age, mo, m (SD) | 127.13 (16.3) | 127.19 (15.1) | 0.99 |
| Pubertal status, % | |||
| Prepubertal | 60.0 | 51.2 | 0.73 |
| Early pubertal | 6.7 | 14.6 | |
| Mid pubertal | 23.3 | 22.0 | |
| Late pubertal | 10.0 | 12.2 | |
| Family income in $, % | |||
| ≤20,000 | 23.3 | 16.7 | 0.80 |
| 20,001–40,000 | 20.0 | 19.0 | |
| 40,001–60,000 | 10.0 | 16.7 | |
| ≥60,001 | 46.7 | 47.6 | |
| Parental education, % | |||
| HS diploma or < | 6.7 | 7.5 | 0.62 |
| Some college | 36.7 | 42.5 | |
| 4-Year college degree | 20.0 | 27.5 | |
| Graduate education or > | 36.7 | 22.5 | |
| Dominant hand at scan, % | |||
| Right | 90.0 | 88.1 | 0.70 |
| Left | 10.0 | 9.5 | |
| Both | 0.0 | 2.4 | |
| Ever taken psychiatric medication, % | 20.0 | 4.8 | 0.04 |
| Psychiatric medication within 48 h of scan, % | 6.7 | 0.0 | 0.09 |
| IQ, mean (SD) | 106.6 (16.6) | 108.5 (11.7) | 0.59 |
| Stressful life events, m (SD) | 10.6 (7.4) | 7.1 (5.9) | 0.03 |
| Traumatic life events, m (SD) | 5.3 (3.8) | 2.5 (2.2) | <0.001 |
| Core depression sum score, m (SD) | 2.6 (1.9) | 1.3 (1.2) | <0.001 |
| Externalizing sum score, m (SD) | 3.1 (4.1) | 0.8 (1.6) | 0.002 |
| Internalizing sum score, m (SD) | 2.7 (2.3) | 0.7 (0.9) | <0.001 |
| CDI (child), m (SD) | 4.6 (4.0) | 3.1 (3.7) | 0.11 |
| CDI (parent), m (SD) | 7.7 (4.2) | 6.4 (4.0) | 0.17 |
| First-degree relative mood/anxiety disorder or suicide attempt, % |
83.3 | 66.7 | 0.11 |
| History of maternal psychiatric illness, % | |||
| Major depression | 31.0 | 26.2 | 0.66 |
| Bipolar disorder | 0.0 | 4.8 | 0.23 |
| Anxiety disorder | 3.4 | 9.5 | 0.42 |
| Suicide attempt | 6.9 | 4.8 | 0.70 |
| ADHD | 0.0 | 2.4 | 0.40 |
| Substance abuse | 6.9 | 2.3 | 0.35 |
Note: Boldface type indicates significant p values. ADHD = attention-deficit/hyperactivity disorder; ANX/DEP = children and adolescents with a history of an anxiety disorder and/or depression; CDI = Children′s Depression Inventory; HC = healthy control subjects; HS = high school.
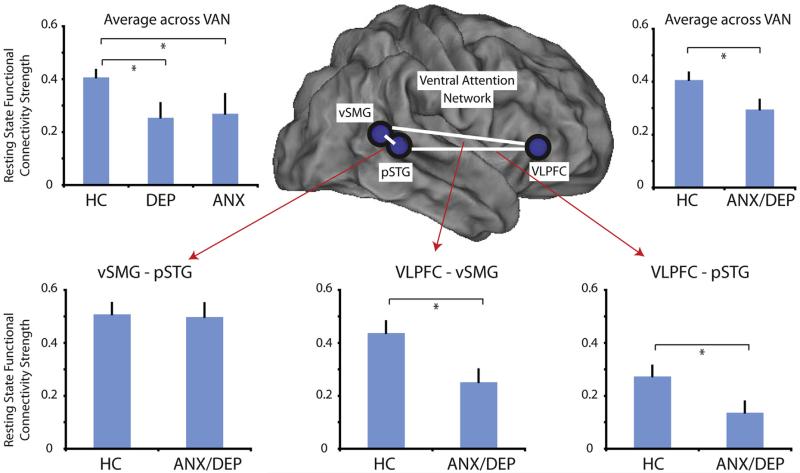
FIGURE 1.
