Abstract
Objectives
To examine alternative models of defining and characterizing successful aging.
Design
A retrospective cohort study
Setting
Olmsted County, MN.
Participants
560 community-dwelling non-demented adults, aged 65 years and older.
Measurements
Three models were developed. Each model examined subtests in four cognitive domains: memory, attention/executive function, language, and visual-spatial skills. A composite domain score was generated for each of the four domains. In Model 1, a global z-score was further generated from the four cognitive domains, and subjects with mean global z-score in the top 10% were classified as “successful agers” whereas those in the remaining 90% were classified as “typical agers”. In Model 2, subjects with all 4 domain scores above the 50th percentile were classified as “successful agers.” In Model 3, a primary neuropsychological variable was selected from each domain, and subjects whose score remained above minus 1 SD compared to norms for young adults were labeled successful agers. Validation tests were conducted to determine the ability of each model to predict survival and conversion to mild cognitive impairment (MCI).
Results
Model 1 showed 65% lower mortality in successful agers compared to typical agers, and also a 25% lower conversion rate to MCI.
Conclusion
Model 1 was most strongly associated with longevity and cognitive decline; as such, it can be useful in investigating various predictors of successful aging, including plasma level, APOE genotype, and neuroimaging measurements.
Keywords: successful aging, optimal aging, longevity, cognitive decline
INTRODUCTION
With the growing of population of older adults, the topic of successful aging has received increasing attention from various aspects of research (1). However, although there is increasing public and research interest in successful aging, the construct awaits a standard definition and there are currently no accepted sets of criteria. The question of which components are essential to the definition of successful aging is still a subject of considerable debate, as is also the term to be used, with descriptors including “healthy aging”, “successful aging”, and “productive aging”. While no single term is more comprehensive or universally accepted over others, in the present study, we will use the term “successful aging”, adopting the model proposed by Rowe and Kahn in 1987, in which a distinction is made between “successful” and “usual” aging(2).
Attempts at characterizing successful aging date back to 1960s, where Havighurst defined it as “adding life to the years” and “getting satisfaction from life” (3). While interest in successful aging remained through the decades, it peaked again with Rowe and Kahn’s 1987 landmark article in Science, in which they distinguished “successful” aging from normal or “usual” aging, and highlighted the need for characterizing the healthiest among the older population. This prevailing model was then advanced in a set of studies conducted by the MacArthur Studies of Successful Aging, in which successful aging was characterized as involving freedom from disability along with high cognitive, physical, and social functioning (4).
A comprehensive review of 28 studies observed at least 10 components of the definition, ranging from physical functioning to personality and self-rated health (5). The common theme that emerged from these definitions was the absence of disability and perceived good health. However, not all investigators (nor study participants) agree that good health is essential to successful aging. A survey of 239 surviving alumni from the class of 1939 at Yale found that participants rated quality of life as good, despite the presence of diseases and disabilities (6). Similarly, other researchers asked participants about their own definitions of successful aging and observed a relatively greater emphasis on social integration and well-being than found in definitions used by investigators (7–9). Other studies also report an association between sleeping well and aging successfuly (10).
As such, operationalization of successful aging can differ depending on who defines it and which components are measured (11). This emphasizes the need for well-developed criteria of successful aging in that it helps better characterize successful aging. We chose cognitive criteria because our goal was to characterize successful aging using resources of Mayo Alzheimer’s Disease Patient Registry (ADPR), a cohort that has a large number of subjects who have been longitudinally followed for a long period of time (1986–2004). Individuals in this cohort underwent cognitive assessments at baseline and in follow-up examinations, and this provided enriched cognitive data that allowed for testing various models of successful cognitive aging. Thus, while we realize that there are different approaches to defining successful aging, we chose to focus on cognitive successful aging in order to utilize this large database that had several repeated cognitive assessments since it allowed us to develop and test several models of successful cognitive aging. In addition, evidence from healthy aging as well as Alzheimer’s disease research indicates that cognitive function is an important factor in aging, and that cognitive integrity is likely a surrogate for successful aging. As such, understanding cognitive aspects of aging can provide important insights into successful aging.
