Abstract
The neurocognitive and behavioral profile of individuals with 47,XYY is increasingly documented; however, very little is known about the effect of a supernumerary Y-chromosome on brain development. Establishing the neural phenotype associated with 47,XYY may prove valuable in clarifying the role of Y-chromosome gene dosage effects, a potential factor in several neuropsychiatric disorders that show a prevalence bias towards males, including autism spectrum disorders. Here, we investigated brain structure in 10 young boys with 47,XYY and 10 age-matched healthy controls by combining voxel-based morphometry (VBM) and surface-based morphometry (SBM). VBM results show the existence of altered grey matter volume in the insular and parietal regions of 47,XYY relative to controls, changes that were paralleled by extensive modifications in white matter bilaterally in the frontal and superior parietal lobes. SBM analyses corroborated these findings and revealed the presence of abnormal surface area and cortical thinning in regions with abnormal grey matter and white matter volumes. Overall, these preliminary results demonstrate a significant impact of a supernumerary Y-chromosome on brain development, provide a neural basis for the motor, speech, and behavior regulation difficulties associated with 47,XYY, and may relate to sexual dimorphism in these areas.
Keywords: Sex chromosome, aneuploidy, brain development, autism, sexual dimorphism
INTRODUCTION
XYY syndrome (47,XYY) is a relatively common sex aneuploidy, occurring in approximately 1 of 1000 live male births (Morris et al., 2008), but remains largely underdiagnosed (Stochholm et al., 2010). Indeed, the condition is not associated with a clearly discernible physical phenotype, although individuals with 47,XYY present with increased growth velocity (Ratcliffe et al., 1992) and adult stature (Ottesen et al., 2010), as well as higher prevalence of neurological disorders (Higgins et al., 2007; Stochholm et al., 2010). Boys with 47,XYY do not typically present marked intellectual impairments (Leggett et al., 2010), but nonetheless display motor (Linden et al., 2002; Ratcliffe, 1999), reading, and speech difficulties (Geerts et al., 2003; Ratcliffe, 1999). Parents of children with 47,XYY also report the presence of significant behavioral problems in their children (Ross et al., 2012; Ratcliff, 1999), characterized by impulsivity and lack of self-control (Ross et al., 2012); as well as a high prevalence of symptoms associated with autism spectrum disorders (ASD) and attention deficits (Cordeiro et al., 2012; Ross et al., 2012).
While the neurocognitive and behavioral phenotype associated with XYY syndrome is increasingly documented, only two studies have used imaging modalities to investigate the impact of a supernumerary Y-chromosome on neuroanatomy. While the first study conducted by Warwick and colleagues (1999) did not reveal significant differences compared to control adult males, a more recent study reported increased total grey matter and white matter volume in 47,XYY (Bryant et al., 2012). In this second study, multivoxel pattern analysis was used to establish that brain morphology of boys with 47,XYY was more similar to boys with Klinefelter syndrome (XXY) than to male controls (XY). While this is informative as to the overall effect of a supernumerary sex chromosome on brain morphology, a statistical analysis of sub-regional differences using a voxel-based morphometric approach is still lacking. Consequently, the precise effect of an extra Y-chromosome on brain structure remains largely unknown. Documenting the neuromorphology associated with 47,XYY would be valuable, not only to understand the neural basis of the cognitive and behavioral difficulties associated with this condition, but more broadly to better understand the role of Y-chromosome gene dosage in neuropsychiatric disorders such as ASD showing a male-biased prevalence.
Here, we used voxel-based morphometry (VBM) in conjunction with surface based morphometry (SBM) and volumetric subcortical measurements to assess the impact of a supernumerary Y-chromosome on brain development in 10 young boys with 47,XYY and 10 age- and IQ-matched healthy controls. Combining VBM and SBM gives the advantage of delineating the relative contribution of cortical area and thickness to potential alterations in regional grey matter volume, while volumetric subcortical analysis gives a precise measure of defined subcortical structures. Considering the documented behavioral difficulties in individuals with 47,XYY and the increased risk for ASD in affected individuals, we hypothesized the existence of aberrant morphology in regions subserving inhibition, namely in the frontal lobe, as well as in circuits related to social cognition and motor and verbal abilities.
MATERIAL AND METHODS
Participants
Ten boys with 47,XYY (mean age 11.91 ±2.31; range 8–16) were recruited from the Thomas Jefferson University sex chromosome disorders clinic, the patient advocacy organization (KS&A), or were referred by other physicians. Genetic diagnoses of boys with 47,XYY were confirmed by a postnatal G-banded peripheral blood karyotype performed on a minimum of 20 cells. Ten healthy control male participants (XY; mean age 11.21 ±0.85; range 8–12) were recruited via public announcements and by referral from other families participating in research studies. Prior to enrollment in this study, all participants were screened with standard forms and interviews for contraindications for magnetic resonance imaging (MRI) as well as past medical history to ensure that there were no instances of neurological injury, psychiatric illness or disease (except for disorders often associated with XYY). Four participants in the 47,XYY group reported having a diagnosis of attention deficit hyperactivity disorder (ADHD). The sample used in the present study overlaps with previously published work (Ross et al., 2012; Bryant et al., 2012). The Institutional Review Boards at Thomas Jefferson University and Stanford University approved the study. Informed consent was obtained from all parents and verbal assent was obtained from all participants.