Resting state functional connectivity (RSFC) of the ventral attention network (VAN) and individual VAN connections by diagnostic category. Note: The VAN is lateralized to the right cerebral hemisphere and no left hemisphere regions are included in this analysis. Lower panels illustrate RSFC strengths (Fisher-z transformed Pearson correlation coefficients) of individual VAN connections by diagnostic category. Upper panels compare the average VAN RSFC strength (average of the 3 individual connections) by diagnostic category. Error bars represent standard errors of the mean. ANX = children with a history of anxiety but not depression; ANX/DEP = children with a history of anxiety and/or depression; DEP = children with a history of depression but not anxiety; HC = healthy control subjects; pSTG = posterior superior temporal gyrus; VLPFC = ventro-lateral prefrontal cortex; vSMG = ventral supramarginal gyrus.
Table S3, available online, provides a comparison between included subjects and subjects excluded on the basis of excessive motion during neuroimaging. Excluded subjects were significantly younger than included subjects and excluded ANX/DEP subjects had significantly higher externalizing sum scores and CDI-P (parent) scores relative to included ANX/DEP subjects. Table S4, available online, compares ANX/DEP subjects with and without comorbid externalizing disorders (ADHD, ODD, CD). ANX/DEP with comorbid externalizing disorders had significantly higher externalizing sum scores and CDI-P scores relative to ANX/DEP without comorbid externalizing disorders.
Ventral Attention Network Functional Connectivity
An ANOVA comparing RSFC strength within the VAN revealed a main effect of diagnostic group (between ANX, DEP, and HC; F2,59 = 3.65, p = .032). Fisher’s least significant difference (LSD) post hoc comparisons indicated a significant difference between ANX and HC (p = .036) as well as a significant difference between DEP and HC (p = .048) (Figure 1). Because there was no difference in VAN functional connectivity between the ANX and DEP children (p = .87), and because depression and anxiety disorders are highly related, we combined these 2 groups (ANX/DEP) for further analyses. Functional connectivity remained significantly different between ANX/DEP and HC (F1,70 = 6.03, p = .017).
Diagnostic grouping (ANX/DEP versus HC) was based on any history of depression or an anxiety disorder from annual assessments beginning when subjects were 3 to 6 years old. In all, 48.0% of ANX-DEP subjects met criteria for depression or an anxiety disorder at the assessment closest to imaging, and ANX-DEP subjects who did not meet diagnostic criteria at the most recent assessment nevertheless had reduced VAN functional connectivity relative to HC (F1,58 = 5.42, p = .023). Tables S1 and S2, available online, provide further information about longitudinal symptoms in ANX-DEP, and Figure S1, available online, illustrates that there was no correlation between number of assessments meeting diagnostic criteria and VAN RSFC (p = .84). Figure S2, available online, demonstrates no correlation between time since most recent diagnosis and VAN RSFC (p = .44).
There was a trend-level interaction between ROI pair and diagnostic group (F2,140 = 2.74, p = .068), and we performed exploratory analyses examining individual functional connections within the VAN (Figure 1). There was a significant group difference between ANX/DEP and HC in functional connectivity between the right VLFPC and the right pSTG [t(70) = 3.22, p = .023] as well as between the right VLPFC and the right vSMG [t(70) = 2.82, p = .006].
Associations Between Ventral Attention Network Functional Connectivity and Threat Bias
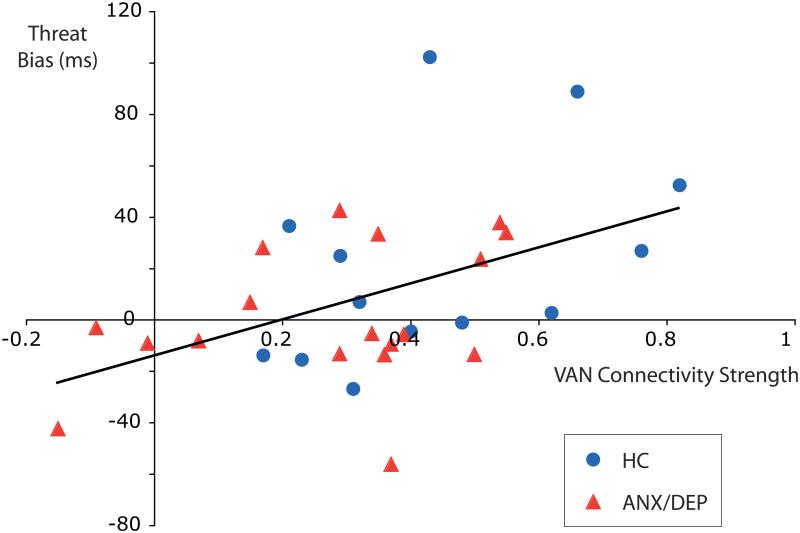
Measures of threat bias were available in a subset of the subjects, collected on average 2.1 years after imaging (18 ANX-DEP and 13 HC; 43% of the total sample). Across all subjects in this subset, there was a significant strong correlation between threat bias and RSFC of the VAN (r = 0.47, p < .01; Figure 2). The correlation between threat bias and RSFC remained significant when controlling for diagnostic group (r = 0.40, p = .028). ANX-DEP subjects had (nonsignificant) lower threat bias relative to HC (1.3 milliseconds versus 21.6 milliseconds, respectively), and lower VAN functional connectivity was associated with lower threat bias. There were no significant relationships between average VAN functional connectivity and any of the clinical dimensional measures collected near the time of scan, including core depression sum score, internalizing sum score, externalizing sum score, or CDI.
