Abstract
Purpose
Little is known about the clinical features of advanced gastric cancer (AGC) combined with disseminated intravascular coagulation (DIC). The main objective of this study was to determine the clinical outcome of patients with AGC complicated by DIC.
Materials and Methods
We conducted a retrospective review of 68 AGC patients diagnosed with DIC at four tertiary medical centers between January 1995 and June 2010.
Results
Sixty eight patients were included. The median age was 55 years (range, 25 to 78 years). Nineteen patients received chemotherapy, whereas 49 patients received only best supportive care (BSC). The median overall survival (OS) of the 68 patients was 16 days (95% confidence interval [CI], 11 to 21 days). Significantly prolonged OS was observed in the chemotherapy group, with a median survival of 61 days compared to 9 days in the BSC group (p<0.001, log-rank test). Age and previous chemotherapy were another significant factors that were associated with OS in univariate analysis. In multivariate analysis, age (≥65 vs. <65; hazard ratio [HR], 0.38; 95% CI, 0.18 to 0.78; p<0.001), chemotherapy (BSC vs. chemotherapy; HR 0.31; 95% CI, 0.15 to 0.63; p<0.001), and previous chemotherapy (yes or no; HR, 0.49; 95% CI, 0.25 to 0.98; p<0.045) were consistently independent prognostic factors that impacted OS.
Conclusion
Our study showed that patients with AGC complicated by DIC had very poor OS, and suggested that chemotherapy might improve OS of these patients.
Keywords: Stomach neoplasms, Disseminated intravascular coagulation, Drug therapy
Introduction
Gastric cancer ranks fourth in cancer incidence in men (640,600 new cases per year) and fifth in women (349,000 new cases per year) [1]. Advanced gastric cancer (AGC) is rarely associated with disseminated intravascular coagulation (DIC); the incidence rate of DIC is 1.6% in AGC patients [2]. Due to its rarity, little is known about the clinical features of patients with AGC and DIC.
DIC is a clinical condition characterized by widespread activation of the coagulation system. It can be caused by various precipitating conditions; trauma, sepsis, toxin, and solid tumors have all been reported to induce DIC complications [3]. DIC from cancer generally has a less fulminant course and follows a more gradual and chronic pattern than DIC complicating sepsis or trauma. However, chronic activation of the coagulation system eventually leads to exhaustion of platelets and coagulation factors and can result in development of a clinical hemorrhagic problem [4].
The prognosis of symptomatic DIC associated with AGC is known to be very poor [5]. Treatment of underlying malignancy is a key measure for restoration of normal coagulation. If the malignancy is improved, then the DIC will usually diminish with time. However, patients often have a poor performance status (PS) accompanied by hematologic abnormalities such as thrombocytopenia, leucopenia, and anemia in AGC associated with DIC. These conditions cause physicians to be hesitant in starting chemotherapy and cause a dilemma in treatment of AGC complicated by DIC. It also may be misinterpreted as a simple hematologic abnormality or long-lasting hematologic toxicity caused by previous chemotherapy in the early stage, resulting in delayed treatment and subsequent aggravation of prognosis. Therefore, knowledge of the disease course is imperative.
Although anecdotal cases and several small studies indicating that the prognosis of AGC associated with DIC might improve with 5-fluorouracil (5-FU) based chemotherapy have been reported [2,6-10], the clinical data are far from sufficient for establishment of a standardized treatment strategy. The main objective of this multicenter retrospective study was to determine the clinical outcome of AGC complicated by DIC. The secondary aim was to identify prognostic factors having an impact on survival.
Materials and Methods
1. Patient eligibility and assessments
Patients were eligible for inclusion in this retrospective study if they had histologically confirmed gastric cancer with at least one metastatic site and if they met the DIC criteria of the Japanese Association for Acute Medicine (JAAM) [11]. DIC was diagnosed in patients with ≥4 points, according to the JAAM criteria [11,12].
Patients were identified at four tertiary medical centers between December 1994 and April 2010. Demographic information, including age, sex, PS, and clinical data, including clinicopathologic features and laboratory findings were collected. Laboratory information included a complete blood count, chemistry panel, carcinoembryonic antigen, and DIC profiles. Radiologic images included chest radiographs, and abdominal and/or chest computed tomography (CT) containing measurable lesions were considered baseline images if they were obtained less than four weeks before starting chemotherapy. Medical records and abdominal and/or chest CTs were reviewed for evaluation of the clinical course and outcomes of chemotherapy. Responses to chemotherapy were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) based on changes in target lesions and non-target lesions [13].