Cognitive and ASD-related Assessment
Participants were administered the Differential Ability Scales (DAS) for verbal, non-verbal, spatial, and conceptual abilities. Boys with 47,XYY were also administered the Autism Diagnostic Interview – Revised (ADI-R) and the Social Responsiveness Scale (SRS). Four of the ten boys with 47,XYY met the ADI-R cutoff for an autism spectrum disorder diagnosis, and seven of them had a SRS-Total T-score of 76 of higher, which is strongly associated with a diagnosis of ASD. The population characteristics are summarized in Table 1.
Table 1.
Demographics and cognitive data of boys with XY (controls) and 47,XYY.
| Control (XY) (n=10) |
47,XYY (n=10) |
|
|---|---|---|
| Age | 11.21 (0.85) | 11.91 (2.31) |
| Socioeconomic status | 50.83 (6.32) | 49.25 (10.97) |
| Height | 0.35 (1.20) | 1.32 (1.20) |
| Weight | 0.42 (1.30) | 0.94 (0.61) |
| Differential Ability Scales | 112.10 (15.35) | 89.00 (10.83) |
| Verbal Abilitya | 112.10 (15.35) | 89.00 (10.83) |
| Non-verbal Reasoning Abilitya | 113.80 (13.06) | 88.60 (11.69) |
| Spatial Abilitya | 106.60 (12.06) | 87.60 (16.37) |
| General Conceptual Abilitya | 113.30 (13.97) | 86.20 (8.91) |
| ADHD diagnosis | 0 | 4 |
| ADI-R cutoff | - | 4 |
| SRS Total T-score | - | 74.60 (15.64) |
Indicates significant differences between groups at p < 0.05. ADHD, Attention Deficit Hyperactivity Disorder; ADI-R, Autism Diagnostic Interview-Revised; SRS, Social Responsiveness Scale.
MRI Data Acquisition
All MRI scanning was carried out at Thomas Jefferson University on a Philips 3.0T whole-body clinical MRI system (Achieva, Philips Medical Systems, Best, The Netherlands) equipped with a Quasar Dual high-performance gradient system capable of on-axis (x, y and z) peak gradient of 80 mT/m and 200 mT/m/ms slew rate, and an 8-channel SENSE (sensitivity encoding) head coil. Structural images were obtained using a conventional, high-resolution three-dimensional (3D) T1-weighted fast-gradient echo sequence (repetition time [TR] = 25ms, echo time [TE] = 2.3ms, flip angle = 30°, 0.96×0.96×1mm voxels, 160 contiguous anterior commissure-posterior commissure [AC-PC]-aligned slices of 1.5 mm thickness, acquisition time = 6 minutes, 9 seconds).
Image processing: Voxel-based morphometry
Volumetric analysis of T1-weighted images was performed using Statistical Parametric Mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm) in Matlab. After manual verification of alignment on the AC-PC axis, images were corrected for magnetic field inhomogeneity and were separated into GM, WM, and cerebrospinal fluid (CSF) image volumes based on a priori adult tissue probability maps (Ashburner and Friston, 2005). Iterative weighting using Hidden Markov Random Fields was concurrently applied to encode spatial information based on constraints of neighboring voxels (Zhang et al., 2001). Inter-subject registration was then achieved via creation of a custom subject-based template, resampled to 1.5×1.5×1.5mm voxels, using the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) toolbox followed by warping into Montreal Neurological Institute (MNI) space. Jacobian-scaled (modulated) tissue classes were subsequently smoothed using a 8mm full-width at half-maximum (FWHM) Gaussian smoothing kernel.
Image processing: Surface-based morphometry and volumetric subcortical segmentation
Cortical reconstruction and volumetric segmentation was performed with the Freesurfer version 5.0 image analysis suite (http://surfer.nmr.mgh.harvard.edu/). The technical details of the procedures used are extensively described in prior publications (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 2004). This processing includes removal of non-brain tissue, segmentation of subcortical white matter and deep gray matter volumetric structures, tessellation of the gray matter-white matter boundary, and automated topology correction (Ségonne et al., 2007). The gray-white and pial surfaces were visually inspected, and appropriate manual corrections were performed, where needed, as per the Freesurfer Tutorial (http://surfer.nmr.mgh.harvard.edu/fswiki/FsTutorial). All raters were trained to achieve inter-rater reliability of ≥0.95 (intraclass correlation coefficient) with gold-standard datasets developed in our laboratory for volumetric regions of interest. Once cortical models were complete, brain surfaces for each hemisphere were parcellated into 34 distinct regions based on gyral and sulcal structure (Fischl et al., 2004; Desikan et al., 2006). Freesurfer calculates gray matter volume (GMV), surface area (SA) of the gray-white boundary, mean cortical thickness (CT), and white matter volume (WMV) for each parcellated region. The gray-white boundary parcellation is used to assign an anatomical label to underlying white matter voxels within 5mm of the cortical surface, allocating these voxels to the nearest cortical parcellation (Salat et al., 2009).