FIGURE 2.
Ventral attention network (VAN) resting state functional connectivity (RSFC) strength is positively correlated with threat bias independent of diagnostic group. Note: Each point represents an individual subject, and healthy control subjects (HC) and children with a history of anxiety and/or depression (ANX/DEP) are labeled separately. VAN connectivity strength is computed as the average of the 3 Fisher-z–transformed Pearson correlation coefficients for the individual VAN functional connections.
Controls for Demographic Variables and Subject Motion
We derived a propensity score40 for each subject to be categorized as ANX/DEP versus HC on the basis of sex, ethnicity, pubertal status, family income, dominant hand, IQ, stressful life events, traumatic life events, use of psychotropic medication, and maternal substance abuse. When this propensity score was included as a covariate in an analysis of covariance (ANCOVA) comparing functional connectivity strength within the VAN between groups, diagnostic grouping remained significant (F1,64 = 3.88, p = .053). Similar results were obtained when each of the above demographic factors were included as individual covariates in an ANCOVA (F1,26 = 4.00, p = .056).
Following volume censoring, there were no group differences between ANX/DEP and HC in average motion parameters, for either average FD (0.111 versus 0.105; t = 1.3, p = .19) or average DVARS (1.67 versus 1.71; t = 0.91, p = .37) As an additional control for subject motion, we used FD as a covariate in an ANCOVA comparing functional connectivity in the VAN between ANX/ DEP and HC; group differences remained significant (F1,69 = 5.83, p = .018).
Functional Connectivity in Regions Homotopic to Ventral Attention Network
To test specificity and to further rule out motion artifact as an explanation for group differences, we examined functional connectivity strength among the 3 regions in the left cerebral hemisphere that are homotopic to the 3 regions in the right hemisphere comprising the VAN. There was not a significant group difference in RSFC strength among these 3 left hemisphere regions when comparing ANX/DEP to HC (F1,70 = 0.02, p = .97), nor was there a group difference when comparing ANX, DEP, and HC (F2,59 = 1.23, p = .30 ), as in the original analysis. Furthermore, none of the individual functional connections (L VLPFC–L vSMG; L VLPFC–L pSTG; L pSTG–L vSMG) showed any significant group difference for ANX/DEP versus HC (all 3 p values >0.60)
Effects of Comorbid Externalizing Disorders
To determine whether results were driven by comorbid psychiatric disorders, we performed an additional ANOVA comparing VAN functional connectivity between HC, ANX/DEP with a history of comorbid externalizing disorder (ADHD, CD, or ODD), and ANX/DEP without externalizing comorbidity. This test revealed a significant main effect of diagnostic group (F2,69 = 3.28, p = .044). A post hoc LSD test indicated a significant difference specifically between HC and the ANX/DEP without a history of an externalizing disorder (p = .019).
In addition to a main effect of diagnostic group, the ANOVA examining externalizing comorbidity revealed a significant interaction between diagnostic group and connection (F4,138 = 4.5, p = .002). Follow-up 1-way ANOVAs revealed a significant effect of diagnostic category on the right VLPFC–right vSMG connection (F2,69 = 4.26, p = .018), with the comorbid ANX/ DEP and externalizing group having significantly lower RSFC than HC (p = .008). In addition, there was a significant effect of diagnostic category on the right pSTG–right vSMG connection (F2,69 = 4.33, p = .017), with the ANX/DEP plus externalizing group having significantly increased connectivity relative to the ANX/DEP-only group (p = .004).
