Abstract
Purpose
The novel heat shock protein tumor necrosis factor receptor-associated protein 1 (TRAP1) is associated with multidrug resistance in colorectal cancer (CRC) cells in vitro. Excision repair cross-complementation group 1 (ERCC1) expression levels in tumor tissues also predict clinical outcomes in metastatic CRC patients receiving combination oxaliplatin and 5-fluorouracil treatment. We investigated whether TRAP1 and ERCC1 protein expression by immunohistochemistry predict clinical outcomes in CRC patients.
Materials and Methods
The study population consisted of 56 patients with metastatic CRC who received first-line oxaliplatin/5-fluorouracil therapy. Clinical response and overall survival (OS) by levels of the markers TRAP1 and ERCC1 were evaluated.
Results
The rates of TRAP1 and ERCC1 expression were 21% and 52%, respectively. Patients negative for ERCC1 expression showed a tendency to respond to chemotherapy (p=0.066). Median OS was significantly longer in patients negative for TRAP1 than those positive for TRAP1 (p=0.023). Patients negative for ERCC1 expression also had a better OS than those positive for ERCC1 (p=0.021). The median OS was 30.9 months for patients negative for TRAP1 and ERCC1 compared to 13.2 months for those positive for TRAP1 and/or positive for ERCC1 expression (p=0.006). The combination of TRAP1 and ERCC1 expression was significantly associated with the response to chemotherapy (p=0.046) and independently predicted median OS in multivariate analysis (hazard ratio, 2.98; 95% confidence interval, 1.18 to 7.49).
Conclusion
The present study demonstrates that the combination of TRAP1 and ERCC1 expression predicts the survival of metastatic CRC patients who were treated with oxaliplatin/5-fluorouracil.
Keywords: Colorectal neoplasms, ERCC1, Fluorouracil, Oxaliplatin, TRAP1
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in males and the second most commonly diagnosed in females, with over 1.2 million new cancer cases and 608,700 CRC-related deaths estimated to have occurred worldwide in 2008 [1]. The addition of oxaliplatin or irinotecan to 5-fluorouracil (5-FU) in systemic palliative chemotherapy improves survival to approximately 20 months in patients with metastatic CRC, and is therefore now indicated as a first-line treatment in these patients. Cytotoxic agents remain the backbone of chemotherapy for metastatic CRC, despite the introduction of newer targeted agents such as bevacizumab and cetuximab. It is important to find biomarkers that might enable the selection of those patients who would benefit most from cytotoxic agents.
Tumor necrosis factor receptor-associated protein 1 (TRAP1) is a mitochondrial heat shock protein homologous to heat shock protein 90 (Hsp90) [2]. TRAP1 functions with Hsp90 to maintain the membrane potential of mitochondria by antagonizing the pore forming properties of cyclophilin D, which induces apoptosis [3]. In addition, TRAP1 protects cancer cells from the oxidative stress and apoptosis induced by granzyme M and reactive oxygen species [4,5]. In vitro studies found that the expression of TRAP1 increases in CRC cells that are resistant to oxaliplatin, irinotecan, and 5-FU [6]. However, clinical studies of the role of TRAP1 expression in metastatic CRC patients are lacking.
The excision repair cross-complementation group 1 (ERCC1) protein of the nucleotide excision repair (NER) pathway modulates the cytotoxic effect of oxaliplatin by contributing to oxaliplatin-DNA adducts repair [7]. Shirota et al. [8] reported that ERCC1 mRNA levels are inversely associated with survival and response to 5-FU and oxaliplatin combination chemotherapy in metastatic CRC patients. More recently, immunohistochemical staining by antibodies specific to ERCC1 showed that positive ERCC1 expression predicted poor clinical outcomes in patients with advanced CRC treated with oxaliplatin.
Thymidylate synthase (TS) is the primary cytotoxicity-inducing target of 5-FU. TS is a possible predictive marker for response to 5-FU-based chemotherapy and a potential prognostic marker in metastatic CRC patients. However, the existing evidence is not adequate to justify the use of TS expression as a predictor or prognostic marker in clinical practice.
We conducted this study to evaluate whether the protein expression of TRAP1, ERCC1, and TS as detected by immunohistochemistry (IHC) can be used to predict clinical outcomes in patients with metastatic CRC treated with first-line oxaliplatin/5-FU.
Materials and Methods
1. Patient selection
Sixty-eight patients with histologically confirmed colorectal adenocarcinoma diagnosed from January 2006 through December 2009 were retrospectively selected at two centers: Kyung Hee University Hospital at Gangdongand Kyung Hee University Medical Center, Republic of Korea. The eligibility criteria were as follows: age 18 years or older; Eastern Cooperative Oncology Group (ECOG) performance scale 0-3 with adequate renal, hepatic, and cardiac function; metastatic disease at initial diagnosis or recurrent disease; no prior palliative chemotherapy; and receipt of first-line oxaliplatin/5-FU chemotherapy for palliation. Patients with no measurable lesions or whose tumor tissues were not available were excluded. Based on these criteria, three patients were excluded for lack of measurable lesions and nine patients were excluded for no available tumor tissue. Fifty-six patients remained eligible for analysis. Of the study patients, both primary and metastatic tumor tissues were available for 18 patients. Clinical data were retrieved from the medical records. This study was approved by the institutional review boards at both institutions (KMC IRB 0920-07, KHNMC IRB 2009-055).
2. Treatment protocol
Oxaliplatin (85 mg/m2) was administered intravenously on day 1 with leucovorin (200 mg/m2) followed by a 5-FU bolus (400 mg/m2) and a 5-FU infusion (600 mg/m2) on days 1 and 2 (FOLFOX-4) in a 2-week cycle. Oxaliplatin (130 mg/m2) was administered intravenously on day 1, then oral capecitabine (1,000 mg/m2) twice daily from the evening of day 1 to the morning of day 15 (XELOX), followed by a 7-day treatment-free interval in a 3-week cycle. Treatment continued until disease progression, unacceptable toxicity, scheduled treatment, or a joint patient/physician decision occurred.