Statistical analysis
Differences in age, height/weight standard deviation scores, socioeconomic status and cognitive abilities were evaluated using one-way ANOVAs. Regarding VBM analysis, differences in total GMV and WMV between groups were evaluated using a two-sample t-test. Between-group voxel-based morphological differences of regional GM and WM in 47,XYY and control subjects were investigated by applying the general linear model (GLM) in SPM8. Accordingly, a voxel-wise two-sample t-test was performed while covarying for the effects total GMV and total WMV in their respective analyses. Statistical inference of significant clusters was then evaluated using the VBM5 toolbox (dbm.neuro.uni-jena.de/vbm/) at a height of P < 0.01, spatial extent P < 0.05 (family wise error (FWE) corrected), while applying non-stationary cluster extent correction to account for non-uniform smoothness across the data (Hayasaka et al., 2004). To more easily localize structural WMV changes, images of significant WMV clusters were resliced to 2×2×2mm voxels for compatibility with FSL white matter atlases to identify specific white matter tracts contained within the clusters.
For SBM and subcortical volumetric data, results of segmentation and parcellation processes were analyzed to compute the between-group effect for every regional GMV, WMV, SA, and CT characteristic, using total brain volume as covariates. Multiple comparisons were controlled using the False Discovery Rate (FDR; Benjamini and Hochberg, 2000). These analyses included 84 regional GMVs (including 16 subcortical regions), 73 WMVs (including 5 corpus callosum regions), 68 SAs and 68 CTs. The FDR threshold was applied separately to the results for each type of analysis – GMV, WMV, SA and CT. The results were considered statistically significant if they passed the FDR threshold of q < 0.05 for multiple comparisons. In addition, we performed post hoc region-of-interest (ROI) analyses on Freesurfer-defined anatomical areas corresponding to brain regions where significant VBM results were obtained, as well as the striatum (putamen, pallidum, caudate), which is often found to be structurally abnormal in ASD (Nickl-Jockschat et al. 2011; Langen et al., 2009).
RESULTS
Sample characteristics and whole brain measures
Groups did not differ significantly in age (p = 0.386), weight (p= 0.267), height (p = 0.087) or socioeconomic status (p = 0.716), but boys with 47,XYY obtained significantly lower scores on all cognitive measures of the Differential Ability Scales (all p ≤ 0.008). (Table 1)
Whole brain VBM results revealed significant differences in total GMV (p = 0.013), WMV (p < 0.001), and total brain volume (p = 0.003), with 47,XYY exhibiting larger volumes than controls. SBM analysis showed a similar pattern of volumetric differences with 47,XYY exhibiting larger GMV, WMV, and total brain volume relative to controls, but these differences did not reach significance (all p ≥ 0.098). Significantly increased total surface area was noted in the 47,XYY group in comparison to controls (p = 0.026), whereas mean cortical thickness was similar in both groups (2.75 (Controls) vs 2.73 (XYY); p=0.650) (Table 2).
Table 2.
Whole brain measures for voxel-based and surface based techniques.
| Control (XY) (n=10) |
47,XYY (n=9) |
p value |
|
|---|---|---|---|
| Voxel Based Measures | |||
| Total Grey Matter Volume | 741.87 (47.23) | 800.23 (46.20) | 0.013 |
| Total White Matter Volume | 501.50 (25.32) | 550.35 (21.72) | 0.001 |
| Total Brain Volume | 1243.41 (67.59) | 1350.58 (62.57) | 0.003 |
| Surface Based Measures† | |||
| Total Surface Area | 1862.05 (121.78)) | 1983.90 (92.75) | 0.026 |
| Mean Cortical Thickness | 2.75 (0.14) | 2.72 (0.12) | 0.650 |
Due to scan characteristics, one participant with 47,XYY could not go through the SBM pipeline and was excluded from theses analyses (n=9). Volumes are expressed in cm3, area in cm2, and thickness in mm. Significant results (p<0.05) are in indicated in bold.
Regional VBM results
Table 3 lists regions and structures for which significant differences (FWE-corrected) were observed in GM and WM volumes between 47,XYY and controls. Briefly, regional increases in GMV in 47,XYY were observed over the superior parietal regions bilaterally, extending from the postcentral gyri and encompassing part of the precuneus regions. In the right hemisphere, boys with 47,XYY also displayed increased GMV relative to controls in the superior portion of the occipital lobe and the cuneus. Conversely, decreased GMV in 47,XYY participants was observed in the right hemisphere, covering the insular cortex, the inferior frontal gyrus and the superior temporal cortex. GMV in the right putamen and lentiform nucleus was also decreased in 47,XYY in comparison to controls (Figure 1A). Regarding WMV, extensive reductions were observed bilaterally in the frontal lobes of boys with 47,XYY. These regions include the bilateral anterior thalamic radiation, uncinate fasciculus, inferior fronto-occipital fasciculus, cingulum, corticospinal tract and the body of the corpus callosum. In the right hemisphere, reductions in WMV were also present in the forceps minor and the superior longitudinal fasciculus of boys with 47,XYY. An increase in WMV was seen in the 47,XYY group in superior parietal cortex bilaterally (Figure 1B).
