Abstract
Purpose
Platinum-based therapy is the mainstay for management of high-risk neuroblastoma. Prevalence of platinum-related ototoxicity has ranged from 13% to 95% in previous reports; variability is attributable to small samples and disparate grading scales. There is no consensus regarding optimal ototoxicity grading. Furthermore, prevalence and predictors of hearing loss in a large uniformly treated high-risk neuroblastoma population are unknown. We address these gaps in our study.
Patients and Methods
Audiologic testing was completed after administration of cisplatin alone (< 400 mg/m2; exposure one) or after cisplatin (400 mg/m2) plus carboplatin (1,700 mg/m2; exposure two). Hearing loss was graded using four scales (American Speech-Language-Hearing Association; Brock; Chang; and Common Terminology Criteria for Adverse Events, version 3 [CTCAEv3]).
Results
Of 489 eligible patients, 333 had evaluable audiologic data. Median age at diagnosis was 3.3 years. Prevalence of severe hearing loss differed by scale. For those in the exposure-one group, prevalence ranged from 8% per Brock to 47% per CTCAEv3 (Brock v CTCAEv3 and Chang, P < .01; CTCAEv3 v Chang, P = .16); for those in the exposure-two group, prevalence ranged from 30% per Brock to 71% per CTCAEv3 (all pair-wise comparisons, P < .01). In patients requiring hearing aids, hearing loss was graded as severe in 49% (Brock), 91% (Chang), and 100% (CTCAEv3). Risk factors for severe hearing loss included exposure to cisplatin and carboplatin compared with cisplatin alone and hospitalization for infection.
Conclusion
Severe hearing loss is prevalent among children with high-risk neuroblastoma. Exposure to cisplatin combined with myeloablative carboplatin significantly increases risk. The Brock scale underestimates severe hearing loss and should be used with caution in this setting.
INTRODUCTION
Neuroblastoma, an embryonal tumor of the autonomic nervous system, is the second most common pediatric solid tumor.1 Patients with high-risk features require dose-intensive therapy, often with cisplatin and myeloablative doses of carboplatin to maximize likelihood of survival,2–7 increasing the risk for ototoxicity.8–10 Platinum-related sensorineural hearing loss is generally irreversible and bilateral, and it manifests initially in the high frequencies, progressing to the speech frequencies with increasing cumulative exposure.11,12 The impact of hearing loss in patients with neuroblastoma is particularly significant because nearly 90% are age < 5 years at exposure to the ototoxic agents,13 and given the short latency between exposure and ototoxicity, hearing loss occurs while language is developing. The high-frequency speech phonemes are critical to speech intelligibility14; thus, even mild hearing loss in the high-frequency ranges (> 2,000 Hz) can adversely affect language development and has been associated with increased academic, social, and emotional difficulties in young children.15–17
The prevalence of cisplatin-related ototoxicity ranges from 13% to 95%.8,18–29 Factors influencing risk are related to platinum treatment (eg, platinum type, dose, infusion duration), host factors (eg, age, genetic susceptibility, renal function), and receipt of additional ototoxic therapy (cranial irradiation, aminoglycosides, loop diuretics).12,18,20,21,23,25,28,30–32 Variability in ototoxicity grading criteria affect reported prevalence and severity of hearing loss and may underestimate the significance of hearing loss in children who are in the process of developing language.25,33 The Common Terminology Criteria for Adverse Events, version 3 (CTCAEv3; as well as prior versions),34 and Brock criteria20 have been used most frequently in these trials18,20,24,25,31,35–37; more recently, the American Speech-Language-Hearing Association (ASHA)38 and Chang39 scales have also been used to evaluate hearing loss in children with cancer,25,39 and evaluations based on the CTCAEv440 and International Society of Pediatric Oncology (SIOP-Boston)33 scales are under way. Concerns have been raised regarding the poor correlation among these ototoxicity grading criteria and the lack of consensus regarding an optimal pediatric ototoxicity scale,41 with the consequent lack of comparability of ototoxicity outcomes among clinical trials.25,33,41,42
In this study, we evaluated audiologic data collected in a large, uniformly treated cohort of patients with neuroblastoma who received platinum-based therapy. We aimed to: one, determine the prevalence and severity of hearing loss using four standard grading scales (ASHA,38 Brock,20 CTCAEv3,34 and Chang39); two, examine clinical and demographic determinants of ototoxicity; and three, evaluate concordance among the scales.