3. Response and survival assessment
Objective tumor response was evaluated by computed tomography after every three cycles of chemotherapy. Based on the Response Evaluation Criteria in Solid Tumors version 1.0, complete response (CR) was defined as the complete disappearance of all target lesions; partial response (PR) was at least a 30% decrease in the sum of the longest diameter of the target lesions; progressive disease was defined as at least a 20% increase in the sum of the longest diameter of the target lesions; and stable disease indicated neither PR nor progressive disease. Overall survival (OS) was assessed as the time from the initiation of first-line chemotherapy to death or last follow-up. Patients who were alive at the last follow-up were censored at that time. Progression-free survival (PFS) was defined as the time from the start of treatment to the time of the first record of progression or to the date of death.
4. IHC for TRAP1, ERCC1, and TS
Immunohistochemical staining was performed using 4-µm-thick tissue sections from paraffin-embedded tissue blocks and a Bond Polymer Intense Detection System (VisionBioSystems, Melbourne, VIC, Australia) according to the manufacturer's instructions with minor modifications. Each formalin-fixed and paraffin-embedded tissue section was deparaffinized with Bond Dewax Solution (VisionBioSystems) and subjected to antigen retrieval using the Bond ER Solution (VisionBioSystems) at 100℃ for 30 minutes. The endogenous peroxidase was subsequently quenched by incubation with hydrogen peroxide for 5 minutes. The sections were then incubated for 15 minutes at room temperature with rabbit polyclonal anti-human TRAP1 (1:100, ab26135, Abcam, Cambridge, UK), mouse monoclonal anti-human ERCC1 (1:400, 8F1, NeoMarkers, Fremont, CA), and mouse monoclonal anti-human TS (1:50, TS106, Dako, Glostrup, Denmark) using a biotin-free polymeric horseradish peroxidase-linker antibody conjugate system in a Bond-max automatic slide stainer (VisionBioSystems) and visualized using a 3.3-diaminobenzidine solution (1 mM DAB, 50 mM Tris-HCl, pH 7.6, and 0.006% H2O2). The slides were counterstained with hematoxylin. A negative control was created by replacing a specific primary antibody with distilled water. Formalin-fixed, paraffin-embedded human kidney cells (epithelial cells of renal tubule) for TRAP1, endothelial cells from the human tonsil for ERCC1, and human colonic adenocarcinoma tissue for TS were used as positive controls.
5. Interpretation of immunohistochemical staining
Immunostaining was assessed in five high-powered fields at ×400 magnification. Immunoreactivity was evaluated semiquantitatively based on staining proportion and intensity. The stained tumor tissues were scored by an expert pathologist (G.Y.K) who was blinded to the patients' clinical data. The proportion of staining was scored on a scale from 0 to 4 as follows: score 4, >50% positive; score 3, 26% to 50% positive; score 2, 6% to 25% positive; score 1, 1% to 5%; score 0, 0%. In addition, the intensity of staining was scored from 0 to 3 (0, absent; 1, weak; 2, moderate; 3, strong). The immunoreactive score (IS) for each sample was determined by multiplying the two individual scores. Positive TRAP1 expression was arbitrarily defined as an IS of 12 with strong intensity and over 50% of cells stained (Fig. 1A and D). Positive ERCC1 and TS expression (Fig. 1B, E and 1C, F, respectively) was defined as an IS≥6 (median), while negative expression was considered IS<6 (median), according to a previous study [9].
Fig. 1.
Immunohistochemical staining of a tumor necrosis factor receptor-associated protein 1 (TRAP1) (A, D), excision repair cross-complementing 1 (ERCC1) (B, E), and thymidylate synthase (TS) (C, F) on paraffin sections of colorectal carcinoma specimens (A-F; ×400).
6. Statistical analysis
The associations between levels of TRAP1, ERCC1, and TS expression and clinical characteristics were analyzed by Pearson's chi-square or Fisher's exact tests, as indicated. The differences in biologic marker expression between primary and matched metastatic tumors were analyzed with Fisher's exact test. PFS by first-line chemotherapy and OS were analyzed with the Kaplan-Meier method. The log-rank test was used to compare survival. Age, ECOG performance status, and variables with p<0.05 on univariate analysis for OS and TRAP1/ERCC1 score were included in a subsequent multivariate Cox proportional hazards model bya forward procedure. Two-sided null hypotheses of no difference were rejected if p-values were less than 0.05, or, equivalently, if the 95% confidence intervals (CIs) of risk point estimates excluded 1. All statistical analyses were performed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL).
Results
1. Patient characteristics
The clinical characteristics of the study patients are shown in Table 1. The median age was 63 years (range, 31 to 84 years) and half of the patients were female. The primary tumor site was the colon in 32 patients (57%) and the rectum in 24 patients (43%). Seventeen patients (30%) had two or more sites of metastasis. The most common metastatic sites were liver (63%), lung (30%), and lymph nodes (39%). Of the 35 patients who had liver metastasis, tumorectomy was performed in four patients. Forty-four patients (79%) received FOLFOX-4 and 12 patients (21%) received XELOX as first-line chemotherapy. Bevacizumab was combined with FOLFOX-4 in five patients (13%). The median number of cycles of chemotherapy administered was 8 (range, 1 to 21). Thirty-five of the 56 patients (63%) received second-line chemotherapy, with 34 patients (97%) receiving irinotecan-based chemotherapy and one patient (3%) receiving capecitabine.