Table 3.
Significant clusters for VBM analysis regarding grey and white matter volume differences between groups.
| Cluster number | FWE Cluster P value |
No. Voxels |
Centroid |
Regions, structures, or WM tracts included in cluster (nearest GM for undetermined WM) |
Broadman Areas included in cluster (nearest for WM) |
||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Grey Matter | |||||||
| XY > XYY | |||||||
| 1 | 0.020 | 4003 | 43 | 0 | −1 | R Insula, R Putamen, R Inferior Frontal Gyrus, R lentiform nucleus, R claustrum, R Superior Temporal | 13, 45, 47 |
| XY < XYY | |||||||
| 1 | <0.001 | 15 566 | 21 | −75 | 36 | L and R precuneus, L and R Superior parietal, L and R post central, R cuneus, R Occipital | 2, 3, 5, 7, 19, 40 |
| White Matter | |||||||
| XY > XYY | |||||||
| 1 | 0.001 | 8 279 | −24 | 13 | −6 | L uncinate fasciculus, Inferior fronto-occipital fasciculus, anterior thalamic radiation, cingulum (cingulate gyrus), Body of corpus callosum, Corticospinal tract (WM beneath Superior frontal gyrus, paracingulate, SMA) | 6, 9, 24, 32, 47 |
| 2 | 0.007 | 4 678 | 27 | 20 | −1 | R forcep minor, R uncinate fasciculus, R Inferior fronto-occipital fasciculus, R Superior Longitudinal Fasciculus, R anterior thalamic radiation, R cingulum (cingulate gyrus), Corticospinal tract | 6, 13, 32, 24 |
| XY < XYY | |||||||
| 1 | 0.016 | 3 573 | 24 | −44 | 48 | (R Superior parietal) | 5, 7 |
| 2 | 0.040 | 2 514 | −27 | −44 | 50 | (L Superior parietal) | 5, 7 |
FWE: Family-wise error corrected; WM: white matter; GM: Grey matter.
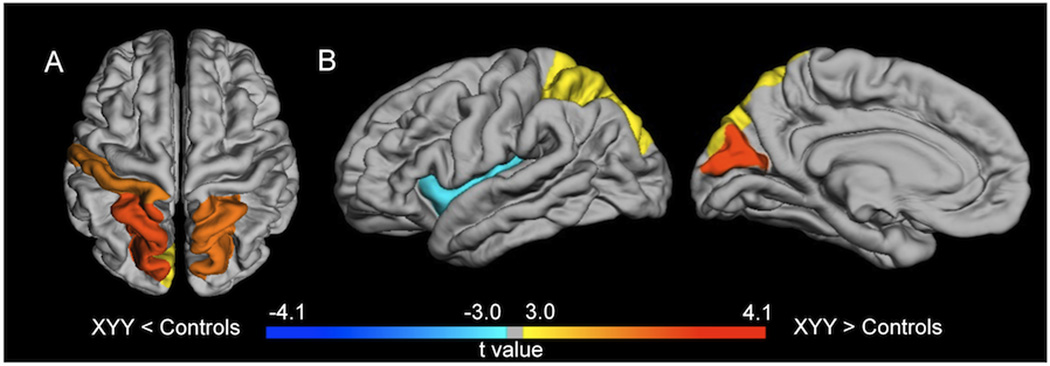
Figure 1. Results of Voxel-Based Morphometry Analysis.

(A) Significant results of VBM analysis for grey matter volume (GMV) difference between groups. Cold colors show regions of decreased GMV in XYY individuals compared to controls (XY), while warm colors show regions of increased GMV in boys with XYY compared to healthy participants. (B) Significant VBM results for white matter volume (WMV) difference between groups. Cold colors show regions of reduced WMV in boys with XYY compared to controls (XY), while warm colors show regions of increased WMV in XYY compared to controls.
Regional SBM and subcortical results
SBM analyses did not reveal the presence of significant differences (FDR corrected) in cortical thickness or GMV. However, significant alterations in WMV and surface area were observed in boys with 47,XYY individuals, including increased WMV relative to controls in bilateral superior parietal regions, right pericalcarine region, and in the cuneus and postcentral regions of the left hemisphere. Boys with 47,XYY also presented with increased surface area in the insula, cuneus and superior parietal regions of the left hemisphere (Table 4, Figure 2).
Table 4. SBM Results: Whole Brain Analysis.