PATIENTS AND METHODS
Study Participants
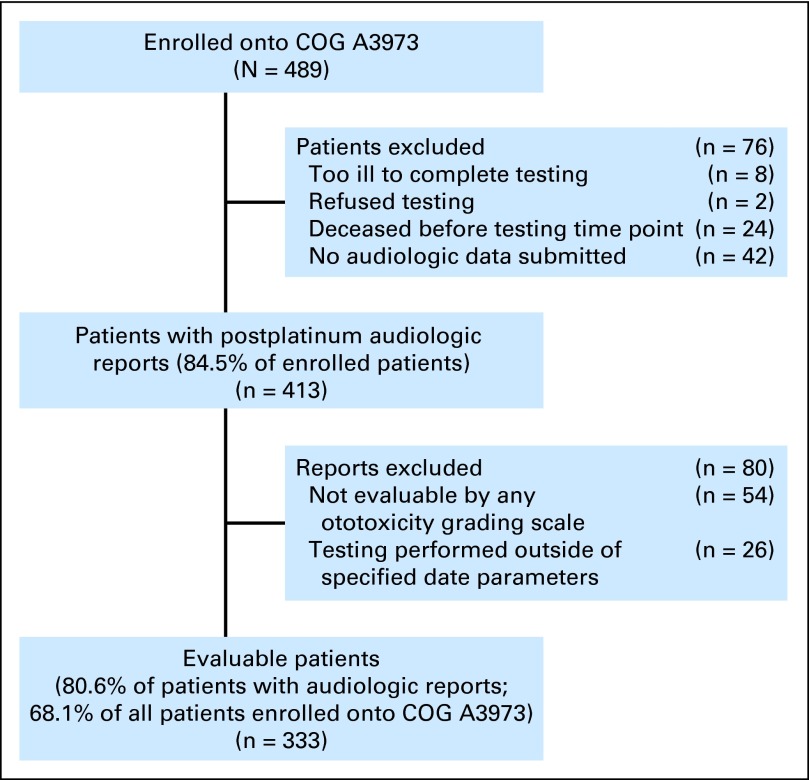
Participants were enrolled between February 9, 2001, and February 24, 2006, onto the COG (Children's Oncology Group) A3973 trial,43 a clinical trial for patients age ≤ 30 years with newly diagnosed high-risk neuroblastoma. Induction therapy included high-dose cisplatin (200 mg/m2 × 2), followed by consolidation with myeloablative carboplatin (1,700 mg/m2; Appendix Table A1, online only). Institutions enrolled patients after obtaining approval from their local institutional review boards and securing written informed consent/assent from patients and/or their legal representatives. Patients with postplatinum audiologic data evaluable by at least one of the four ototoxicity grading scales (Fig 1; Appendix Table A2, online only) were included in this analysis. Institutions that contributed audiologic data are listed in the Appendix (online only).
Fig 1.
COG (Children's Oncology Group) A3973 trial evaluable postplatinum audiologic data.
Audiologic Assessments
Audiology reports, obtained for toxicity monitoring (conducted before first platinum exposure, after cumulative cisplatin exposure of 200 and 400 mg/m2, and after myeloablative doses of carboplatin for transplantation) were submitted by the treating institutions to the COG Statistics and Data Center (audiologic evaluation methods summarized in Appendix Table A3, online only). Air- and bone-conduction thresholds (tone-burst thresholds for auditory brainstem response) for each tested frequency and details of testing (eg, type of testing performed, masking, tympanometry, use of hearing aids or assistive devices) were obtained from the audiology reports. A standardized process for audiology report interpretation was developed by the investigators (Appendix Table A3). Audiology reports were graded independently by two investigators (W.L., K.K.) according to each of the four grading scales. A grade was assigned to each ear with evaluable thresholds or to the soundfield; in cases where disagreement in grading between investigators existed, a consensus grading determination was made.
For each patient, the most recent postplatinum audiologic assessment that was evaluable by at least one of the four grading scales and paired with an evaluable preplatinum test (when available, to allow for grading by scales requiring preplatinum studies) was selected for inclusion in the analysis. Reports were deemed inevaluable in the presence of evident or suspected middle-ear pathology, insufficient range of evaluable thresholds across frequencies to allow for definitive grading, lack of tone-burst thresholds for auditory brainstem response testing (ie, clicks only), or testing completed outside acceptable date parameters (Appendix Table A4, online only).
Main Outcome Measures
Patients were categorized by total planned per-protocol platinum dose exposure before the index audiologic assessment: exposure one (≤ cisplatin 400 mg/m2) or exposure two (cisplatin 400 mg/m2 plus carboplatin 1,700 mg/m2; Fig 2). Evaluable postplatinum audiologic assessments were categorized as follows: presence of hearing loss (yes v no) according to each of the four grading scales and severity (grade) of hearing loss according to three of the four scales (ASHA does not grade severity). Evaluable preplatinum assessments were required for evaluation per ASHA (all assessments) and CTCAEv3 (if rated < grade 3) criteria, but not for Brock or Chang. When hearing aid recommendation/use was missing from the report (required for assignment of CTCAEv3 grade ≥ 3), a recommendation was assigned using pre-established criteria (Appendix Table A3, online only); these patients were designated as requiring hearing aids. Severity grading was assigned based on the better ear. Criteria for classification of hearing loss are summarized in Table 1.
Fig 2.
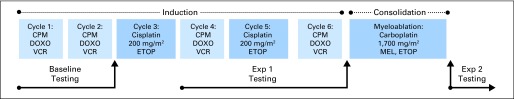
Audiometric testing time points in relation to protocol-delivered platinum. CPM, cyclophosphamide; DOXO, doxorubicin; ETOP, etoposide; Exp 1, exposure one (cisplatin ≤ 400 mg/m2); Exp 2, exposure two (cisplatin 400 mg/m2 and carboplatin 1,700 mg/m2); MEL, melphalan; VCR, vincristine.
Table 1.
Criteria for Classification of Hearing Loss Rating by Scale
| Hearing Loss Rating | ASHA Grade* | Brock Grade | Chang Grade | CTCAEv3 Grade |
|---|---|---|---|---|
| Presence | ||||
| No | Absence of A, B, or C | 0 | 0 | 0 |
| Yes | A, B, or C | 1-4 | 1-4 | 1-4 |
| Severity | ||||
| None/negligible | NA | 0 | 0 | 0 |
| Mild | NA | 1 | 1a, 1b | 1 |
| Moderate | NA | 2 | 2a | 2 |
| Severe | NA | 3, 4 | 2b, 3, 4 | 3, 4 |
Abbreviations: ASHA, American Speech-Language-Hearing Association; CTCAEv3, Common Terminology Criteria for Adverse Events, version 3; NA, not applicable.