2. Chemotherapy regimens
All eligible patients received one of the following chemotherapy regimens: A institute-FOLFOX: oxaliplatin 85 mg/m2 (day [D] 1), leucovorin 30 mg (D1, 2), 5-FU 1,000 mg/m2 (D1, 2) every two weeks, DCF: docetaxel 70 mg/m2 (D1), cisplatin 40 mg/m2 (D2, 3), 5-FU 1,200 mg/m2 (D1, 2, 3) every three weeks, FOLFIRI: irinotecan 180 mg/m2 (D1), leucovorin 30 mg (D1, 2), 5-FU 1,000 mg/m2 (D1, 2) every two weeks, docetaxel: docetaxel 30 mg/m2 (D1, 8) every three weeks, DP: docetaxel 75 mg/m2 (D1), cisplatin 60 mg/m2 (D1) every three weeks; B institute-DP: docetaxel 75 mg/m2 (D1), cisplatin 75 mg/m2 (D1) every three weeks; C institute-TP: paclitaxel 175 mg/m2 (D1), cisplatin 80 mg/m2 (D1) every three weeks, FOLFIRI: irinotecan 150 mg/m2 (D1, leucovorin 20 mg/m2 (D1, 2), 5-FU 400 mg/m2 intravenous (IV) push (D1, 2), 5-FU 600 mg/m2 over 22 hours (D1, 2) every two weeks; D institute-FPL: leucovorin 20 mg/m2 IV push (D1-D5), 5-FU 800 mg/m2 (D1-D5), cisplatin 75 mg/m2 (D1), every three weeks, docetaxel: docetaxel 75 mg/m2 (D1) every three weeks, FL: leucovorin 20 mg/m2 IV push (D1-D5), 5-FU 800 mg/m2 (D1-D5) every three weeks, DP: docetaxel 75 mg/m2 (D1), cisplatin 75 mg/m2 (D1) every three weeks.
Standard pre-medications were administered appropriately prior to treatment depending on the protocol of the specific institution. Relative dose intensity (RDI) was defined as the actual chemotherapeutic dose administered divided by the total planned dose in a given period.
3. Statistical analysis
Baseline demographics, including medians, ranges, and frequencies were summarized using descriptive statistics. Patients were divided into a chemotherapy group and a best supportive care (BSC) group. A chi-squared test or Fisher's exact test were used as appropriate for comparison of categorical variables (sex, site, metastatic site, PS), and a Wilcoxon rank sum test was used for comparison of quantitative variables (age) between the two groups.
Survival was calculated according to the Kaplan-Meier method and the survival curves were compared by univariate analysis with the long-rank test. Cox's proportional hazard model was used for multivariate survival analyses. Overall survival (OS) was defined as the time from the date of first chemotherapy administration to the date of death. Statistical significance was defined as a p-value<0.05. All analyses were performed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL) or SAS ver. 9.2 (SAS Institute Inc., Cary, NC).
Results
1. Patient characteristics
A total of 68 patients were enrolled in this study. A description of the clinical characteristics of the patients is provided in Table 1. Nineteen patients (28%) underwent chemotherapy, while 49 patients (72%) received only BSC. Of the 19 patients who underwent chemotherapy, nine (47%) were treated with 5-FU-based chemotherapy and 10 (53%) were treated with taxane-based chemotherapy. The median age of patients was 55 years (range, 25 to 78 years). Patients who received chemotherapy were significantly younger than patients in the BSC group (51 years vs. 58 years, p=0.03). More frequent bone metastasis (79% vs. 29%, p<0.01), previous chemotherapy (63% vs. 18%, p<0.01) and prolonged prothrombin time (61% vs. 26%, p<0.01) were observed in the chemotherapy group. No significant difference in other factors, including sex, PS, baseline hemoglobin, platelet count, and lactate dehydrogenase level was observed between the two groups. The median follow-up duration was 0.5 months (range, 0.3 to 0.7 months).
Table 1.