Table 1.
Clinical characteristics
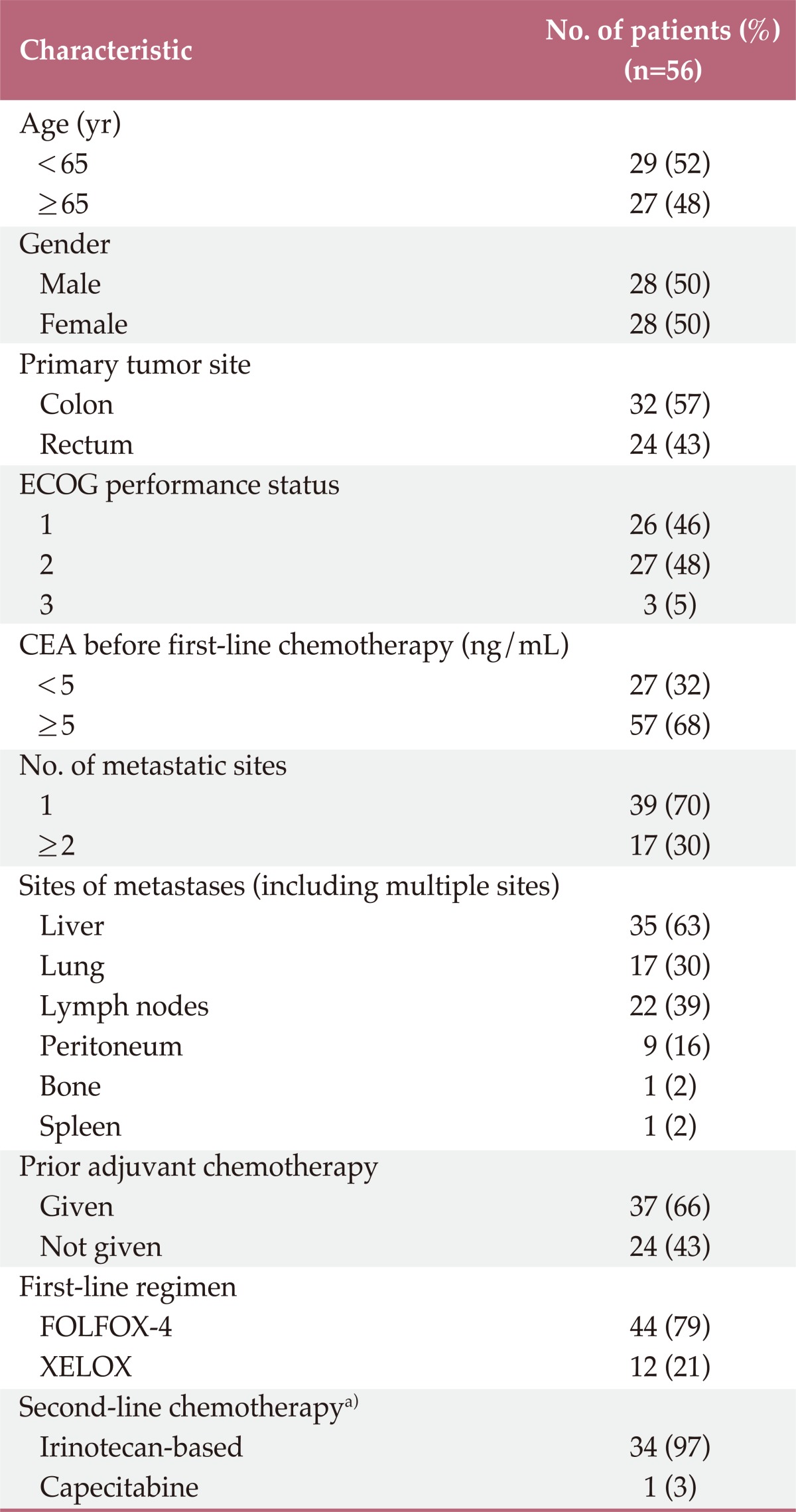
ECOG, Eastern Cooperative Oncology Group; CEA, carcino embryonic antigen; FOLFOX-4, 5-fluorouracil/folinic acid plus oxaliplatin; XELOX, capecitabine plus oxaliplatin. a)Thirty-five of 56 patients (63%) received second-line chemotherapy.
2. TRAP1, ERCC1, and TS expression
Twelve patients (21%) had positive intratumoral TRAP1 expression and 44 patients (79%) had negative TRAP1 expression according to the definition given above (Fig. 1A and D). Patients positive for TRAP1 expression had significantly worse ECOG performance scores than those negative for TRAP1 expression (p=0.003).
Twenty-nine (52%) of the 56 patients had positive intratumoral ERCC1 expression and 27 (48%) were negative for ERCC1 expression (Fig. 1B and E). Male patients had more positive ERCC1 expression in tumor tissue than did female patients (p=0.016).
Positive TS expression was observed in 33 tumors (59%), and 23 (41%) were negative for TS expression (Fig. 1C and F). No significant association was found between the level of TRAP1, ERCC1, or TS expression and other clinical parameters. TRAP1, ERCC1, and TS expression in primary tumor tissues was not significantly different from that in matched metastatic tumors in 18 analyzable patients (p=0.596, p=0.638, and p=0.890, respectively).
3. Response according to TRAP1, ERCC1, and TS expression
Response rates were measurable in 53 out of 56 patients (95%). The overall response rate for first-line FOLFOX chemotherapy was 62.3% (33 of 53 cases). Table 2 outlines the overall response by the levels of TRAP1, ERCC1, and TS expression. ERCC1 expression in tumor tissues and response were marginally associated (p=0.066). All four patients who achieved CR were negative for ERCC1 expression in tumor tissues, while all patients with progressive disease were positive for ERCC1 expression (Table 2). Neither TRAP1 nor TS expression were associated with response. However, when TRAP1 and ERCC1 were considered in combination, we found that patients who had both negative TRAP1 and negative ERCC1 expression responded better than patients positive for TRAP1 and/or ERCC1 expression (p=0.046) (Table 2).