ASHA scale does not grade severity of hearing loss.
Statistical Analyses
Inter-rater reliability was assessed using Cohen's kappa statistic.44 The prevalence of hearing loss for each scale was calculated and compared pairwise using the generalized linear mixed-effects model.45,46 For patients with any hearing loss, distribution by severity of hearing loss (mild, moderate, or severe) was tabulated. Pairwise concordance among scales was evaluated using McNemar's test.47 Between-group means were compared using the t test, and medians were compared using the Kruskal-Wallis test.47 Unconditional multivariable logistic regression48 was conducted to determine the risk factors of hearing loss, considering age at diagnosis, sex, race/ethnicity, cumulative platinum exposure (exposure one, cisplatin ≤ 400 mg/m2; exposure two, cisplatin 400 mg/m2 plus carboplatin 1,700 mg/m2), time interval between platinum and testing, preconsolidation glomerular filtration rate (marker for impaired platinum clearance49), chemotherapy dose reduction during induction, and hospitalization for infection during induction (surrogate for exposure to ototoxic agents such as nonanthracycline aminoglycoside antibiotics30,50) associated with hearing loss defined by each scale. The criterion for selection of explanatory variables was .05 for entry and .10 for removal. All analyses were performed using SAS software (version 9.2; SAS Institute, Cary, NC).
RESULTS
Participant Characteristics
Of the 489 patients enrolled onto COG A3973, 333 (68.1%) were evaluable; postplatinum audiologic reports were not submitted for 76 patients; 54 were excluded because the data were not evaluable by any of the four grading scales, and 26 were excluded because testing was performed outside of the specified date parameters (Fig 1). Of the 333 evaluable patients, 56% were male, and the median age at diagnosis was 3.3 years (range, 0.3 to 29.1 years); the most recent evaluable postplatinum audiologic testing occurred after exposure one for 66 patients (19.8%; cumulative cisplatin dose, 200 mg/m2 for six patients and 400 mg/m2 for 60 patients) and after exposure two for 267 patients (80.2%; cumulative cisplatin 400 mg/m2 plus carboplatin 1,700 mg/m2; Table 2; Appendix Table A5, online only). There were fewer male patients in the exposure-one group (P = .03), and as expected, age at testing was older (5.2 v 3.7 years; P < .001) and time interval between platinum exposure and testing was longer (273 v 34 days; P < .001) in the exposure-two group. All other characteristics were comparable. For patients with available clinical data (Table 2), 82.5% required hospitalization for infection during induction, 12% required a platinum dose reduction of ≥ 25%, and 19% had compromised renal function (glomerular filtration rate < 100 mL/min/1.73 m2) at the end of induction.
Table 2.
Demographic and Clinical Characteristics of Study Participants
| Characteristic | All COG A3973 Patients (N = 489) |
Evaluable Patients (n = 333) |
Inevaluable Patients (n = 156) |
P | Exposure-One Patients (n = 66)* |
Exposure-Two Patients (n = 267)† |
P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |||
| Male sex | 280 | 57.3 | 186 | 55.9 | 94 | 60.3 | .51 | 29 | 43.9 | 157 | 58.8 | .030 |
| Race/ethnicity | .49 | .10 | ||||||||||
| Non-Hispanic white | 336 | 68.7 | 235 | 70.6 | 101 | 64.7 | 46 | 69.7 | 189 | 70.8 | ||
| Hispanic | 42 | 8.6 | 27 | 8.1 | 15 | 9.6 | 5 | 7.6 | 22 | 8.2 | ||
| African American | 59 | 12.1 | 35 | 10.5 | 24 | 15.4 | 12 | 18.2 | 23 | 8.6 | ||
| Asian | 20 | 4.1 | 15 | 4.5 | 5 | 3.2 | 2 | 3.0 | 13 | 4.9 | ||
| Other/unknown | 32 | 6.5 | 21 | 6.3 | 11 | 7.1 | 1 | 1.5 | 20 | 7.5 | ||
| Age at diagnosis, years‡ | .008 | .87 | ||||||||||
| Mean | 3.93 | 4.03 | 3.71 | 4.48 | 3.92 | |||||||
| SD | 3.31 | 3.28 | 3.37 | 4.57 | 2.87 | |||||||
| Median | 3.14 | 3.31 | 2.57 | 3.31 | 3.31 | |||||||
| Range | 0.30-29.09 | 0.30-29.09 | 0.69-20.91 | 0.62-29.09 | 0.30-22.78 | |||||||
| Age < 5 years at diagnosis | 396 | 81.0 | 267 | 80.2 | 129 | 82.7 | .54 | 51 | 77.3 | 216 | 80.9 | .51 |
| Age at testing, years‡ | < .001 | |||||||||||
| Mean | 5.60 | — | 5.07 | 5.73 | ||||||||
| SD | 3.39 | 4.68 | 2.99 | |||||||||
| Median | 4.94 | — | 3.73 | 5.16 | ||||||||
| Range | 1.01-29.56 | 1.01-29.56 | 1.37-24.05 | |||||||||
| Interval (most recent platinum to test), days‡ | < .001 | |||||||||||
| Mean | — | 402.24 | — | 87.23 | 480.10 | |||||||
| SD | 474.60 | 204.46 | 490.10 | |||||||||
| Median | — | 240 | — | 34 | 273 | |||||||
| Range | 8-2,517 | 8-1,156 | 47-2,517 | |||||||||
| Platinum dose reduction ≥ 25%§ | 53 | 11.9 | 40 | 12.4 | 13 | 10.8 | .95 | 10 | 15.6 | 30 | 11.6 | .35 |
| Infection requiring hospitalization‖ | 373 | 82.5 | 272 | 83.2 | 101 | 80.8 | .84 | 56 | 87.5 | 216 | 82.1 | .54 |
| Preconsolidation GFR‡ | .48 | .24 | ||||||||||
| Mean | 136.4 | 135.9 | 138.5 | 139.3 | 135.3 | |||||||
| SD | 46.8 | 44.8 | 54.1 | 58.5 | 41.9 | |||||||
| Median | 129 | 129 | 126 | 136 | 129 | |||||||
| Range | 27-400 | 43-400 | 27-400 | 43-349 | 50-400 | |||||||
| GFR < 100 mL/min/1.73 m2¶ | 76 | 19.2 | 58 | 18.5 | 18 | 22.0 | .53 | 12 | 24.5 | 46 | 17.4 | .24 |
NOTE. Bold font indicates significance.