DISCUSSION
The current study investigated alterations in the VAN in children with a history of depression and/or anxiety as a putative neural correlate of changes in attention associated with these disorders. Results indicated that children with a history of an anxiety disorder or depression had decreased RSFC among some regions of the VAN relative to healthy control subjects. In addition, the magnitude of RSFC of the VAN was correlated with measures of attention bias toward threat in subjects tested across diagnostic categories. These results remained significant when controlling for a wide range of demographic factors, and results were not attributable to comorbid externalizing disorders (ADHD, ODD, CD).
Reduced VAN Connectivity in Children With History of Depression/Anxiety
Anxiety disorders and depression were associated with decreased VAN functional connectivity. The VAN is 1 of at least 2 networks involved in the processing of novel stimuli and stimulus-driven attention, orienting toward suddenly appearing, unexpected stimuli.41 A current model proposes that the dorsal attention network, which includes regions in the dorsal frontal and parietal lobes, first detects novel stimuli and initiates reorienting, whereas the VAN is involved in more complex adjustments taking place after this initial reorientation, such as reconfiguring stimulus–response relationships, expectancies, and reward contingencies.20 These results therefore provide a plausible neurobiological correlate for some of the changes in attention associated with depression and anxiety.
The magnitude of VAN functional connectivity was strongly correlated with measures of threat bias independent of diagnostic status. Notably, VAN functional connectivity was not significantly correlated with dimensional measures of depression, anxiety, or externalizing symptoms collected near the time of neuro-imaging. This pattern of results suggests that children with a history of depression and/or anxiety have an altered VAN and that having an altered VAN is specifically associated with changes in attention bias and not with current psychiatric symptoms. The specificity of this association is important because depression and anxiety are associated with a range of symptoms and are highly comorbid with other psychiatric illnesses, making it challenging to link neuro-imaging findings to specific underlying bio-markers. Changes in attention toward negative stimuli have been associated with children and adults with anxiety disorders and major depression8-10 and also in children who are at risk for these disorders.42 One possibility is that an altered VAN and changes in attention toward negative stimuli are associated with a predisposition for depression and anxiety disorders even if symptoms are not currently present.
Children with a history of depression or anxiety in this study had nonsignificant lower threat bias (i.e., threat avoidance) compared to study subjects with no history of psychiatric illness. Although the majority of studies in adults and children find a bias toward negative stimuli in individuals with depression and/or anxiety disorders, threat avoidance is also a well-known phenomenon.10,16-18 Whether children with anxity and depression experience threat bias versus threat avoidance likely depends on experimental conditions and may vary across development, context, and disorder stage; further work is needed to clarify these points.
In the current study, threat bias was strongly positively correlated with the magnitude of VAN RSFC. Although this result is supportive of the general statement that threat bias is associated with VAN RSFC, caution is warranted when interpreting the direction of this effect. Although higher anxiety is typically associated with higher threat bias, under some conditions, higher distress is associated with lower threat bias (higher threat avoidance).43 One possibility, therefore, is that the same individual with high anxiety who exhibits high bias toward threat under 1 set of conditions may exhibit high bias away from threat under another set of conditions. The magnitude of VAN RSFC, therefore, might correlate with measures of attention to threat; but the direction of this effect could depend on experimental conditions, stage of development, and disorder stage.
Role of VAN in Development
The current results suggest that changes in the VAN may be an early feature of depression and anxiety disorders. Given the myriad brain changes taking place during adolescence, an altered VAN is likely to affect neurodevelopment and may influence adult brain structure and function. One possibility, which is highly speculative at this point, is that a VAN with high reactivity to novel stimuli (e.g., in infants with high behavioral inhibition) is associated with increased physiological response to these stimuli, whereas decreased VAN RSFC is related to maladaptive responses to novel stimuli. Over the course of development, this pattern of responses to novel stimuli may naturally evolve into an attention bias either toward or away from (avoidance) negative stimuli, because interactions with the environment and/or other unfolding disease processes may selectively reinforce maladaptive responses to negative stimuli.
Groups in this study were based on having depression or an anxiety disorder during early childhood at any time before the scanning. Future work should measure the VAN in children at risk for, or immediately after the onset of, depression or an anxiety disorder, to more directly sort out whether VAN changes are a cause or an effect of having these illnesses. Movement artifact has recently become recognized as a major problem in the analysis of resting state functional connectivity data, especially in children, older adults, and clinical populations.36,44,45 We mitigated this concern in the current study by volume censoring our data using very strict movement criteria. There were no group differences in movement parameters, and results were unchanged when residual movement (surviving volume censoring) was used as a covariate when comparing groups. As an additional safeguard, we examined functional connectivity strength in left hemisphere regions homotopic to the VAN, because movement should affect both sets of regions similarly. There were no significant group differences among these homotopic left hemisphere regions, bolstering our confidence that the current results are not due to movement artifacts.