Table 2.
Response assessment by TRAP1, ERCC1, and TS expression
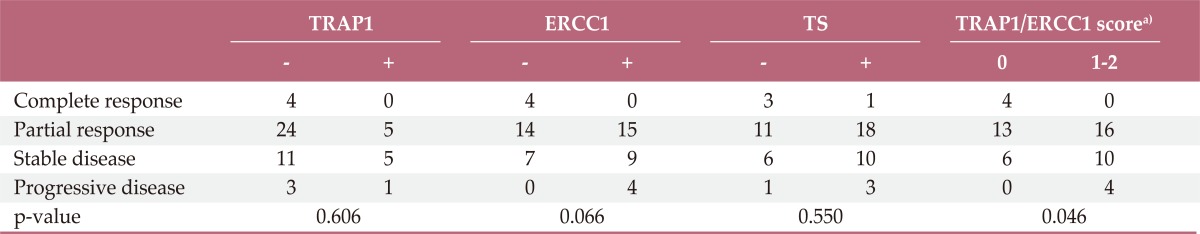
TRAP1, tumor necrosis factor receptor-associated protein 1; ERCC1, excision repair cross-complementing 1; TS, thymidylate synthase. a)ERCC1/TRAP1: score 0, negative TRAP1 and negative ERCC1; score 1, either positive TRAP1 or positive ERCC1; score 2, both positive TRAP1 and positive ERCC1.
The number of metastatic sites was significantly associated with response to chemotherapy (p=0.009 by Fisher's exact test, data not shown). Other clinical characteristics, including age, gender, primary tumor site, adjuvant chemotherapy, first-line regimen, history of tumorectomy, site of metastasis, ECOG performance score, and carcinoembryonic antigen level, had no significant association with response to chemotherapy.
4. Survival according to TRAP1, ERCC1, and TS expression
The median follow-up period for all 56 patients was 27.0 months (95% CI, 22.1 to 31.9 months). The median PFS for these patients was 6.0 months (95% CI, 4.8 to 7.2 months) and the median OS was 18.5 months (95% CI, 8.6 to 28.5 months). Patients with more than one site of metastasis had worse PFS (5.0 months vs. 7.0 months; p=0.006 by log-rank test). Patients with liver metastasis had shorter PFS than those with other metastatic sites with marginal significance (5.0 months vs. 7.0 months; p=0.082 by log-rank test). There was no significant association between other clinical characteristics and PFS. No significant association between TRAP1, ERCC1, and TS expression and PFS was observed (Table 3).
Table 3.
PFS and OS by patient characteristics (univariate analysis)
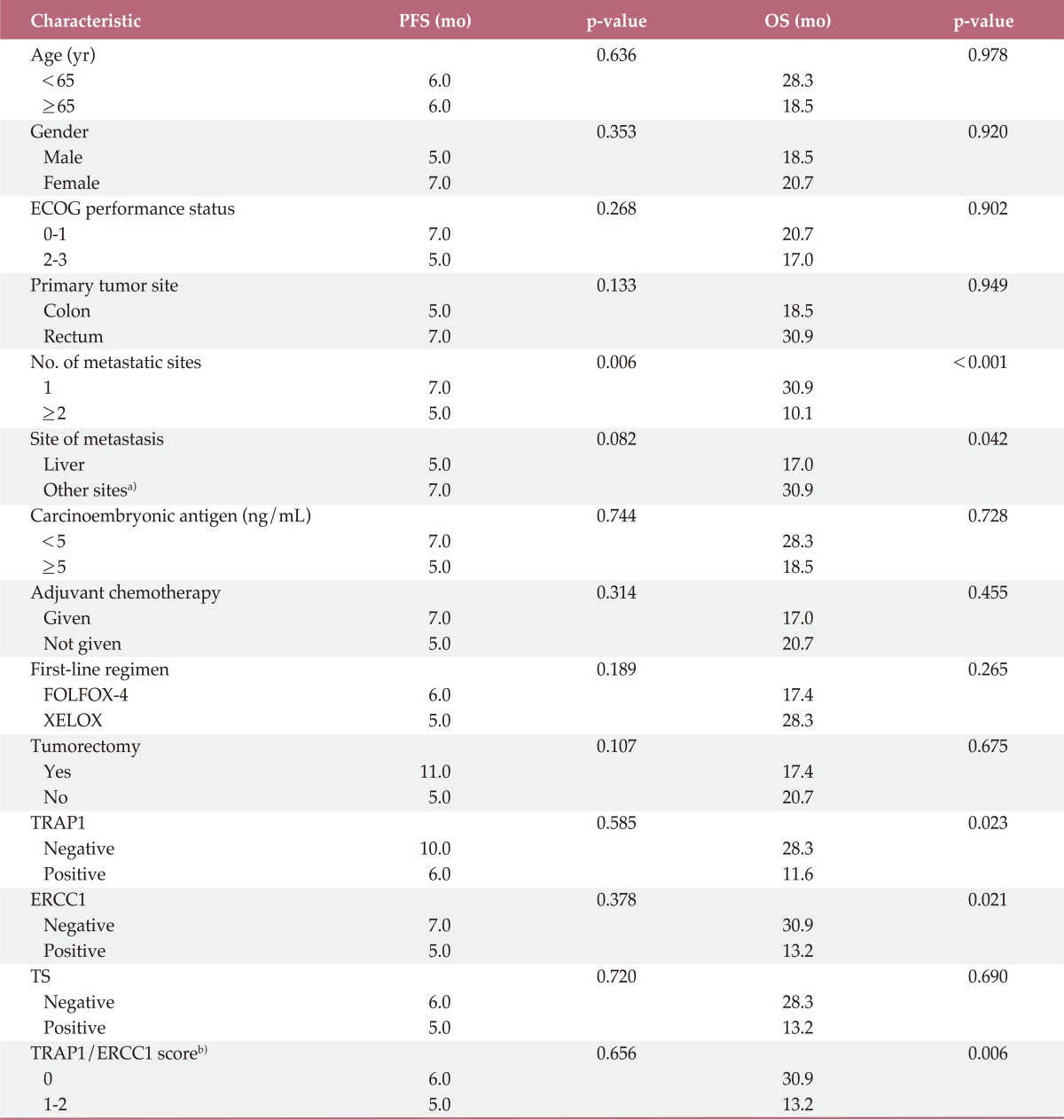
PFS, progression-free survival; OS, overall survival; ECOG, Eastern Cooperative Oncology Group; FOLFOX-4, 5-fluorouracil/folinic acid plus oxaliplatin; XELOX, capecitabine plus oxaliplatin; TRAP1, tumor necrosis factor receptor-associated protein 1; ERCC1, excision repair cross-complementing group 1; TS, thymidylate synthase. a)Other sites include lung, lymph nodes, peritoneum, bone, and spleen, b)Score 0, patients negative for TRAP1 and ERCC1 expression; score 1, patients positive for either TRAP1 or positive ERCC1 expression; score 2, patients positive for TRAP1 and ERCC1.