Abbreviations: COG, Children's Oncology Group; GFR, glomerular filtration rate; SD, standard deviation.
Exposure one: cisplatin ≤ 400 mg/m2.
Exposure two: cisplatin 400 mg/m2 and carboplatin 1,700 mg/m2.
P values compare the medians between the various groups.
In induction, for 444 patients with available data.
In induction, for 452 patients with available data.
For 396 patients with available data.
Quality of Audiologic Assessments
Significantly fewer auditory tests were evaluable by ASHA than by any other scale (ASHA, 42.9%; CTCAEv3, 70.2%; Chang, 73.8%; Brock, 74.3%; P < .001).
Inter-Rater Reliability
A total of 1,989 ratings were independently completed by two investigators (W.L., K.K.); these included ratings of individual ears and/or soundfields according to the four scales (ASHA, 329; Brock, 570; Chang, 563; CTCAEv3, 527 ratings). Inter-rater reliability was ≥ 0.95 for all scales; discrepant ratings between investigators totaled 19 (0.96%) of 1,989.
Prevalence of Hearing Loss
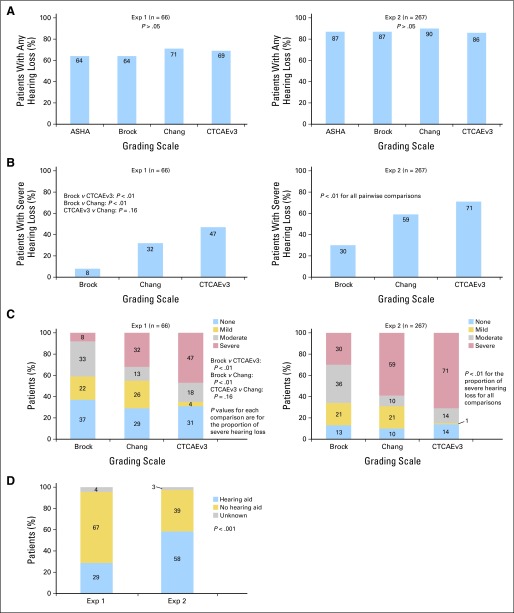
Prevalence of any hearing loss (Brock, CTCAEv3, and Chang, grades 1 to 4; ASHA, grades A to C) was comparable across the four grading scales (P > .05; Fig 3A), ranging from 64% to 71% for the exposure-one group to 86% to 90% for the exposure-two group. Prevalence of severe hearing loss differed by scale (Figs 3B and 3C). Among exposure-one patients, prevalence of severe hearing loss was 8% per Brock, 32% per Chang, and 47% per CTCAEv3 (Brock v CTCAEv3 and Chang, P < .01; CTCAEv3 v Chang, P = .16); comparable prevalences for the exposure-two cohort were 30%, 59%, and 71%, respectively (all pairwise comparisons, P < .01). Prevalence of patients requiring hearing aids was 28.8% at exposure one and 58.4% at exposure two (P < .001; Fig 3D).
Fig 3.
Prevalence of (A) any hearing loss and (B) severe hearing loss and (C) degree of hearing loss by exposure and grading scale; (D) percentage of children requiring a hearing aid by exposure. ASHA, American Speech-Language-Hearing Association; CTCAEv3, Common Terminology Criteria for Adverse Events, version 3; Exp 1, exposure one (cisplatin ≤ 400 mg/m2); Exp 2, exposure two (cisplatin 400 mg/m2 and carboplatin 1,700 mg/m2).
Risk Factors for Severe Hearing Loss
Multivariable analysis revealed that the odds of developing severe hearing loss in exposure-two patients ranged from odds ratios (ORs) of 3.2 per Brock (95% CI, 1.1 to 9.8; P = .038) to 3.8 per CTCAEv3 (95% CI, 1.7 to 8.6; P = .002) compared with exposure-one patients. Additionally, ORs of developing severe hearing loss in patients hospitalized at least once for infection during induction ranged from 1.8 per CTCAEv3 (95% CI, 0.86 to 3.7, P = .124) to 5.1 per Brock (95% CI, 1.7 to 14.9; P = .004) compared with those never hospitalized during induction for infection (Fig 4).
Fig 4.