METHODS
Selection of study subjects
Subjects were Olmsted County, MN, residents ages 65 years or older who were identified at the time of their routine general medical examination in the Division of Primary Care Internal Medicine at the Mayo Clinic. The details of subject recruitment have been previously published and are briefly described here (12–14). The study protocol was approved by the Institutional Review Board and informed consent was obtained from all participants.
Subjects were identified from a community-based cohort of healthy individuals recruited from 1986–2004 to serve as normal comparison groups and a longitudinal normal cohort for the Mayo Alzheimer ’s Disease Patient Registry (ADPR). All eligible persons were evaluated using an identical standardized clinical protocol that included a neurologic examination by a behavioral neurologist and neuropsychological testing. Functional status was assessed using the Record of Independent Living. Laboratory tests included CBC count, thyroid function tests, Vitamin B-12 and folic acid levels, sensitive thyroid stimulating hormone level, and syphilis serology. A blood draw for APOE-ε4 genotyping was also performed. A Charlson Comorbidity Index (14, 15) was calculated from diagnoses assessed electronically using the records-linkage system of the Rochester Epidemiology Project, an extensive indexing system based on surgical and medical diagnoses maintained by the Mayo Clinic.
At the completion of the assessment, a consensus meeting was held involving behavioral neurologists, neuropsychologists, nurses, and geriatricians. A final clinical diagnosis was made based on all available information. Participants became qualified as healthy individuals if they were deemed by their clinician to be functioning normally in the community and did not have a cognitive impairment and, in addition, did not have any active neurologic, psychiatric, or other illnesses believed to affect cognition (as determined by the neurologist’s interview), and were not taking any psychoactive medications.
Neuropsychological evaluation
Domain specific measures of cognitive function were ascertained from the Neuropsychology Screening Battery (NSB). The NSB consists of two or three measures in each of the following cognitive domains: memory, executive function, language and visuospatial skills. The tests in each domain were as follows: Memory: Logical Memory-II % retention and Visual Reproduction-II % retention from the Weschler Memory Scale-Revised (WMS-R)(16), Rey Auditory Verbal Learning Test(17), and Free and Cued Selective Reminding Test(18); Attention: Trail Making Tests(19) and Digit Span and Digit Symbol subtests from the Wechsler Adult Intelligence Scale-Revised (WAIS-R)(20); Language: Boston Naming Test(21), Category Fluency, and Controlled Oral Word Association Test (22–24), and Visuospatial: Block Design, Picture Completion and Object Assembly subtests from the WAIS-R(20).
Each component test of a specific domain generated a raw score and this score was converted to a Mayo’s Older American Normative Studies (MOANS) value that corrected for age and transformed the raw scores to a standardized score with a mean of 10 and standard deviation of 3, and adjusted to population norms (25). The MOANS scores on each measure were then combined within a domain to yield a composite domain score which is tantamount to a z score for that domain.
Selection of criteria for successful aging
Candidate criteria for successful aging were examined using three models. The models were based on participants’ performance on the Neuropsychology Screening Battery, where a composite domain score was obtained as described above.
In Model 1, a mean global z-score was generated by calculating the mean of the four domain scores. Participants with mean global z-score in the top 10% were then classified as “successful agers” whereas those in the remaining 90% were classified as “typical agers”.
In Model 2, participants’ domain scores in each of the cognitive domains were assessed and those with all 4 domain scores above the 50th percentile were classified as “successful agers”. Participants with at least one domain score below the 50th percentile were classified as “typical agers”.
Model 3 was based on age-associated memory impairment (AAMI), a term proposed by NIMH in 1986 to characterize memory changes in aging that are considered to be manifestation of normal cognition.(26) The AAMI referenced memory function in older individuals to the performance of younger adults, where diagnostic criteria involved 1 SD below the mean established norms for young adults on standardized neuropsychological tests. Thus, in Model 3, ‘reverse-age-associated memory impairment (r-AAMI) criteria were generated using cutoff RAWscores for young adults aging 24–35 years old. Subjects whose RAWscore remained above -1 SD compared to norms for young adults were labeled successful agers. As such, the following cutoff RAWscores in each of the four cognitive domains were used to identify successful agers: Logical Memory II (Memory domain), with cutoff score > 12; Boston Naming (Language domain), with cutoff score >= 53; Block Design (Visuospatial domain), with cutoff score >= 21; and Trail Making B (Attention domain), with cutoff score < 91.