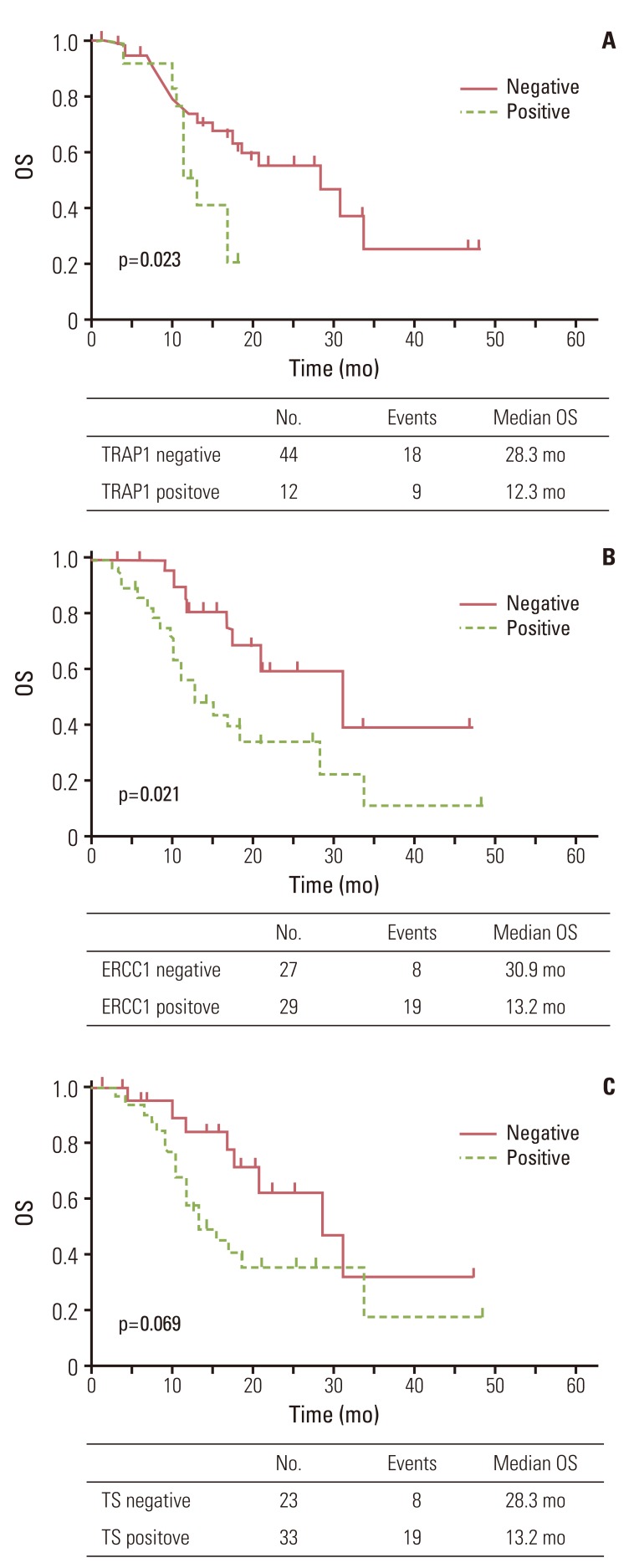
Patients negative for TRAP1 expression had significantly better OS than those positive for TRAP1 (p=0.023) (Fig. 2A). Patients negative for ERCC1 expression also had a better OS than those positive for ERCC1 (p=0.021) (Fig. 2B). However, patients negative for TS expression only showed a trend for better OS than those positive for TS (p=0.069) (Fig. 2C). Patients with more than one metastatic site or liver metastasis had worse OS (p<0.001 and p=0.042, respectively). Other clinical characteristics did not differ significantly in OS (Table 3).
Fig. 2.
Overall survival (OS) curve for 56 patients according to tumor necrosis factor receptor-associated protein 1 (TRAP1) (A), excision repair cross-complementing 1 (ERCC1) (B), and thymidylate synthase (TS) (C) expression.