Results of unconditional multivariable logistic regression of risk factors for severe hearing loss (carboplatin conditioning, hospitalization for infection); controlled for race/ethnicity, sex, age at diagnosis, time from most recent platinum to auditory testing, preconsolidation glomerular filtration rate, and chemotherapy dose reduction during induction. CTCAEv3, Common Terminology Criteria for Adverse Events, version 3.
Exposure-two patients were 3.7× more likely to require a hearing aid (95% CI, 1.8 to 7.9; P = .001) compared with exposure-one patients. Furthermore, patients with a history of hospitalization for infection were 2.3× more likely to require a hearing aid (95% CI, 1.2 to 4.4; P = .01).
Concordance Among Grading Scales
There was high concordance among the scales in detecting normal versus impaired hearing (concordant pairs: ASHA v Brock, 99.3%; ASHA v CTCAEv3, 100%; ASHA v Chang, 99.3%; Brock v CTCAEv3, 100%; Brock v Chang, 99.6%; CTCAEv3 v Chang; 100%; P > .05 for all comparisons). However, concordance of severe hearing loss among scales was only 48.4% for Brock versus CTCAEv3 and 52.8% for Brock versus Chang, with Chang and CTCAEv3 demonstrating the maximum degree of concordance for severe hearing loss rating at 89% (P < .001 for all comparisons). Among patients requiring hearing aids, only 49.1% were graded as having severe hearing loss by Brock, compared with 91.3% by Chang and 100% by CTCAEv3 (P < .001).
DISCUSSION
In this large study using a homogeneously treated cohort of children with high-risk neuroblastoma, we found that 67% of patients had severe hearing loss (per CTCAEv3). Consistent with previous studies,37,51 the risk for severe hearing loss was increased > three-fold by all scales after consolidation with myeloablative carboplatin, and there was up to five-fold increased risk for severe hearing loss (by Brock scale) in those hospitalized for infection during induction, a surrogate for additional ototoxic exposures (eg, nonanthracycline aminoglycoside antibiotics). Finally, this study quantified the discordance among grading scales in identifying severe hearing loss.
Dose-intensive cisplatin induction followed by myeloablative carboplatin-based consolidation are among the most efficacious agents for management of high-risk neuroblastoma1,3,4,52–56; thus, use of these agents continues despite their known ototoxic properties. In addition, these patients often experience complications necessitating exposure to additional ototoxic agents, including nonanthracycline aminoglycoside antibiotics.30,37,50,57
Mild to moderate high-frequency hearing loss has significant implications for young children because of the high-frequency fricative sounds (such as th, k, and s) required for speech discrimination14 and identification of verb tense and pleural forms,58 and it has been associated with academic difficulties.15–17 In this study, > 80% of patients had at least mild hearing loss, regardless of grading scale used, with a large majority (from 61% per Brock to 81% per CTCAEv3) experiencing moderate or severe hearing loss. The functional implications of this hearing loss are substantial, with > 50% of the cohort requiring a hearing aid. Although hearing aid technology continues to improve, no hearing aid is able to restore normal hearing14,59; children with these devices require close monitoring, and most will need intensive speech/language services and specialized educational accommodations to maximize their educational potential.25,60 Hearing loss has significant educational and psychosocial consequences. Using data from a survey completed by parents of neuroblastoma survivors, patients with hearing loss were 2× more likely to have academic problems requiring special educational services than those with intact hearing. Hearing loss was also associated with lower patient-reported school and psychosocial functioning.61
Our study found no significant differences in discriminating normal from impaired hearing (range, 99.3% to 100% concordant pairs) across grading scales. However, significant discordance was identified among the scales in identifying severe hearing loss, with > 50% discordance in assignment of severe ratings between the two most commonly used scales: CTCAEv3 and Brock. For children who received cisplatin and myeloablative carboplatin, hearing loss was rated as severe in only 30% by the Brock scale, but it was rated as severe in 59% by Chang and 71% by CTCAEv3. A similar pattern was observed for patients who received cisplatin only, with 8% receiving a rating of severe by Brock, 32% by Chang, and 47% by CTCAEv3.
The difference in severity rating between the Brock and CTCAEv3 scales is in large part a result of the reliance of the latter scale on functional outcome in defining severe hearing loss (ie, patients requiring a hearing aid are by definition assigned severe [grade 3] rating according to CTCAEv334). The Chang scale39 is also structured to align severity ratings with functional outcomes through its rating schema, which includes evaluations of key interval frequencies (3,000 and 6,000 Hz) and thresholds < 40 dB that are typically used by audiologists in making clinical determinations regarding the need for hearing aids. Of note, the prevalence of hearing aids at both exposure one (28.8%) and exposure two (58.4%) closely approximated the prevalence of Chang severe ratings at those time points (32% and 59%, respectively).
The Brock scale20 was the first ototoxicity scale specifically developed to assess platinum-related hearing loss, and its design was based on audiograms from 41 children with high-frequency hearing loss that sloped an average of 45 dB per octave over the impaired frequencies. Given these large decrements per octave, and assuming that hearing would be normal or only minimally impaired at the octave below, 40 dB was selected as the cutoff for significant loss at each frequency.20 The Chang scale was built on the design employed by Brock to assess the typical pattern of hearing loss seen in platinum-based regimens, but it included modifications that addressed functional deficits caused by losses < 40 dB and at interval frequencies not assessed by Brock, with the goal of aligning objective severity grading with audiologists' clinical recommendations for amplification.39 The importance of the inclusion of modest (< 40 dB) decrements and key interval frequencies in assessing the functional implications of hearing loss (particularly with regard to need for amplification) is underscored by our finding that only 49% of children requiring a hearing aid were rated as having severe hearing loss according to the Brock scale, whereas 91% and 100% of these children received a severe rating according to the Chang and CTCAEv3 scales, respectively. Thus, the commonly used Brock scale significantly underestimated functionally severe hearing loss in this cohort.