Outcome measures
The primary outcome measures were mortality and conversion to MCI. Mortality was determined by assessing all-cause mortality using the records linked resources of the Rochester Epidemiology Project information in ADPR records, and State of Minnesota death tapes. The diagnosis of MCI was made by the adjudicating team based on the psychometric data and also using the Petersen et al criteria (memory complaint, normal activities of daily living, normal general cognitive function, abnormal memory for age, and not demented) (12).
Statistical analyses
For each model, Kaplan-Meier curves were constructed for successful agers and compared to curves constructed for typical agers using the log-rank test. Hazard ratio (HR) and 95% CI were estimated using Cox proportional hazards models adjusting for age, education, gender, and comorbidity. Statistical testing was conducted at two-tailed alpha level of 0.05. Analyses were performed using SAS (SAS Institute, Cary, NC).
RESULTS
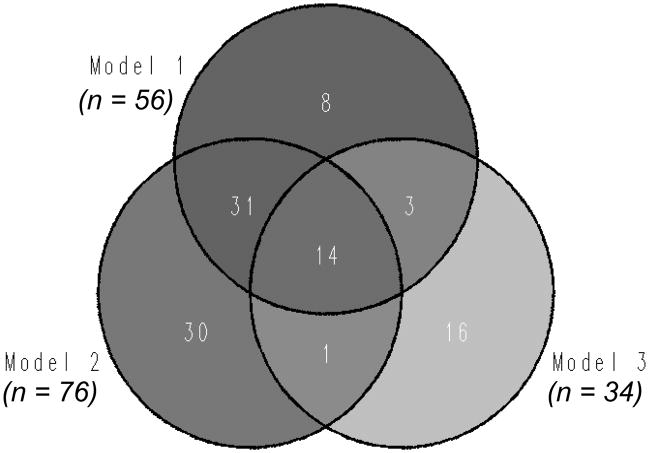
The Venn diagram in Figure 1 indicates the number of participants identified as successful agers using the three models. For this analysis, only participants who had all the neuropsychological data needed to generate all the three models were selected, and there were 560 such participants. As the Venn Diagram indicates, Model 1 yielded 56 participants that were classified as successful agers, whereas the remaining 504 participants were typical agers. Model 2 generated 76 successful agers and 484 typical agers, whereas Model 3 generated 34 successful agers and 526 typical agers. As the figure also shows, there were some overlap between models, where 45 participants met the criteria for both Models 1 and 2; 17 met the criteria for Models 1 and 3, and 15 met the criteria for Models 1 and 3. In addition, 14 participants met the criteria for all three models. This suggests that some participants were highly “successful” in that they met the cutoff scores for all three models. However, since the number of such subjects is small, it is hard to make further predictions about these subgroups.
Figure 1.
Number of subjects identified as successful agers in the three models.
Participant characteristics
Table 1 shows the characteristics of participants as a whole and grouped into the three models. As the table indicates, for each model, education level was significantly higher for successful agers compared to typical agers. In Model 3, successful agers were also significantly younger than typical agers.
Table 1.
Participant Characteristics of Successful and Typical Agers in the Three Models.
| Subjects | Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Successful Agers | Typical Agers | p-value | Successful Agers | Typical Agers | p-value | Successful Agers | Typical Agers | p-value | ||
| Age, y mean (SD) | 79.7 (6.5) | 79.2 (6.8) | 79.8 (6.5) | 0.52 | 79.0 (6.2) | 79.8 (6.6) | 0.33 | 71.7 (7.8) | 80.2 (6.1) | <.001 |
| Education, y mean (SD) | 13.1 (2.9) | 15.0 (2.9) | 12.8 (2.9) | <.001 | 14.5 (2.9) | 12.8 (2.9) | <.001 | 14.7 (2.5) | 12.9 (2.9) | 0.003 |
| Gender (M/F) | 192/368 | 20/36 | 172/332 | 0.81 | 26/50 | 166/318 | 0.99 | 11/23 | 181/345 | 0.81 |
| APOE status (ε4+/ε4−) | 124/436 | 15/41 | 109/395 | 0.38 | 18/58 | 106/378 | 0.73 | 12/22 | 112/414 | 0.06 |
Standard deviations are indicated in parentheses. Continuous variables were tested using the Wilcoxon rank sum test, and categorical variables were tested using the Wald chi-square test (1 df), n = 560.