Although the current study indicates an altered VAN in children with a history of early childhood anxiety and depression, future work is needed to clarify the larger role of the VAN in typical childhood development and in the development of other psychiatric disorders. Previous work on the VLPFC in adolescents has highlighted its role in emotion regulation and activity increases have been interpreted as a compensatory response16 to regulate an over-active amygdala.46 Future work should examine the developmental trajectories of stimulus-driven attention, emotion regulation, VAN functional connectivity, and VAN functional activity in typically developing children to elucidate the function of this network and to serve as a basis for understanding alterations in normal development. In addition, future work should address the relationship between the VAN and other functional networks associated with depression and anxiety disorders in children and adolescents, such as the default mode network.47 &
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health (NIH) RO1 MH090786 (J.L., D.B.), NIH RO1 MH090786-03S1 (C.S.), and an American Academy of Child and Adolescent Psychiatry (AACAP) Pilot Research Award for Attention Disorders supported by the Elaine Schlosser Lewis Fund (C.S.).
The authors thank Edward Spitznagel, Ph.D., of Washington University, for his assistance with statistical analyses.
Footnotes
Supplemental material cited in this article is available online.
Disclosures: Dr. Barch has received funding from Novartis and Dainepon, and has served as a consultant for Pfizer and Amgen. Dr. Corbetta has received funding from the National Institutes of Health (NIH) and the McDonnell Foundation. Dr. Schlaggar has received funding from NIH and the Tourette Syndrome Foundation. He has served on the scientific advisory board for the John Merck Fund and the medical advisory board for the Tourette Syndrome Association. Dr. Luby has received grant or research support from the National Institute of Mental Health, the National Alliance for Research on Schizophrenia and Depression, the Communities Healing Adolescent Depression and Suicide (CHADS) Coalition, and the Sidney R. Baer Foundation. She has served as a consultant to the Food and Drug Administration. Mr. Power has received funding from NIH. Dr. Sylvester reports no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Klimkeit EI, Tonge B, Bradshaw JL, Melvin GA, Gould K. Neuropsychological deficits in adolescent unipolar depression. Arch Clin Neuropsychol. 2011;26:662–676. doi: 10.1093/arclin/acr051. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Hu Y, Liu T, Wu D. Dipole source analysis of auditory P300 response in depressive and anxiety disorders. Cogn Neurodynam. 2011;5:221–229. doi: 10.1007/s11571-011-9156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruder GE, Kayser J, Tenke CE, et al. Cognitive ERPs in depressive and anxiety disorders during tonal and phonetic oddball tasks. Clin EEG. 2002;33:119–124. doi: 10.1177/155005940203300308. [DOI] [PubMed] [Google Scholar]
- 4.Kimble M, Kaloupek D, Kaufman M, Deldin P. Stimulus novelty differentially affects attentional allocation in PTSD. Biol Psychiatry. 2000;47:880–890. doi: 10.1016/s0006-3223(99)00258-9. [DOI] [PubMed] [Google Scholar]
- 5.lv J, Zhao L, Gong J, Chen C, Miao D. Event-related potential based evidence of cognitive dysfunction in patients during the first episode of depression using a novelty oddball task. Psychiatry Res. 2010;182:58–66. doi: 10.1016/j.pscychresns.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Qiao Z, Yu Y, Wang L, et al. Impaired pre-attentive change detection in major depressive disorder patients revealed by auditory mismatch negativity. Psychiatry Res. 2013;211:78–84. doi: 10.1016/j.pscychresns.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Sylvester CM, Corbetta M, Raichle ME, et al. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35:527–535. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol. 2004;113:121–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- 9A.Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 10.Shechner T, Britton JC, Perez-Edgar K, et al. Attention biases, anxiety, and development: toward or away from threats or rewards? Depress Anxiety. 2012;29:282–294. doi: 10.1002/da.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. J Abnorm Psychol. 2002;111:107–123. [PubMed] [Google Scholar]