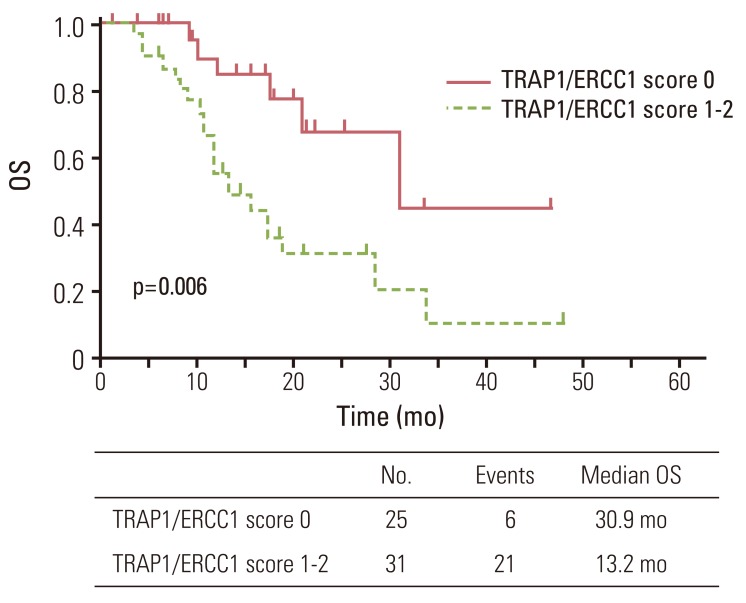
Since TRAP1 and ERCC1 expression were significantly associated with OS, we further investigated OS in relation to combined TRAP1 and ERCC1 expression. Negative TRAP1 and ERCC1 expression was detected in 25 of the patients (45%); 31 patients (55%) were positive for TRAP1 and/or ERCC1 expression. The median survival for patients negative for TRAP1 and ERCC1 was longer than those positive for TRAP1 and/or positive for ERCC1 expression (30.9 months vs. 13.2 months, p=0.006) (Fig. 3). The relative risk of dying for patients positive for expression of either TRAP1 or ERCC1 was 3.37 (95% CI, 1.35 to 8.38) compared with patients who had low expression for both markers (p=0.009).
Fig. 3.
Overall survival (OS) curve for 56 patients according to tumor necrosis factor receptor-associated protein 1 (TRAP1)/excision repair cross-complementing 1 (ERCC1) score (score 0, negative TRAP1 and negative ERCC1; score 1, either positive TRAP1 or positive ERCC1; score 2, positive TRAP1 and positive ERCC1).
Final multivariate analysis was performed with the number of metastatic sites, sites of metastasis, TRAP1/ERCC1 score, age, and ECOG performance status by a forward stepwise method (Table 4). In this model, TRAP1/ERCC1 score (hazard ratio, 2.98; 95% CI, 1.18 to 7.49; p=0.020) and the number of metastatic sites (hazard ratio, 5.03; 95% CI, 2.16 to 11.71; p<0.001) remained significant independent prognostic indicators for OS.
Table 4.
Cox proportional hazards model for overall survival (multivariate analysis)
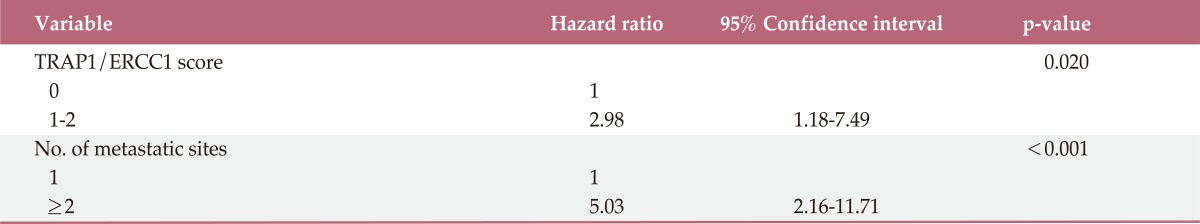
TRAP1, tumor necrosis factor receptor-associated protein 1; ERCC1, excision repair cross-complementation group 1.
Discussion
Combination oxaliplatin and 5-FU is a standard chemotherapy regimen in metastatic CRC patients. Given its widespread use, finding tumor markers that predict clinical outcomes is critical. Costantino et al. [6] recently reported that TRAP1 is responsible for resistance to oxaliplatin, irinotecan, and 5-FU. We found that patients negative for TRAP1 expression survived longer than those positive for TRAP1 expression. This is an intuitive result, as TRAP1 contributes to inhibition of pore formation by cyclophilin D in mitochondrial permeability transition, thus promoting cancer cell survival and preventing apoptosis [3]. In addition, TRAP1 has inhibitors such as shepherd in that inhibit TRAP1-ATPase, increasing the sensitivity of CRC cells to oxaliplatin and irinotecan [6]. Another targeted agent, gamitrinib, accumulates in mitochondria and inhibits Hsp90 activity, resulting in prostate tumor cell death without affecting the normal prostate epithelium [10-12].
Although TRAP1 is strongly expressed in adenocarcinoma cells of the pancreas, breast, and lung, normal matched epithelia contain very low levels of TRAP1 [3]. TRAP1 protein and mRNA expression is upregulated in about 60% of CRC tumors [6], while 21% of patients were positive for TRAP1 expression in our study. First, this difference in expression may have been due to the definition of positive expression used in this study. We defined positive TRAP1 expression as strong intensity and over 50% of cells affected, to exclude false positive TRAP1 expression and to allow for more strict measurement of the association between TRAP1 expression and clinical outcomes. Second, a study by Constantino et al. [6] included CRC patients, 65.4% of whom had no metastasis. TRAP1 was recently found to contribute to the inhibition of metastasis, indicating that the expression of TRAP1 in metastatic tumors may differ from primary tumors [13]. However, additional research is needed to define the optimal cutoff value of positive TRAP1 expression in metastatic CRC.