Patient characteristics
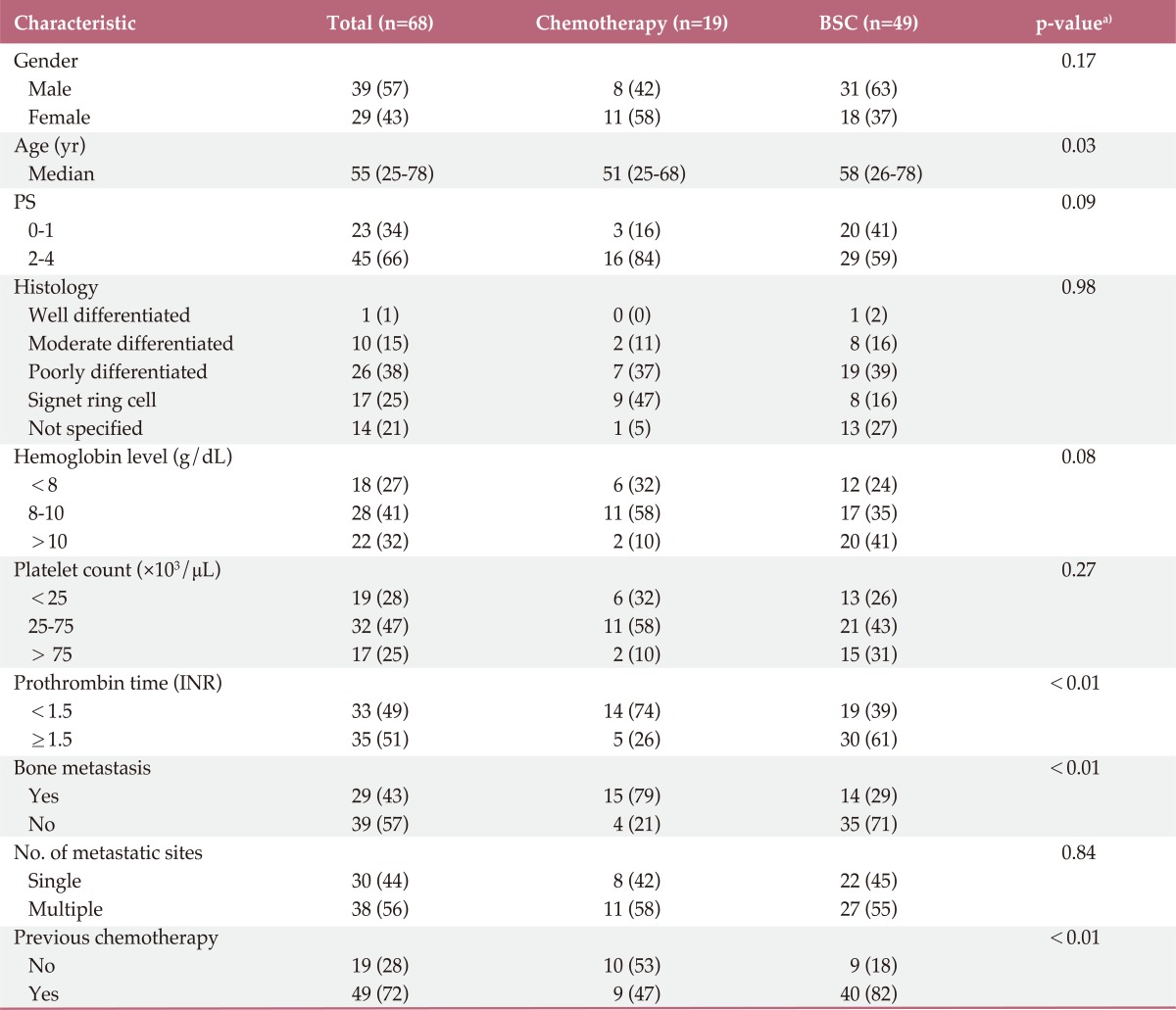
Values are presented as number (%). BSC, best supportive care; PS, performance status; INR, international normalized ratio. a)p-values are for comparison between BSC and chemotherapy.
2. Therapeutic outcomes and prognostic factors
A total of 47 cycles (median, 2 cycles) were delivered to patients in the chemotherapy group. The mean dose intensitiesand RDIs were 1,077.99 mg/m2/wk (range, 750 to 1,333.3 mg/m2/wk) and 95.8% for 5-FU-based regimens and 24.25 mg/m2/wk (range, 23.3 to 25 mg/m2/wk) and 100% for taxane-based regimens. Of the 19 chemotherapy patients, only seven could be assessed for their responses. Two patients (10.5%) had partial responses, one patient (5.2%) had stable disease, and four patients (21.0%) had progressive disease.