The entire cohort was followed for a median of 7.9 years. In Model 1, the median follow up was 8.6 years (range: 2.1–16.2 years) for successful agers, and 7.8 years (range: 0.1–18.0 years) for typical agers. In Model 2, successful agers were followed for a median of 7.8 years (range: 0.6–16.1 years), while typical agers were followed for 8.6 years (range: 0.1–18.0 years). In Model 3, the median follow up was 8.9 years (range: 1.0–18.0 years) for successful agers, and 7.8 years (range: 0.1–17.8 years) for typical agers.
Validation tests
Once the models were developed, the next question was to determine the utility of the models in investigating various aspects of successful aging. As such, we wanted to examine whether the models were able to predict (1) survival, and (2) conversion to MCI.
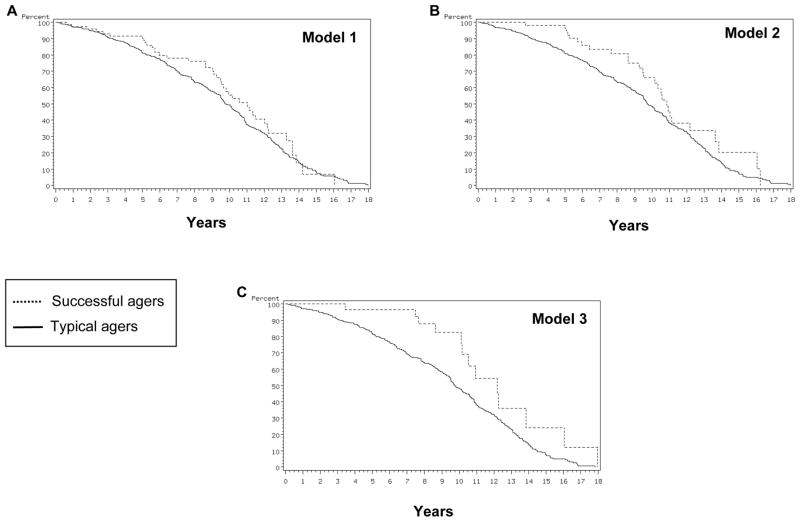
Figure 2 shows Kaplan-Meier survival curves for successful and typical agers in the three models. As the figures indicate, all models showed higher survival for successful agers than for typical agers. For Model 1, in a multivariate analyses adjusted for age, gender, and education, mortality was 35% lower in successful agers compared to typical agers (Table 2). When comorbidity, as measured by Charlson Index, was added to the analyses, the effect did not reach significance. Model 3 showed 48% lower mortality in successful agers compared to typical agers, and when comorbidity was added to the analyses, the effect was not significant (Table 2). Model 2, on the other hand, did not reveal significant effect for either analysis. These findings indicate that Models 1 and 3 were able to predict survival in that those identified as successful agers by these models tended to live longer than those identified as typical agers. Further, when the analyses were adjusted for comorbidities, some of these effects were diminished. This suggests that these models of successful aging share explanatory variance with comorbidities in predicting longevity, and in so doing, capture physical as well as cognitive health.
Figure 2.
Kaplan-Meir survival curves for successful and typical agers in the three models.
Table 2.
Median Survival for Successful and Typical Agers in the Three Models and Hazard of Death Associated with Typical Agers.
| Median survival, y | Adjusted for age, gender, & education | Adjusted for age, gender, education & Charlson Index | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Successful Agers | Typical Agers | p-value | HR (95% CI) | p-value | HR (95% CI) | ||
| Model 1 | 10.8 | 9.8 | 0.04 | 0.65 (0.4–0.9) | 0.08 | 0.70 (0.47–1.05) | |
| Model 2 | 11.0 | 9.8 | 0.49 | 0.89 (0.6–1.2) | 0.50 | 0.89 (0.63–1.25) | |
| Model 3 | 12.2 | 9.7 | 0.04 | 0.52 (0.3–0.9) | 0.16 | 0.65 (0.35–1.19) | |
HR = hazard ratio. Wald chi-square test (1 df).