Quantification of clinically meaningful hearing loss using standardized grading scales is of critical importance in assessing the relative toxicity of ototoxic agents used in oncology trials. In this study, we used all available data obtained during clinical auditory assessments of children participating in the COG A3973 trial to compare four commonly used ototoxicity grading scales. Our finding that postplatinum test results were inevaluable by ASHA in > 50% and by CTCAEv3 in 30% of the patients, in large part because of incomplete or inevaluable preplatinum data, is indicative that a requirement for preplatinum testing could limit the clinical applicability of a grading scale, particularly in young seriously ill children presenting with high-risk disease. Furthermore, ignoring pre-existing hearing loss and reporting grades purely as a change from preplatinum hearing status may obscure the true clinical impact of the hearing loss.62 Although the Chang and Brock scales do not require preplatinum testing, and the proportion of patients inevaluable by these scales was lower than that by the ASHA and CTCAEv3 scales, audiologic data were inevaluable by these scales in > 25% of children, underscoring the critical need for a grading scale that maximizes understanding of a child's auditory status using an efficiently obtained minimal data set.33
The study needs to be considered in the context of its limitations. Audiologic reports submitted by 90 institutions introduced some variability in quality and also affected completeness of data submitted (68% evaluable patients). However, other than slightly older age at diagnosis (3.3 v 2.6 years), all other characteristics were comparable between the two groups. The assessors were aware of the timing of audiograms with respect to platinum exposure, but they were unaware of transplantation status or total platinum dose when they assigned scores. The use of a cross-sectional study design precluded the examination of the trajectory of decline in auditory function over time; this aspect of the study will be addressed in the future. Finally, details regarding other ototoxic agents and irradiation were not available. We used hospitalization for infection as a surrogate for exposure to additional ototoxic agents during induction, but there was no surrogate measure available after consolidation. We expect that only a small minority of patients would have received radiation therapy involving the ear, given the typical presentation of neuroblastoma in the abdomen.
In conclusion, severe hearing loss is prevalent in children with high-risk neuroblastoma treated with platinum-based chemotherapy. Vulnerability is increased with carboplatin conditioning and hospitalization for infection. There is significant discordance among audiologic grading scales in categorizing severe platinum-related hearing loss in children; the Brock scale underestimates severe hearing loss and should be used with caution in this setting. Results from this study could inform selection of grading scales for use in future studies. Data from the current study are being used to evaluate CTCAEv440 and the newly proposed SIOP-Boston grading scale33—scales that are attempting to overcome the limitations of currently available scales.33,41 Platinum remains one of the mainstays of induction therapy for high-risk neuroblastoma; thus, auditory monitoring, avoidance of additional ototoxic agents, and early aural rehabilitation will remain essential in optimizing long-term audiologic outcomes for these patients.
Acknowledgment
We thank Dina Willis, COG (Children's Oncology Group) A3973 research coordinator at the COG Statistics and Data Center (Gainesville, FL) for her invaluable assistance in obtaining the audiologic data from the participating institutions and the patients and families who participated in the COG A3973 study.
Appendix
Participating Institutions
COG (Children's Oncology Group) A3973 participating institutions contributing audiologic data:
A.B. Chandler Medical Center, University of Kentucky; Alberta Children's Hospital; British Columbia Children's Hospital; Broward General Medical Center; C.S. Mott Children's Hospital, University of Michigan; Cabell Huntington Hospital; Cancer Research Center of Hawaii; Carilion Medical Center for Children at Roanoke; Cedars-Sinai Medical Center; Centre Hospitalier Universitaire de Quebec; Children's Hospital and Regional Medical Center; Children's Hospital Central California; Children's Hospital Los Angeles; Children's Hospital of Austin; Children's Hospital of Eastern Ontario; Children's Hospital of Orange County; Children's Hospital of Pittsburgh; Children's Hospital of Philadelphia; Children's Hospital of Western Ontario; Children's Hospital San Diego; Children's Hospitals and Clinics of Minnesota; Children's Medical Center Dayton; Children's Memorial Hospital of Omaha; Children's National Medical Center, Washington, DC; Children's Hospital of New Orleans/Louisiana State University Medical Center Community Clinical Oncology Program; Christiana Care Health Services/A.I. duPont Institute; Cincinnati Children's Hospital Medical Center; City of Hope National Medical Center; Columbus Children's Hospital; Cook Children's Medical Center; Dartmouth-Hitchcock Medical Center; DeVos Children's Hospital; Doernbecher Children's Hospital; Duke University Medical Center; East Tennessee Children's Hospital; Geisinger Medical Center; Georgetown University Medical Center; Hackensack University Medical Center; Hospital Sainte-Justine; Hospital for Sick Children; Indiana University, Riley Childrens Hospital; Inova Fairfax Hospital; Joe DiMaggio Children's Hospital at Memorial; Kaiser Permanente Medical Group, Northern California; Kalamazoo Center for Medical Studies; Kosair Children's Hospital; Loma Linda University Medical Center; Marshfield Clinic; McGill University Health Center, Montreal Children's Hospital; Medical University of South Carolina; Mercy Children's Hospital; Methodist Children's Hospital of South Texas; Michigan State University; Midwest Children's Cancer Center; Miller Children's Hospital/Harbor–University of California Los Angeles; Montefiore Medical Center, Moses Campus; New York Medical College; New York University Medical Center; Newark Beth Israel Medical Center; Raymond Blank Children's Hospital; Rhode Island Hospital; Sacred Heart Children's Hospital; San Jorge Children's Hospital; Sinai Hospital of Baltimore; South Carolina Cancer Center; Southern Illinois University School of Medicine; St John Hospital and Medical Center; St Mary's Hospital; St Vincent Children's Hospital, Indiana; St Vincent Hospital, Wisconsin; Stanford University Medical Center; Tampa Children's Hospital; The Children's Hospital, Denver, Colorado; The Children's Hospital at The Cleveland Clinic; The Children's Mercy Hospital; Tod Children's Hospital, Forum Health; Toledo Children's Hospital; Tulane University Health Sciences Center; University of California San Francisco School of Medicine; University of Alabama at Birmingham; University of Florida; University of Illinois; University of Iowa Hospitals and Clinics; University of Minnesota Medical Center, Fairview; University of Texas Health Science Center at San Antonio; University of Vermont College of Medicine; University of Wisconsin Hospital and Clinics; Virginia Commonwealth University Health System, Medical College of Virginia; Washington University School of Medicine; and West Virginia University Health Sciences Center, Morgantown.