Significant results for whole-brain SBM analysis.
| Measures and Regions | P value | FDR p value |
|---|---|---|
| XYY > XY | ||
| WM Volume | ||
| L Cuneus | 0.004 | 0.044 |
| L Postcentral | ≤ 0.001 | 0.016 |
| L Superior parietal | ≤ 0.001 | 0.016 |
| R Pericalcarine | ≤ 0.001 | 0.003 |
| R Superior parietal | ≤ 0.001 | 0.032 |
| Surface Area | ||
| L Cuneus | ≤ 0.001 | 0.018 |
| L Superior parietal | 0.004 | 0.041 |
| Subcortical | ||
| L Lateral Ventricule | ≤ 0.001 | 0.007 |
| R Lateral Ventricule | ≤ 0.001 | 0.007 |
| XY > XYY | ||
| Surface Area | ||
| L Insula | 0.004 | 0.041 |
| Subcortical | ||
| Central Corpus Callosum | ≤ 0.001 | 0.009 |
Columns show, respectively, region or structure, original p value, and FDR corrected (p) value.
Figure 2.
Regions showing significant differences at a whole brain level (FDR corrected) between groups regarding (A) white matter volume and (B) surface area. Cold colors show regions of reduced WMV/surface area in boys with XYY compared to controls (XY), while warm colors show regions of increased WMV/surface in XYY compared to controls. Color bar shows t value.
Post hoc
ROI analyses based on SBM results using a less stringent threshold (p < 0.05) supported the existence of clinically meaningful differences between controls and participants with 47,XYY. Notably, boys with 47,XYY had a reduction in GMV in insular regions bilaterally, which was also accompanied by a reduction in surface area in the left hemisphere. Increased WMV was seen bilaterally in the postcentral and superior parietal regions in 47,XYY, and surface area was also significantly increased for these regions in the left hemisphere. WMV was larger in the left superior temporal and right lateral occipital regions. Cortical thinning was observed in the left superior parietal and lateral occipital regions, while increases in surface area were present bilaterally in lateral occipital regions in boys with 47,XYY. This group also presented larger pallidum in the right hemisphere in comparison to controls (Table 5).
Table 5.
Results of region of interest (ROI) analysis.
| Regions of interest (ROIs) |
Left Hemisphere |
Right Hemisphere |
||||||
|---|---|---|---|---|---|---|---|---|
| GMV | WMV | CT | SA | GMV | WMV | CT | SA | |
| Cortical regions | ||||||||
| Insula | 0.004 (−) | 0.155 | 0.951 | 0.004 (−) | 0.016 (−) | 0.074 | 0.726 | 0.130 |
| Lateral occipital | 0.120 | 0.170 | 0.027 (−) | 0.020 (+) | 0.777 | 0.015 (+) | 0.320 | 0.048 (+) |
| Precuneus | 0.533 | 0.262 | 0.153 | 0.400 | 0.676 | 0.957 | 0.355 | 0.790 |
| Postcentral | 0.020 (+) | 0.001 (+) | 0.583 | 0.015 (+) | 0.408 | 0.033 (+) | 0.995 | 0.210 |
| Superior Parietal | 0.107 | 0.001 (+) | 0.023 (−) | 0.004 (+) | 0.138 | 0.002 (+) | 0.165 | 0.002 (+) |
| Superior Temporal | 0.400 | 0.044 (+) | 0.767 | 0.070 | 0.479 | 0.712 | 0.549 | 0.620 |
| Subcortical | ||||||||
| Caudate | 0.476 | − | − | − | 0.109 | − | − | − |
| Pallidum | 0.074 | − | − | − | 0.021 (+) | − | − | − |
| Putamen | 0.202 | − | − | − | 0.199 | − | − | − |
Numbers represent p values, with significant between group differences (p< 0.05) in bold. Plus (+) and minus (−) signs indicated respectively and increase and decrease in the variable of interest in the XYY group compared to healthy individuals.
DISCUSSION
In this study, we quantify for the first time brain modifications associated with the presence of a supernumerary Y chromosome in children using complementary image processing strategies. VBM analyses showed that boys with 47,XYY had prominent differences from controls with lesser WM in the frontal region combined with an increase in GM in the right insula, whereas increased WM and reduced GM were observed in the superior parietal, post-central and occipital regions. Whole brain SBM analysis largely corroborated these observations, while complementary post hoc ROI analyses further showed the existence of abnormal GMV, WMV, surface areas and cortical thickness in multiple brain regions of boys with 47,XYY.