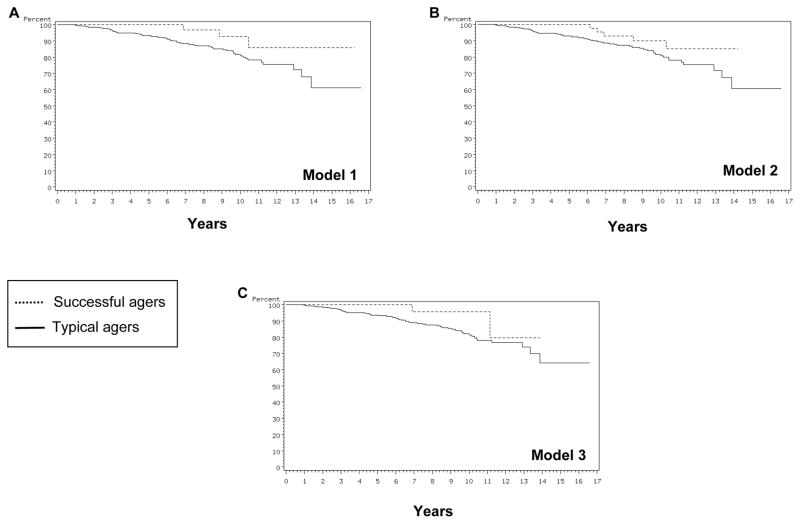
Figure 3 shows Kaplan-Meier curves of conversion to MCI in the three models. As the figures indicate, all models showed higher conversion rate for typical agers than for successful agers. For Model 1, multivariate analyses adjusted for age, gender, and education showed that conversion to MCI was 75% lower in successful agers than in typical agers. Further, this effect remained the same even when comorbidity was added to the analyses (Table 3). Model 2 showed a 58% lower conversion rate in successful agers, which also remained when comorbidity was added to the analyses. Model 3 did not reveal significant difference for either analysis. These findings indicate that Model 1 was able to predict conversion to MCI in that those identified as successful agers by these models tended to convert to MCI at a lower rate than those identified as typical agers. Interestingly, analyses involving comorbidities did not significantly change the results, indicating that the effect of comorbidities on conversion to MCI was minimal (Table 3).
Figure 3.
Kaplan-Meir curves of conversion to MCI in the three models.
Table 3.
Hazard of Conversion to MCI Associated with Typical Agers in the Three Models.
| Adjusted for age, gender, & education | Adjusted for age, gender, education & Charlson Index | |||
|---|---|---|---|---|
|
| ||||
| p-value | HR (95% CI) | p-value | HR (95% CI) | |
| Model 1 | 0.02 | 0.25 (0.07–0.83) | 0.02 | 0.26 (0.08–0.84) |
| Model 2 | 0.06 | 0.42 (0.16–1.06) | 0.06 | 0.41 (0.16–1.05) |
| Model 3 | 0.38 | 0.53 (0.12–2.22) | 0.40 | 0.54 (0.13–2.29) |
HR = hazard ratio. Wald chi-square test (1 df).
DISCUSSION
The present study aimed to generate criteria for successful aging by developing and testing parallel models of successful aging. A longitudinal cohort of healthy individuals followed in the Mayo ADPR was utilized, where participants’ performance in four cognitive domains was assessed and three models of successful aging were generated.
Results indicated that, first, in all the models, successful agers were significantly more educated than typical agers. This is consistent with evidence showing the link between education levels and aging, where individuals with higher education levels perform better on tests of cognitive function, and also show less decline over time compared to those with low education levels(27). Secondly, Model 3 yielded successful agers that were slightly different from the other models, where successful agers were significantly younger than typical agers.