Details of COG A3973 Therapy
COG A3973 therapy consisted of an intensive induction regimen, including a cumulative cisplatin dose of 400 mg/m2, peripheral stem-cell harvest, surgery, and myeloablative consolidation with a conditioning regimen containing carboplatin 1,700 mg/m2 followed by purged versus nonpurged autologous peripheral stem-cell reinfusion. Local radiation therapy (216 Gy in 12 fractions) was administered to primary and persistently active metastatic sites after the myeloablative phase. After consolidation, patients went on to receive 13-cis-retinoic acid for 6 months or were enrolled onto the COG ANBL0032 trial and randomly assigned to receive chimeric anti-GD2 plus 13-cis-retinoic acid versus 13-cis-retinoic acid alone. A subset of patients not eligible for or refusing myeloablative therapy received a modified regimen that included three cycles of maintenance therapy with topotecan and cyclophosphamide administered at 3- to 4-week intervals followed by 13-cis-retinoic acid. Details of COG A3973 protocol phases are summarized in Appendix Table A1.
Audiologic Evaluation Methods
Audiologic evaluation methods included behavioral (conventional, conditioned play, or visual reinforcement) audiometry or auditory brainstem response testing; evaluation technique was determined at the local site based on the patient's age, developmental and clinical status, and ability to cooperate. Testing was performed with insert earphones or headphones when possible to obtain ear-specific thresholds. The instructions developed by the investigators to guide evaluation of submitted audiometric data are summarized in Appendix Table A3.
Table A1.
Details of COG A3973 Therapy43
| Protocol Phase | Therapeutic Agents |
|---|---|
| Induction | Course one: cycles one to three; course two: cycles four to six |
| Cycles one, two, four, and six | Cyclophosphamide 2.1 g/m2 IV with mesna over 6 hours on days 0 and 1 (total dose 4.2 g/m2/cycle) |
| Doxorubicin 25 mg/m2 IV over 24 hours on days 0, 1, and 2 (total dose 75 mg/m2/cycle) | |
| Vincristine 0.67 mg/m2 IV over 24 hours on days 0, 1, and 2 (total dose 2 mg/m2/cycle) | |
| Cycles three and five | Cisplatin 50 mg/m2 IV over 1 hour on days 0, 1, 2, and 3 (total dose 200 mg/m2/cycle) |
| Etoposide 200 mg/m2 IV over 2 hours on days 0, 1, and 2 (total dose 600 mg/m2/cycle) | |
| Consolidation (myeloblative therapy) | Melphalan 70 mg/m2/day IV × 3 days (total dose 210 mg/m2; dose adjusted if GFR < 100 mL/min/1.73 m2) |
| Etoposide 338 mg/m2/day IV × 4 days (total dose 1,352 mg/m2; dose adjusted if GFR < 100 mL/min/1.73 m2) | |
| Carboplatin 425 mg/m2/day IV × 4 days (total dose 1,700 mg/m2; dose adjusted if GFR < 100 mL/min/1.73 m2) | |
| Maintenance* | Topotecan 0.75 mg/m2/day IV × 5 days (total dose 3.75 mg/m2) |
| Cyclophosphamide 250 mg/m2/day IV × 5 days with mesna (total dose 1,250 mg/m2) | |
| Cis-retinoic acid† | 13-cis-retinoic acid 80 mg/m2/dose PO 2×/day for six cycles (total dose 160 mg/m2/day, given for 2 weeks on and 2 weeks off during each cycle) |
Abbreviations: COG, Children's Oncology Group; GFR, glomerular filtration rate; IV, intravenous; PO, orally.
Only for patients refusing or ineligible for myeloablative consolidation therapy; three cycles at 3- to 4-week intervals.
All patients after consolidation or maintenance; six cycles of 2 weeks on and 2 weeks off.
Table A2.