Our regional results contrast sharply with the only previous imaging study using volumetric analyses of cortical and subcortical structures in 47,XYY, where no significant differences from healthy controls were found (Warwick et al., 1999). Many factors may have contributed to this discrepancy. Most notably, the study by Warwick and colleagues was conducted in adults using manual tracing, which encompassed large cortical areas. Here, both VBM and SBM techniques were used on a homogenous cohort of boys with 47,XYY, revealing regional differences that might have been missed using manual tracing approaches. However, our observations of increased total GM and WM are in agreement with previous work using a subset of the present sample (Bryant et al., 2012), and agree with observations of enlarged head circumference in individuals with 47,XYY (Geerts et al., 2003; Nicolson et al., 1998). Interestingly, increased brain volume is one of the few consistent findings in ASD neuroimaging research (Courchesne, 2001; Freitag et al., 2009; Redcay and Courchesne, 2005), and enlarged head circumference has been linked with delayed speech acquisition and higher ADI-R score in the ASD population (Lainhart et al., 2006). Considering that both language difficulties and ASD are frequent in 47,XYY populations (Bishop et al., 2011; Ross et al., 2012), it is possible that the enlarged brain volume associated with the presence of supernumerary Y chromosome constitutes a mediating risk factor for ASD symptoms and associated speech deficits. While we can only speculate as to the precise genetic basis of speech impairments in XYY, a likely genetic mechanism underlying these difficulties would be the overexpression of genes either in homologous regions on the X and Y chromosomes, or alternatively of male-specific genes exclusively located on the Y chromosome. In that regard, it is interesting to note that recent work has identified a potential candidate gene, protocadherin11, with copies on both sex chromosomes, which is linked to neural structures involved in language processing (Priddle et al., 2012). Also, the Y gene neuroligin 4Y (NLGN4Y) is involved in synaptic function and is therefore a compelling XYY speech and language candidate (Bishop et al., 2011). It is plausible that in XYY syndrome, overexpression of these genes in brain regions crucial for language acquisition could detrimentally impact speech acquisition and performance.
With regard to other sex chromosome aneuploidies, it is interesting to note that our findings are both highly similar to what is observed in individuals with Klinefelter syndrome (47,XXY), and strikingly dissimilar to VBM and SBM findings in Turner syndrome (45,XO). In fact, the structural changes seen in XYY are almost the exact opposite to what is observed in females with Turner syndrome. Indeed, boys with Klinefelter syndrome present a increase in bilateral GMV and left WMV in superior parietal regions, while simultaneously showing a extensive reduction of WMV in the frontal regions (Bryant et al., 2011). Conversely, girls with Turner syndrome show bilateral reduction in GMV and WMV in parietal regions, combined with an increase of GMV in the insular cortex (Marzelli et al., 2011; Lepage et al., 2012). These patterns of similarities and dissimilarities suggest a general dosage effect of a supernumerary sex chromosome on brain structure and development. Indeed, behavioral (Ross et al., 2009; 2012) and neuroimaging evidence (Bryant et al., 2012) point to similar patterns of abnormalities between 47,XYY and Klinefelter syndromes. It is plausible that these similarities come from shared gene-dosage abnormalities, as both X and Y chromosomes have identical genes in pseudoautosomal regions (Ross et al., 2009). Nonetheless, males with XYY and XXY syndromes present some notable differences in cognitive abilities; 47,XYY are more severely impaired in the language domain and are at greater risk to develop ASD (Ross et al., 2012), while individuals with Klinefelter syndrome have more difficulties in visuospatial and motor functions (Ross et al., 2009). These differences are informative as to the precise impact of an extra X versus Y chromosome on the brain, but potential differences in brain anatomy still remain to be investigated through a direct comparison between these two disorders.
It is interesting to note that many differences between healthy male controls and boys with 47,XYY were found in brain regions critically involved in cognitive domains of known difficulties in 47,XYY. Of particular interest are VBM analyses indicating aberrant WM volumes in prefrontal-striatal areas (WM located between prefrontal cortex and striatum), as the striatum plays a role in language, executive and motor functions (Robbins, 2007), which are often impaired in XYY (Ross et al., 2009). Furthermore, reduction in GM and WM volumes respectively seen in the insula and uncinate fasciculus could also be linked with expressive language difficulties present in 47,XYY, as the uncinate fasciculus is involved in naming (Papagno et al., 2011), and the anterior insula is part of the articulatory network (Dronkers, 1996; Hickok, 2012). Abnormal development of these white matter tracts, namely in the frontal regions, could also be associated with difficulties in behavior regulation and executive functions, similarly to what is seen in ADHD (Liston et al., 2011). Finally, WM, GMV, surface area and cortical thickness abnormalities observed in the posterior parietal cortex could negatively impact attention, sensorimotor processing and integration of visual and auditory constructs (Arnsten & Rubia, 2012; Cohen et al., 2005; Iacoboni, 2006). Taken together, it is possible that the cognitive profile resulting from these brain alterations could contribute to the increased risk for a diagnosis of autism spectrum disorder (Ross et al., 2012) and the relatively decreased socioeconomic outcome (Stochholm et al., 2012) reported in the XYY population.
Limitations of the present study include the small sample size, which did not allow us to control for IQ or age, and did not permit investigation of brain-behavior correlations. Considering this and to reduce the probability of a type I error, we elected to adopt relatively stringent statistical criteria (FDR) for SBM results combined with specific ROI analyses. However, it is possible that this approach did not allow detection of subtle differences that were present in other cortical regions, as they did not reach the statistical significance threshold established by FDR. For these reasons, our results may be viewed as preliminary. Moreover, considering the fact that XYY syndrome is rarely diagnosed (Stochholm et al., 2010), it is plausible that participants included in the present study might present a more severe phenotype than most individuals with the syndrome, possibly limiting the external validity of our results. To address these issues, future studies investigating the effects of a supernumerary chromosome on brain development and function should be performed with larger sample size. Ideally, these studies should directly compare individuals with XYY and Klinefelter syndrome in order to precisely delineate the relative contribution of genes located on the X and Y chromosomes on brain maturation and cognition in a context of sex chromosome trisomies.