Oxaliplatin is a 1,2-diaminocyclohexaneoxalato-platinum that differs chemically from cisplatin and is effective in the treatment of colorectal cancer. Oxaliplatin/DNA adducts are repaired by the NER pathway, and ERCC1 and the XPF heterodimer act in this pathway by executing the incision of the DNA strand. ERCC1 expression was predictive of oxaliplatin sensitivity in colorectal cell lines [14]. In addition, low mRNA ERCC1 expression is related to better survival in irinotecan-resistant CRC patients treated with oxaliplatin [8]. Kim et al. [15] reported similar results by IHC. Consistent with these reports, our study showed that patients with high ERCC1 expression had poor OS and response.
Two large recent studies that assessed predictive and prognostic markers by IHC reported negative results [16,17]. Ethnic differences in ERCC1 polymorphisms between Asians and Caucasians may explain the differences with our study. The T allele in rs1165C>T was associated with reduced response and poor survival in Asian patients, but not in Caucasian patients [18]. High intratumoral ERCC1 expression was associated with sensitivity to irinotecan-based chemotherapy in CRC patients in a prior report [19]. Although 60.7% of patients received irinotecan as secondline chemotherapy in our study, levels of ERCC1 expression and receipt of irinotecan were not significantly associated.
ERCC1 expression was significantly lower in female patients than male patients in this study. A report on non-small cell lung cancer patients found that ERCC1 expression is different between sexes [20]. However, other researchers who reported different survival by ERCC1 expression did not find significant differences in ERCC1 levels between sexes in non-small cell lung cancer [21,22]. The interpretation of our results is limited by the small number of patients in this study; therefore, differences in ERCC1 expression between sexes in patients with CRC will require more attention in the future.
Although our results showed no significant difference in response rates and PFS according to TRAP1 and ERCC1 expression, this may have been due to the small sample size in this study. We hypothesize that TRAP1 and ERCC1 expression may act synergistically to predict clinical outcomes because these two markers are involved in different drug resistance mechanisms. Risk stratification by TRAP1/ERCC1 score showed a more significant association with response rate and differentiated OS than either marker alone, with a p-value of 0.006 by log-rank test. In multivariate models, the TRAP1/ERCC1 score remained an independent predictor of OS, with a hazard ratio of 5.53. PFS was 6, 5, and 6 months for each risk group, and the response rate to second-line chemotherapy was 29.4%, 15.4%, and 0%, respectively. Although not statistically significant, the high-risk group had a lower response rate to second-line chemotherapy. This may contribute to the differences in OS between groups. Furthermore, all patients but one who received second-line chemotherapy had irinotecan-based chemotherapy. As TRAP1 expression was increased in cells resistant to irinotecan, the effect of irinotecan-based second-line chemotherapy possibly increased the association of TRAP1/ERCC1 score with OS in this study.
5-FU is an analog of uracil that disrupts RNA synthesis and inhibits the action of TS. As a main target of drug therapy, TS expression has been studied extensively. Although a recent meta-analysis showed the predictive [23] and prognostic [24] role of TS in CRC patients receiving 5-FU-based chemotherapy, TS measurement is not currently recommended in clinical practice. The use of a combination of ERCC1 and TS was associated with clinical outcomes in a previous study. In our study, TS expression by IHC was marginally associated with OS; however, combining TS with ERCC1 did not significantly improve clinical outcome prediction (data not shown). Consistent with previous studies [15,25], this negative result may be in part due to prior use of 5-FU-based adjuvant chemotherapy (66% of patients received adjuvant 5-FU-based chemotherapy in this study).
The limitations of immunohistochemical staining methods include their semiquantitative nature, the aging of paraffin-embedded tissue specimens, varying staining techniques, interobserver variation, and recent concerns over the adequate targeting of the 8F1 ERCC1-antibody used in our study.
Conclusion
Our study results suggest that the expression of the novel marker TRAP1 was associated with OS. In addition, the use of a combination of TRAP1 and ERCC1 expression was significantly associated with OS in multivariate analysis. To the best of our knowledge, this is the first in vivo study of TRAP1 in CRC patients. However, further prospective studies with a large number of patients will be required to confirm the results.
Acknowledgments
This study was supported by a grant from Kyung Hee University in 2011 (KHU-20111743).