Once the models were developed, the next step was to determine the utility of these models in clinical research, i.e., the extent to which the developed criteria can be useful in assessing various aspects of successful aging. To that end, we examined the ability of the models to predict two endpoints.
First, with regards to survival, we found that Models 1 and 3 showed significant effects in that those identified as successful agers by these models lived longer than the typical agers. Secondly, it was again Model 1 that showed a significant association with lower conversion to MCI.
Model 1 generated the most reliable criteria for successful aging in that those identified as successful agers by this model lived longer and also converted to MCI at a lower rate. This model has the potential to be useful in investigating various aspects of successful aging, including biological determinants. One major endeavor of successful aging research has been to determine which factors predict successful aging, and whether there are measures that can differentiate successful agers from typical agers. Various studies have investigated different predictors of successful aging (including plasma level, APOE genotype, and neuroimaging measurements), and have used outcome measures such as longevity and cognitive decline (5, 28, 29). Models that are sensitive to these outcome measures (such as Model 1) can be useful in investigating such predictors of successful aging, and we plan to explore these biomarkers in future studies using this cohort.
The present study has potential limitations. The models used to generate criteria for successful aging were based only on cognitive functioning, and other domains such as physical and social functioning were not assessed. While this was done to gain flexibility in assessing whether physical and social activities serve as predictors, the criteria for successful aging remains a cognitive one and thus may not be comparable to other studies that have included other domains in their definitions.
The strengths of the study include a longitudinal cohort with a large number of subjects who have been followed for a median of 4.5 years (range: 1–15 years). In addition, the models were developed using performance in four cognitive domains, thus allowing for a more comprehensive evaluation of cognitive function. The parallel construction of three models also allows for comparison of the models using the same cohort to determine which criteria best define successful aging.
Acknowledgments
This work was supported by grants P50 AG16574, U01 AG06786, K12 RR24151, K01 MH 68351, and the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program of the Mayo Foundation. Dr. Boeve has received grant support from Myriad Pharmaceuticals for clinical trial and honorarium from GE Healthcare. Dr. Knopman has received grant from Elan Pharmaceuticals for clinical trial and is consultant for Lilly. Dr. Petersen is a consultant for Elan Pharmaceuticals, Wyeth Pharmaceuticals, and GE Healthcare.
Footnotes
The remaining authors have no conflict of interest to disclose.
Preliminary reports of these findings were presented at the Society for Neuroscience Meeting, 2008, Washington, D.C.
Drs. Negash, Smith, Pankratz and Petersen contributed to the study concept and design, interpretation of data, and preparation of manuscript. Drs. Geda, Roberts, Knopman, Boeve, and Ivnik contributed to interpretation of data and preparation of manuscript. Drs. Pankratz, Negash and Mr. Aakre contributed to acquisition of data and data analysis.
References
- 1.Blazer DG. Successful aging. Am J Geriatr Psychiatry. 2006 Jan;14(1):2–5. doi: 10.1097/01.JGP.0000195222.93655.d1. [DOI] [PubMed] [Google Scholar]
- 2.Rowe JW, Kahn RL. Human aging: Usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- 3.Havighurst RJ. Successful aging. The Gerontologist. 1961;1(1):8–13. [Google Scholar]
- 4.Rowe JW, Kahn RL. Successful aging. 1. New York: Pantheon Books; 1998. [Google Scholar]
- 5.Depp CA, Jeste DV. Definitions and predictors of successful aging: a comprehensive review of larger quantitative studies. Am J Geriatr Psychiatry. 2006 Jan;14(1):6–20. doi: 10.1097/01.JGP.0000192501.03069.bc. [DOI] [PubMed] [Google Scholar]