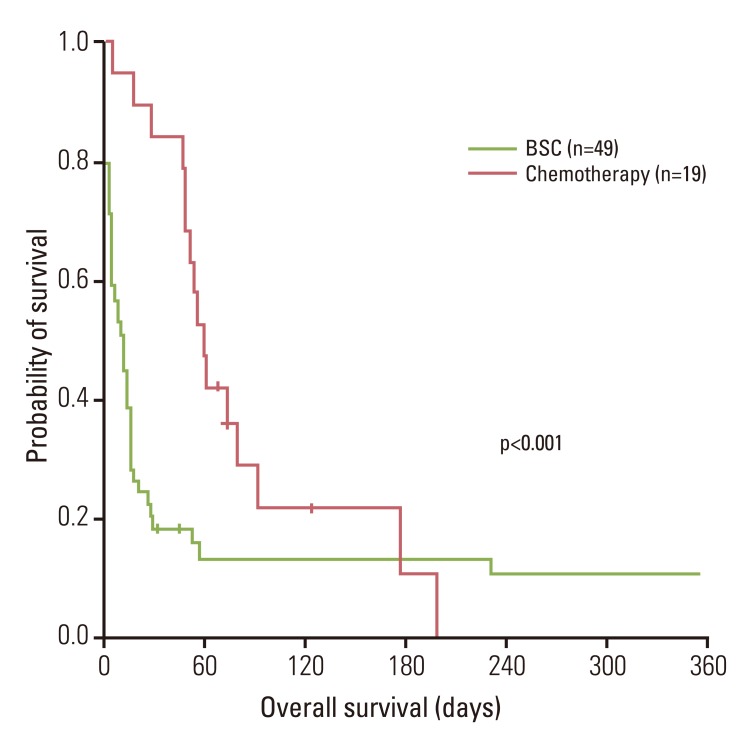
Among the patients who underwent chemotherapy, DIC-related symptoms showed improvement in eight patients (42%). The median OS was 16 days (95% CI, 11 to 21 days). Patients undergoing chemotherapy had a significantly longer OS (median, 61 days; 95% CI, 50 to 72 days; p<0.001) than BSC patients (median, 9 days; 95% CI, 6 to 16 days) (Fig. 1).
Fig. 1.
Overall survival. Patients undergoing chemotherapy had a significantly longer overall survival than best supportive care (BSC) patients (median, 61 days vs. 9 days; p<0.001).
According to univariate analysis, younger age (cut off value; 65 years, p<0.001), chemotherapy (p<0.001), and previous chemotherapy (p<0.001) showed an association with superior survival. However, no statistically significant differences were observed with regard to gender, PS, histology, prothrombin time, number of metastases, baseline hemoglobin, platelet count, fibrinogen, alkaline phosphatase, and chemotherapy regimen. On multivariate analysis, chemotherapy (p<0.001; hazard ratio [HR], 0.31), younger age (p<0.001; HR, 0.38) and previous chemotherapy (p<0.045; HR, 0.49) showed an independent association with a longer OS (Table 2).
Table 2.
Multivariate analysis for overall survival
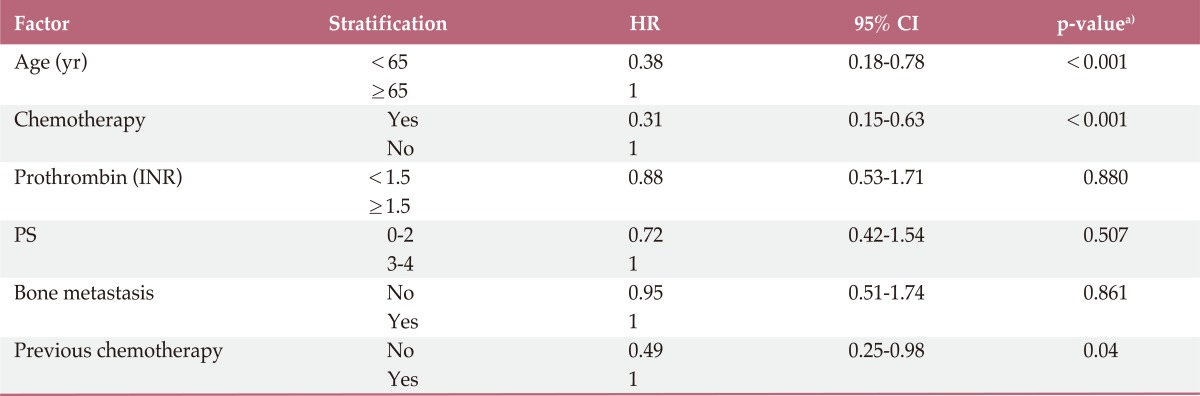
HR, hazard ratio; CI, confidence interval; INR, international normalized ratio; PS, performance status. a)Cox proportional hazard regression model.