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Felts SJ, Owen BA, Nguyen P, Trepel J, Donner DB, Toft DO. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J Biol Chem. 2000;275:3305–3312. doi: 10.1074/jbc.275.5.3305. [DOI] [PubMed] [Google Scholar]
- 3.Kang BH, Plescia J, Dohi T, Rosa J, Doxsey SJ, Altieri DC. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell. 2007;131:257–270. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Hua G, Zhang Q, Fan Z. Heat shock protein 75 (TRAP1) antagonizes reactive oxygen species generation and protects cells from granzyme M-mediated apoptosis. J Biol Chem. 2007;282:20553–20560. doi: 10.1074/jbc.M703196200. [DOI] [PubMed] [Google Scholar]
- 5.Montesano Gesualdi N, Chirico G, Pirozzi G, Costantino E, Landriscina M, Esposito F. Tumor necrosis factor-associated protein 1 (TRAP-1) protects cells from oxidative stress and apoptosis. Stress. 2007;10:342–350. doi: 10.1080/10253890701314863. [DOI] [PubMed] [Google Scholar]
- 6.Costantino E, Maddalena F, Calise S, Piscazzi A, Tirino V, Fersini A, et al. TRAP1, a novel mitochondrial chaperone responsible for multi-drug resistance and protection from apoptotis in human colorectal carcinoma cells. Cancer Lett. 2009;279:39–46. doi: 10.1016/j.canlet.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291–1295. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- 8.Shirota Y, Stoehlmacher J, Brabender J, Xiong YP, Uetake H, Danenberg KD, et al. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol. 2001;19:4298–4304. doi: 10.1200/JCO.2001.19.23.4298. [DOI] [PubMed] [Google Scholar]
- 9.Kim MK, Cho KJ, Kwon GY, Park SI, Kim YH, Kim JH, et al. ERCC1 predicting chemoradiation resistance and poor outcome in oesophageal cancer. Eur J Cancer. 2008;44:54–60. doi: 10.1016/j.ejca.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Leav I, Plescia J, Goel HL, Li J, Jiang Z, Cohen RJ, et al. Cytoprotective mitochondrial chaperone TRAP-1 as a novel molecular target in localized and metastatic prostate cancer. Am J Pathol. 2010;176:393–401. doi: 10.2353/ajpath.2010.090521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang BH, Siegelin MD, Plescia J, Raskett CM, Garlick DS, Dohi T, et al. Preclinical characterization of mitochondria-targeted small molecule hsp90 inhibitors, gamitrinibs, in advanced prostate cancer. Clin Cancer Res. 2010;16:4779–4788. doi: 10.1158/1078-0432.CCR-10-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang BH, Plescia J, Song HY, Meli M, Colombo G, Beebe K, et al. Combinatorial drug design targeting multiple cancer signaling networks controlled by mitochondrial Hsp90. J Clin Invest. 2009;119:454–464. doi: 10.1172/JCI37613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu D, Hu J, Agorreta J, Cesario A, Zhang Y, Harris AL, et al. Tumor necrosis factor receptor-associated protein 1 (TRAP1) regulates genes involved in cell cycle and metastases. Cancer Lett. 2010;296:194–205. doi: 10.1016/j.canlet.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Arnould S, Hennebelle I, Canal P, Bugat R, Guichard S. Cellular determinants of oxaliplatin sensitivity in colon cancer cell lines. Eur J Cancer. 2003;39:112–119. doi: 10.1016/s0959-8049(02)00411-2. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Kwon HC, Oh SY, Lee DM, Lee S, Lee JH, et al. Prognostic value of ERCC1, thymidylate synthase, and glutathione S-transferase pi for 5-FU/oxaliplatin chemotherapy in advanced colorectal cancer. Am J Clin Oncol. 2009;32:38–43. doi: 10.1097/COC.0b013e31817be58e. [DOI] [PubMed] [Google Scholar]
- 16.Koopman M, Venderbosch S, van Tinteren H, Ligtenberg MJ, Nagtegaal I, Van Krieken JH, et al. Predictive and prognostic markers for the outcome of chemotherapy in advanced colorectal cancer, a retrospective analysis of the phase III randomised CAIRO study. Eur J Cancer. 2009;45:1999–2006. doi: 10.1016/j.ejca.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Braun MS, Richman SD, Quirke P, Daly C, Adlard JW, Elliott F, et al. Predictive biomarkers of chemotherapy efficacy in colorectal cancer: results from the UK MRC FOCUS trial. J Clin Oncol. 2008;26:2690–2698. doi: 10.1200/JCO.2007.15.5580. [DOI] [PubMed] [Google Scholar]
- 18.Yin M, Yan J, Martinez-Balibrea E, Graziano F, Lenz HJ, Kim HJ, et al. ERCC1 and ERCC2 polymorphisms predict clinical outcomes of oxaliplatin-based chemotherapies in gastric and colorectal cancer: a systemic review and meta-analysis. Clin Cancer Res. 2011;17:1632–1640. doi: 10.1158/1078-0432.CCR-10-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallbohmer D, Iqbal S, Yang DY, Rhodes KE, Zhang W, Gordon M, et al. Molecular determinants of irinotecan efficacy. Int J Cancer. 2006;119:2435–2442. doi: 10.1002/ijc.22129. [DOI] [PubMed] [Google Scholar]
- 20.Holm B, Mellemgaard A, Skov T, Skov BG. Different impact of excision repair cross-complementation group 1 on survival in male and female patients with inoperable non-small-cell lung cancer treated with carboplatin and gemcitabine. J Clin Oncol. 2009;27:4254–4259. doi: 10.1200/JCO.2008.18.8631. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Z, Chen T, Li X, Haura E, Sharma A, Bepler G. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med. 2007;356:800–808. doi: 10.1056/NEJMoa065411. [DOI] [PubMed] [Google Scholar]
- 22.Lord RV, Brabender J, Gandara D, Alberola V, Camps C, Domine M, et al. Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer Res. 2002;8:2286–2291. [PubMed] [Google Scholar]
- 23.Qiu LX, Tang QY, Bai JL, Qian XP, Li RT, Liu BR, et al. Predictive value of thymidylate synthase expression in advanced colorectal cancer patients receiving fluoropyrimidine-based chemotherapy: evidence from 24 studies. Int J Cancer. 2008;123:2384–2389. doi: 10.1002/ijc.23822. [DOI] [PubMed] [Google Scholar]
- 24.Popat S, Matakidou A, Houlston RS. Thymidylate synthase expression and prognosis in colorectal cancer: a systematic review and meta-analysis. J Clin Oncol. 2004;22:529–536. doi: 10.1200/JCO.2004.05.064. [DOI] [PubMed] [Google Scholar]
- 25.Kwon HC, Roh MS, Oh SY, Kim SH, Kim MC, Kim JS, et al. Prognostic value of expression of ERCC1, thymidylate synthase, and glutathione S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in advanced gastric cancer. Ann Oncol. 2007;18:504–509. doi: 10.1093/annonc/mdl430. [DOI] [PubMed] [Google Scholar]