- 6.Eiseman B. When blue turns gray: postwarranty performance. Am J Geriatr Psychiatry. 2006 Jan;14(1):21–26. doi: 10.1097/01.JGP.0000192509.53109.92. [DOI] [PubMed] [Google Scholar]
- 7.Bowling A, Dieppe P. What is successful ageing and who should define it? Bmj. 2005 Dec 24;331(7531):1548–1551. doi: 10.1136/bmj.331.7531.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reichstadt J, Depp CA, Palinkas LA, et al. Building blocks of successful aging: a focus group study of older adults’ perceived contributors to successful aging. Am J Geriatr Psychiatry. 2007 Mar;15(3):194–201. doi: 10.1097/JGP.0b013e318030255f. [DOI] [PubMed] [Google Scholar]
- 9.Montross LP, Depp C, Daly J, et al. Correlates of self-rated successful aging among community-dwelling older adults. Am J Geriatr Psychiatry. 2006 Jan;14(1):43–51. doi: 10.1097/01.JGP.0000192489.43179.31. [DOI] [PubMed] [Google Scholar]
- 10.Driscoll HC, Serody L, Patrick S, et al. Sleeping well, aging well: a descriptive and cross-sectional study of sleep in “successful agers” 75 and older. Am J Geriatr Psychiatry. 2008 Jan;16(1):74–82. doi: 10.1097/JGP.0b013e3181557b69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng TP, Broekman BF, Niti M, et al. Determinants of successful aging using a multidimensional definition among Chinese elderly in Singapore. Am J Geriatr Psychiatry. 2009 May;17(5):407–416. doi: 10.1097/JGP.0b013e31819a808e. [DOI] [PubMed] [Google Scholar]
- 12.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 13.Petersen RC, Kokmen E, Tangalos E, et al. Mayo Clinic Alzheimer’s Disease Patient Registry. Aging. 1990;2:408–415. doi: 10.1007/BF03323961. [DOI] [PubMed] [Google Scholar]
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a Clinical Comorbidity Index for Use with ICD-9-CM Administrative Databases. Journal of Clinical Epidemiology. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 15.Melton LJ. History of the Rochester Epidemiology Project. Mayo Clinic Proceedings. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 16.Wechsler DA. Wechsler Memory Scale-Revised. New York: Psychological Corporation; 1987. [Google Scholar]
- 17.Rey A. L’examen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 18.Grober E, Buschke H. Genuine Memory Deficits in Dementia. Developmental Neuropsychology. 1987;3:13–36. [Google Scholar]
- 19.Reitan R. Validity of the Trail-Making Test as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 20.Wechsler DA. Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 21.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 22.Benton AL, Hamsher K, Varney NR, et al. Contributions to Neuropsychological Assessment. New York: Oxford University Press; 1983. [Google Scholar]
- 23.Monsch AU, Bondi MW, Butters N, et al. A comparison of category and letter fluency in Alzheimer’s disease and Huntington’s disease. Neuropsychology. 1994;8:25–30. [Google Scholar]
- 24.Benton AL, Hamsher K. Multilingual Aphasia Examination. Iowa City, IA: AJA Associates; 1989. [Google Scholar]
- 25.Ivnik RJ, Malec JF, Smith GE, et al. WAIS-R, WMS-R and AVLT norms for ages 56 through 97. Clin Neuropsychol. 1992;6 (suppl):1–104. [Google Scholar]
- 26.Crook T, Bartus RT, Ferris SH, et al. Age-associated memory impairment: Proposed diagnostic criteria and measures of clinical change - Report of a National Institute of Mental Health Work Group. Developmental Neuropsychology. 1986;2:261–267. [Google Scholar]
- 27.Evans DA, Beckett LA, Albert MS, et al. Level of education and change in cognitive function in a community population of older persons. Ann Epidemiol. 1993 Jan;3(1):71–77. doi: 10.1016/1047-2797(93)90012-s. [DOI] [PubMed] [Google Scholar]
- 28.Willcox DC, Willcox BJ, Shimajiri S, et al. Aging gracefully: a retrospective analysis of functional status in Okinawan centenarians. Am J Geriatr Psychiatry. 2007 Mar;15(3):252–256. doi: 10.1097/JGP.0b013e31803190cc. [DOI] [PubMed] [Google Scholar]
- 29.Barzilai N, Atzmon G, Derby CA, et al. A genotype of exceptional longevity is associated with preservation of cognitive function. Neurology. 2006 Dec 26;67(12):2170–2175. doi: 10.1212/01.wnl.0000249116.50854.65. [DOI] [PubMed] [Google Scholar]