- 12.Browning M, Holmes EA, Charles M, Cowen PJ, Harmer CJ. Using attentional bias modification as a cognitive vaccine against depression. Biol Psychiatry. 2012;72:572–579. doi: 10.1016/j.biopsych.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakamata Y, Lissek S, Bar-Haim Y, et al. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biol Psychiatry. 2010;68:982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hogan AM, Butterfield EL, Phillips L, Hadwin JA. Brain response to unexpected novel noises in children with low and high trait anxiety. J Cogn Neurosci. 2007;19:25–31. doi: 10.1162/jocn.2007.19.1.25. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Edgar K, Reeb-Sutherland BC, McDermott JM, et al. Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. J Abnorm Child Psychol. 2011;39:885–895. doi: 10.1007/s10802-011-9495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monk CS, Nelson EE, McClure EB, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 17.Pine DS, Mogg K, Bradley BP, et al. Attention bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. Am J Psychiatry. 2005;162:291–296. doi: 10.1176/appi.ajp.162.2.291. [DOI] [PubMed] [Google Scholar]
- 18.Stirling LJ, Eley TC, Clark DM. Preliminary evidence for an association between social anxiety symptoms and avoidance of negative faces in school-age children. J Clin Child Adolesc Psychol. 2006;35:431–439. doi: 10.1207/s15374424jccp3503_9. [DOI] [PubMed] [Google Scholar]
- 19.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 20.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koric L, Volle E, Seassau M, et al. How cognitive performance-induced stress can influence right VLPFC activation: an fMRI study in healthy subjects and in patients with social phobia. Hum Brain Mapp. 2012;33:1973–1986. doi: 10.1002/hbm.21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyer AE, Lau JYF, McClure-Tone EB, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry. 2008;65:1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luby JL, Si X, Belden AC, Tandon M, Spitznagel E. Preschool depression: homotypic continuity and course over 24 months. Arch Gen Psychiatry. 2009;66:897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luby JL, Belden AC, Pautsch J, Si X, Spitznagel E. The clinical significance of preschool depression: impairment in functioning and clinical markers of the disorder. J Affect Disord. 2009;112:111–119. doi: 10.1016/j.jad.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaffrey MS, Luby JL, Repovŝ G, et al. Subgenual cingulate connectivity in children with a history of preschool-depression. Neuroreport. 2010;21:1182–1188. doi: 10.1097/WNR.0b013e32834127eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luking KR, Repovs G, Belden AC, et al. Functional connectivity of the amygdala in early-childhood-onset depression. J Am Acad Child Adolesc Psychiatry. 2011;50:1027–1041. doi: 10.1016/j.jaac.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egger HL, Ascher B, Angold A. The Preschool Age Psychiatric Assessment: version 1.4. Duke University Medical Center; Durham, NC: 1999. p. 2003. [Google Scholar]
- 29.Bird HR, Gould MS, Staghezza B. Aggregating data from multiple informants in child psychiatry epidemiological research. J Am Acad Child Adolesc Psychiatry. 1992;31:78–85. doi: 10.1097/00004583-199201000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Angold A, Costello EJ. The Child and Adolescent Psychiatric Assessment (CAPA) J Am Acad Child Adolesc Psychiatry. 2000;39:39–48. doi: 10.1097/00004583-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Gaffrey MS, Belden AC, Luby JL. The 2-week duration criterion and severity and course of early childhood depression: implications for nosology. J Affect Disord. 2011;133:537–545. doi: 10.1016/j.jad.2011.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovacs M. The Children’s Depression, Inventory (CDI) Psychopharmacol Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 33.Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- 34.Talairach J, Tournoux P. Thieme Medical; New York: 1988. Co-planar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- 35.Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage. 2013;76:439–441. doi: 10.1016/j.neuroimage.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Power Jonathan D, Cohen Alexander L, Nelson Steven M, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fair DA, Schlaggar BL, Cohen AL, et al. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 41.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 42.Mogg K, Wilson KA, Hayward C, Cunning D, Bradley BP. Attentional biases for threat in at-risk daughters and mothers with lifetime panic disorder. J Abnorm Psychol. 2012;121:852–862. doi: 10.1037/a0028052. [DOI] [PubMed] [Google Scholar]
- 43.Bar-Haim Y, Holoshitz Y, Eldar S, et al. Life-threatening danger and suppression of attention bias to threat. Am J Psychiatry. 2010;167:694–698. doi: 10.1176/appi.ajp.2009.09070956. [DOI] [PubMed] [Google Scholar]
- 44.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satterthwaite TD, Wolf DH, Loughead J, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monk CS, Telzer EH, Mogg K, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaffrey MS, Luby JL, Botteron K, Repovs G, Barch DM. Default mode network connectivity in children with a history of preschool onset depression. J Child Psychol Psychiatry. 2012;53:964–972. doi: 10.1111/j.1469-7610.2012.02552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.