Discussion
Our study included the largest number of patients with AGC complicated by DIC to date, and also compared clinical outcomes between a chemotherapy group and a BSC group. Although similar studies on these issues have been reported, those studies included a small number of cases or only evaluated treatment outcomes (Table 3).
Table 3.
Studies or case series on AGC with DIC
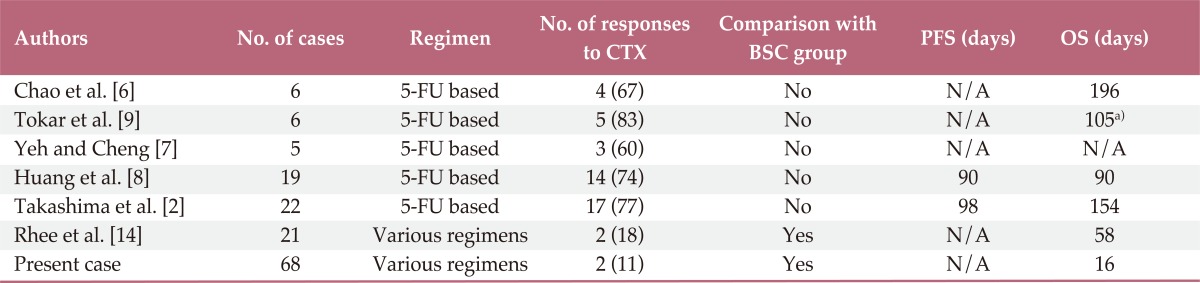
Values are presented as number (%). AGC, advanced gastric cancer; DIC, disseminated intravascular coagulation; CTX, chemotherapy; BSC, best supportive care; PFS, progression free survival; OS, overall survival; 5-FU, 5-fluorouracil; N/A, not available or not applicable. a)Survival was reported as mean value.
We found that patients who underwent chemotherapy had outcomes superior to those who received BSC. Our finding is consistent with the report by Rhee et al. [14], which favored chemotherapy in AGC patients with DIC. Until now, that was the only study comparing a chemotherapy group with a BSC group [14]. However, that study included only seven patients in the BSC group, which weakens the conclusion that chemotherapy provides superior outcomes to BSC in AGC with DIC. In our study, we included 49 patients in the BSC group, which strengthens our assertion that chemotherapy can prolong survival in patients with AGC and DIC. Other studies without BSC also reported higher responses to chemotherapeutic treatment, from 74% to 100% of cases, and favorable survival times, which supports the notion that chemotherapy could be beneficial to patients with AGC complicated by DIC [2,6-10]. In addition, although statistical significance was marginal (p=0.045), patients who had not undergone prior chemotherapy had better survival than those who had undergone previous chemotherapy.
In our current study, the median OS (16 days) was very poor. Compared with other studies, the poorest survival was observed in this study [2,6-10]. In this study, patients with BSC had a median OS of only nine days. As reported in previous studies, we assert that this short life expectancy reflects the aggressive nature of AGC with DIC [5]. However, in this study, selection bias may have contributed to the relatively poorer survival. The patient cohort was comprised of poor PS (proportion of PS 2-4 was 66%) and the majority of patients were in the BSC group (72%). Because other studies on AGC with DIC usually included only patients who underwent chemotherapy [2,6-10], this selection bias could have resulted in the difference in survival.
Biopsy reports showed that the majority (80%) of patient cohort in our study had signet ring cells (SRC) or poorly differentiated cells (PD) type and this result is similar to another studies [7,8,15]. Considering the fact that SRC and PD are not major histologic types of gastric cancer [16], this may be a notable finding. Conduct of future studies on the association between the histologic type of gastric cancer and DIC is warranted.
There are several limitations to this study. First, due to the inherent nature of retrospective studies and limited accessibility to all of the centers, we were unable to fully identify changes in the clinical manifestations of the disease or adverse effects of chemotherapy. The information collected on chemotherapy was not sufficient. Because patients were recruited regardless of treatment status in the trajectory of gastric cancer, the possibility exists that a high proportion of patients were receiving salvage chemotherapy at the time of their diagnosis with DIC, which may have contributed to the poor survival of our cohort. In addition, although cases were recruited from six tertiary cancer centers, the patient cohort was not sufficient to determine whether chemotherapy is really helpful for gastric cancer patients with DIC.