CONCLUSION
Using both VBM and SBM, significant brain alterations are identifiable in young boys with XYY syndrome. Specifically, our results show extensive modifications in GMV in the insular and parietal regions and alterations in WMV in the frontal and superior parietal regions in children with XYY syndrome. Our analyses also reveal abnormal surface area and aberrant cortical thickness in some regions showing abnormal tissue volume. These findings lay the groundwork for further investigations of brain-behavior correlations, white matter connectivity, and delineating the contribution of genes located on the X and Y chromosomes to brain development and function during childhood.
Acknowledgements
This study was funded in part by Delaware Health Science Alliance Pilot Award (JR) and NIH R01HD049653 (AR). JFL is supported by a Postdoctoral Fellowship from the Canadian Institutes of Health Research (CIHR). DH is supported by an award from the National Institute of Mental Health (MH097120). The NIH did not participate in study design, data collection, data analysis, manuscript preparation, or publication decisions. We would like to acknowledge the support of the families involved in the study. We thank the Department of Radiology, Thomas Jefferson University for providing the MRI scanner time via a research support initiative to SL.
Footnotes
Disclosure statement: JFL, DH, DR, SL, MR and MM have nothing to declare.
REFERENCES
- Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2012;51:356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav. 2000;25:60–83. [Google Scholar]
- Bishop DV, Jacobs PA, Lachlan K, Wellesley D, Barnicoat A, Boyd PA, Fryer A, Middlemiss P, Smithson S, Metcalfe K, Shears D, Leggett V, Nation K, Scerif G. Autism, language and communication in children with sex chromosome trisomies. Arch Dis Child. 2011;96:954–959. doi: 10.1136/adc.2009.179747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Hoeft F, Lai S, Lackey J, Roeltgen D, Ross J, Reiss AL. Neuroanatomical phenotype of Klinefelter syndrome in childhood: a voxel-based morphometry study. J Neurosci. 2011;31:6654–6660. doi: 10.1523/JNEUROSCI.5899-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Hoeft F, Lai S, Lackey J, Roeltgen D, Ross J, Reiss AL. Sex chromosomes and the brain: a study of neuroanatomy in XYY syndrome. Dev Med Child Neurol. 2012;54:1149–1156. doi: 10.1111/j.1469-8749.2012.04418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen YE, Russ BE, Gifford GW., 3rd Auditory processing in the posterior parietal cortex. Behav Cogn Neurosci Rev. 2005;4:218–231. doi: 10.1177/1534582305285861. [DOI] [PubMed] [Google Scholar]
- Cordeiro L, Tartaglia N, Roeltgen D, Ross J. Social deficits in male children and adolescents with sex chromosome aneuploidy: a comparison of XXY, XYY, and XXYY syndromes. Res Dev Disabil. 2012;33:1254–1263. doi: 10.1016/j.ridd.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag CM, Luders E, Hulst HE, Narr KL, Thompson PM, Toga AW, Krick C, Konrad C. Total brain volume and corpus callosum size in medication-naïve adolescents and young adults with autism spectrum disorder. Biol Psychiatry. 2009;66:316–319. doi: 10.1016/j.biopsych.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts M, Steyaert J, Fryns JP. The XYY syndrome: a follow-up study on 38 boys. Genet Couns. 2003;14:267–279. [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. NeuroImage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Hickok G. Computational neuroanatomy of speech production. Nat Rev Neurosci. 2012;13:135–145. doi: 10.1038/nrn3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins CD, Swerdlow AJ, Schoemaker MJ, Wright AF, Jacobs PA On behalf of the UK clinical cytogenetics group. Mortality and cancer incidence in males with Y polysomy in Britain: a cohort study. Hum Genet. 2007;121:691–696. doi: 10.1007/s00439-007-0365-8. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Visuo-motor integration and control in the human posterior parietal cortex: evidence from TMS and fMRI. Neuropsychologia. 2006;44:2691–2699. doi: 10.1016/j.neuropsychologia.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, Deutsch CK, Dunn M, Estes A, Tager-Flusberg H, Folstein S, Hepburn S, Hyman S, McMahon W, Minshew N, Munson J, Osann K, Ozonoff S, Rodier P, Rogers S, Sigman M, Spence MA, Stodgell CJ, Volkmar F. Head circumference and height in autism: a study by the Collaborative Program of Excellence in Autism. Am J Med Genet A. 2006;140:2257–2274. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen M, Schnack HG, Nederveen H, Bos D, Lahuis BE, de Jonge MV, van Engeland H, Durston S. Changes in the developmental trajectories of striatum in autism. Biol Psychiatry. 2009;66:327–333. doi: 10.1016/j.biopsych.