Conclusion
Findings of our study suggest that AGC patients with DIC could benefit from chemotherapy. Therefore, chemotherapy maybe considered among the first treatment options in AGC patients who also present with DIC.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Takashima A, Shirao K, Hirashima Y, Takahari D, Okita NT, Nakajima TE, et al. Sequential chemotherapy with methotrexate and 5-fluorouracil for chemotherapy-naive advanced gastric cancer with disseminated intravascular coagulation at initial diagnosis. J Cancer Res Clin Oncol. 2010;136:243–248. doi: 10.1007/s00432-009-0655-8. [DOI] [PubMed] [Google Scholar]
- 3.Pasquini E, Gianni L, Aitini E, Nicolini M, Fattori PP, Cavazzini G, et al. Acute disseminated intravascular coagulation syndrome in cancer patients. Oncology. 1995;52:505–508. doi: 10.1159/000227520. [DOI] [PubMed] [Google Scholar]
- 4.Levi M. Disseminated intravascular coagulation in cancer patients. Best Pract Res Clin Haematol. 2009;22:129–136. doi: 10.1016/j.beha.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Sallah S, Wan JY, Nguyen NP, Hanrahan LR, Sigounas G. Disseminated intravascular coagulation in solid tumors: clinical and pathologic study. Thromb Haemost. 2001;86:828–833. [PubMed] [Google Scholar]
- 6.Chao Y, Teng HC, Hung HC, King KL, Li CP, Chi KH, et al. Successful initial treatment with weekly etoposide, epirubicin, cisplatin, 5-fluorouracil and leucovorin chemotherapy in advanced gastric cancer patients with disseminated intravascular coagulation. Jpn J Clin Oncol. 2000;30:122–125. doi: 10.1093/jjco/hyd038. [DOI] [PubMed] [Google Scholar]
- 7.Yeh KH, Cheng AL. Gastric cancer associated with acute disseminated intravascular coagulation: successful initial treatment with weekly 24-hour infusion of high-dose 5-fluorouracil and leucovorin. Br J Haematol. 1998;100:769–772. doi: 10.1046/j.1365-2141.1998.00613.x. [DOI] [PubMed] [Google Scholar]
- 8.Huang TC, Yeh KH, Cheng AL, Hsu CH. Weekly 24-hour nfusional 5-fluorouracil as initial treatment for advanced gastric cancer with acute disseminated intravascular coagulation. Anticancer Res. 2008;28:1293–1297. [PubMed] [Google Scholar]
- 9.Tokar M, Bobilev D, Ariad S, Geffen DB. Disseminated intravascular coagulation at presentation of advanced gastric cancer. Isr Med Assoc J. 2006;8:853–855. [PubMed] [Google Scholar]
- 10.Kim SH, Lee GW, Go SI, Cho SH, Kim HJ, Kim HG, et al. A phase II study of irinotecan, continuous 5-fluorouracil, and leucovorin (FOLFIRI) combination chemotherapy for patients with recurrent or metastatic gastric cancer previously treated with a fluoropyrimidine-based regimen. Am J Clin Oncol. 2010;33:572–576. doi: 10.1097/COC.0b013e3181bead7b. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko T, Wada H. Diagnostic criteria and laboratory tests for disseminated intravascular coagulation. J Clin Exp Hematop. 2011;51:67–76. doi: 10.3960/jslrt.51.67. [DOI] [PubMed] [Google Scholar]
- 12.Gando S, Iba T, Eguchi Y, Ohtomo Y, Okamoto K, Koseki K, et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med. 2006;34:625–631. doi: 10.1097/01.ccm.0000202209.42491.38. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Rhee J, Han SW, Oh DY, Im SA, Kim TY, Bang YJ. Clinicopathologic features and clinical outcomes of gastric cancer that initially presents with disseminated intravascular coagulation: a retrospective study. J Gastroenterol Hepatol. 2010;25:1537–1542. doi: 10.1111/j.1440-1746.2010.06289.x. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi T, Sasaki T, Ibuka T, Imai K, Monma K, Sakaki N, et al. Sequential MTX and 5-FU therapy of gastric cancer with systemic bone metastasis and disseminated intravascular coagulation. Gan To Kagaku Ryoho. 1992;19:69–74. [PubMed] [Google Scholar]
- 16.Park JM, Jang YJ, Kim JH, Park SS, Park SH, Kim SJ, et al. Gastric cancer histology: clinicopathologic characteristics and prognostic value. J Surg Oncol. 2008;98:520–525. doi: 10.1002/jso.21150. [DOI] [PubMed] [Google Scholar]