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Leggett V, Jacobs P, Nation K, Scerif G, Bishop DV. Neurocognitive outcomes of individuals with a sex chromosome trisomy: XXX, XYY, or XXY: a systematic review. Dev Med Child Neurol. 2010;52:119–129. doi: 10.1111/j.1469-8749.2009.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage JF, Mazaika PK, Hong DS, Raman M, Reiss AL. Cortical Brain Morphology in Young, Estrogen-Naive, and Adolescent, Estrogen-Treated Girls with Turner Syndrome. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs195. [Epub ahead of print] PMID:22806268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden MG, Bender BG, Robinson A. Genetic counseling for sex chromosome abnormalities. Am J Med Genet. 2002;110:3–10. doi: 10.1002/ajmg.10391. [DOI] [PubMed] [Google Scholar]
- Liston C, Malter Cohen M, Teslovich T, Levenson D, Casey BJ. Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: pathway to disease or pathological end point? Biol Psychiatry. 2011;69:1168–1177. doi: 10.1016/j.biopsych.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Marzelli MJ, Hoeft F, Hong DS, Reiss AL. Neuroanatomical Spatial Patterns in Turner Syndrome. Neuroimage. 2011;55:439–447. doi: 10.1016/j.neuroimage.2010.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl-Jockschat T, Habel U, Michel TM, Manning J, Laird AR, Fox PT, Schneider F, Eickhoff SB. Brain structure anomalies in autism spectrum disorder--a meta-analysis of VBM studies using anatomic likelihood estimation. Hum Brain Mapp. 2012;33:1470–1489. doi: 10.1002/hbm.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson R, Bhalerao S, Sloman L. 47,XYY karyotypes and pervasive developmental disorders. Can J Psychiatry. 1998;43:619–622. doi: 10.1177/070674379804300611. [DOI] [PubMed] [Google Scholar]
- Ottesen AM, Aksglaede L, Garn I, Tartaglia N, Tassone F, Gravholt CH, Bojesen A, Sørensen K, Jørgensen N, Rajpert-De Meyts E, Gerdes T, Lind AM, Kjaergaard S, Juul A. Increased number of sex chromosomes affects height in a nonlinear fashion: a study of 305 patients with sex chromosome aneuploidy. Am J Med Genet A. 2010;152A:1206–1212. doi: 10.1002/ajmg.a.33334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Alberman E, Scott C, Jacobs P. Is the prevalence of Klinefelter syndrome increasing? Eur J Hum Genet. 2008;16:163–170. doi: 10.1038/sj.ejhg.5201956. [DOI] [PubMed] [Google Scholar]
- Papagno C. Naming and the role of the uncinate fasciculus in language function. Curr Neurol Neurosci Rep. 2011;11:553–559. doi: 10.1007/s11910-011-0219-6. [DOI] [PubMed] [Google Scholar]
- Priddle TH, Crow TJ. Protocadherin 11X/Y a human-specific gene pair: an immunohistochemical survey of fetal and adult brains. Cereb Cortex. 2012;23:1933–1941. doi: 10.1093/cercor/bhs181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe SG, Pan H, McKie M. Growth during puberty in the XYY boy. Ann Hum Biol. 1992;19:579–587. doi: 10.1080/03014469200002392. [DOI] [PubMed] [Google Scholar]
- Ratcliffe S. Long-term outcome in children of sex chromosome abnormalities. Arch Dis Child. 1999;80:192–195. doi: 10.1136/adc.80.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psychiatry. 2005;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JL, Zeger MP, Kushner H, Zinn AR, Roeltgen DP. An extra X or Y chromosome: contrasting the cognitive and motor phenotypes in childhood in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Dev Disabil Res Rev. 2009;15:309–317. doi: 10.1002/ddrr.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JL, Roeltgen DP, Kushner H, Zinn AR, Reiss A, Bardsley MZ, McCauley E, Tartaglia N. Behavioral and social phenotypes in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Pediatrics. 2012;129:769–778. doi: 10.1542/peds.2011-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Greve DN, Pacheco JL, Quinn BT, Helmer KG, Buckner RL, Fischl B. Regional white matter volume differences in nondemented aging and Alzheimer's disease. Neuroimage. 2009;44:1247–1258. doi: 10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ségonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Stochholm K, Juul S, Gravholt CH. Diagnosis and mortality in 47,XYY persons: a registry study. Orphanet J Rare Dis. 2010;29:5–15. doi: 10.1186/1750-1172-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stochholm K, Juul S, Gravholt CH. Socio-Economic Factors Affect Mortality in 47,XYY Syndrome-A Comparison With the Background Population and Klinefelter Syndrome. Am J Med Genet Part A. 2012;158A:2421–2429. doi: 10.1002/ajmg.a.35539. [DOI] [PubMed] [Google Scholar]
- Warwick MM, Doody GA, Lawrie SM, Kestelman JN, Best JJ, Johnstone EC. Volumetric magnetic resonance imaging study of the brain in subjects with sex chromosome aneuploidies. J Neurol Neurosurg Psychiatry. 1999;66:628–632. doi: 10.1136/jnnp.66.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]