Comparison of Ototoxicity Grading Scales Used in This Analysis
| Grading Scale | ASHA | Brock | Chang | CTCAEv3 |
|---|---|---|---|---|
| Brief description | Ototoxicity defined in absolute terms (presence/absence of hearing loss) Assesses decrease in hearing compared with preplatinum hearing status |
Developed by pediatric oncologist specifically for evaluation of platinum-related hearing loss Five-point scale based on progression of loss from high to low frequencies |
Modification of Brock scale Addresses functional deficits caused by losses < 40 dB and at intervals not assessed by Brock scale Seven-point scale (high to low frequencies) |
Developed by NCI/CTEP Four-point scale combines assessment of subjective and objective hearing loss |
| Criteria for grading determinations | Hearing loss is present if ≥ one of the following criteria are met: Grade A: ≥ 20 dB decrease in pure tone threshold at one test frequency Grade B: ≥ 10 dB decrease in pure tone threshold at two consecutive test frequencies Grade C: loss of response at three consecutive test frequencies where responses were previously obtained Hearing loss is absent if none of the above criteria are met |
Grade 0: thresholds < 40 dB HL at all tested frequencies Grade 1: thresholds ≥ 40 dB HL at 8,000 Hz Grade 2: thresholds ≥ 40 dB HL at 4,000 to 8,000 Hz Grade 3: thresholds ≥ 40 dB HL at 2,000 to 8,000 Hz Grade 4: thresholds ≥ 40 dB HL at 1,000 to 8,000 Hz |
Grade 0: ≤ 20 dB at 1,000, 2,000, and 4,000 Hz Grade 1a: ≥ 40 dB at any frequency 6,000 to 12,000 Hz Grade 1b: > 20 and < 40 dB at 4,000 Hz Grade 2a: ≥ 40 dB at ≥ 4,000 Hz Grade 2b: > 20 and < 40 dB at any frequency < 4,000 Hz Grade 3: ≥ 40 dB at 2,000 or ≥ 40 dB at 3,000 Hz Grade 4: ≥ 40 dB at ≥ 1,000 Hz |
Grade 0: does not meet criteria for grades 1-4 Grade 1: threshold shift or loss of 15-25 dB relative to preplatinum hearing status, averaged at ≥ two contiguous test frequencies in at least one ear, or subjective change in absence of grade 1 threshold shift Grade 2: threshold shift or loss of > 25 to 90 dB, averaged at two contiguous test frequencies in at least one ear Grade 3: hearing loss sufficient to indicate therapeutic intervention, including hearing aids (eg, ≥ 20 dB bilateral HL in speech frequencies; ≥ 30 dB unilateral HL), and requiring additional speech-language–related services Grade 4: audiologic indication for cochlear implant and requiring additional speech-language–related services |
| Strengths | Detection of early hearing loss | Simplicity of use No preplatinum hearing assessment needed |
Identifies earlier/milder hearing loss Useful for assessing clinical implications of loss No preplatinum hearing assessment needed |
NCI standard |
| Limitations | Requires comparison with preplatinum hearing status Does not measure severity or clinical impact of hearing loss |
Does not consider interval frequencies key to clinical applications (eg, 3,000 Hz) Does not measure losses < 40 dB at any frequency |
Complexity of use Requires extensive data set Not widely validated |
Complexity of use Requires comparison with preplatinum hearing status (unless hearing loss is grade ≥ 3 based on functional outcomes) Not specific to configuration of platinum-related loss Clinical impact of grades 1-2 unclear |
Abbreviations: ASHA, American Speech-Language-Hearing Association; CTCAEv3, Common Terminology Criteria for Adverse Events, version 3; CTEP, Cancer Therapy Evaluation Program; HL, hearing level; NCI, National Cancer Institute.
Table A3.
Audiologic Evaluation Methods
| Grading Scale | Evaluation Considerations |
|---|---|
| All | If more than one evaluable postplatinum audiometric test is available, use the most recent evaluable test |
| For ambiguous markings on submitted handwritten audiometric graphs, apply the following rules: if any part of the symbol touches the line, assume the primary value (eg, 30, 40, 50 dB, and so on); if no part of the symbol touches the line, assume the intermediate value (eg, 35, 45 dB, and so on) | |
| Use all available thresholds evaluable at 1,000, 2,000, 3,000, 4,000, 6,000, and 8,000 Hz (as indicted by the particular scale) to complete the rating | |
| If both soundfield and ear-specific testing were submitted for the same time point, preferentially use ear-specific testing (if evaluable) | |
| If threshold absent (ie, nonresponse, loss of response), record as 130 dB HL | |
| If all air-conduction thresholds are normal, assessment is complete (ie, no need to evaluate bone conduction and tympanogram results) | |
| For reports with any abnormal air-conduction thresholds, bone conduction and tympanogram results are evaluated as follows: | |
| —If both right and left unmasked bone conduction values are present, use the better threshold | |
| —If air/bone discrepancy > 20 dB, mark data as inevaluable | |
| Tympanogram is rated normal if peak is present and pressure is between −250 and +300; compliance, if included, is normal if ≥ 0.2 and ≤ 1.2 cm3; if tympanogram or immittance data are not included, but audiologist rating is present, use rating (eg, type A, normal); if no tympanogram report is submitted, assume normal result (ie, audiologists typically report pertinent positives) | |
| Soundfield testing: if 25 dB is considered abnormal per the scale, record as such (ie, even though 25 dB may be considered clinically normal in the soundfield, there are no accommodations in the grading scales for this) | |
| ABR testing: normal threshold values: ≤ 40 dB nHL at 500 Hz; ≤ 20 dB nHL at 1,000, 2,000, 3,000, and 4,000 Hz; ≤ 35 dB nHL at 6,000 Hz; ≤ 40 dB nHL at 8,000 Hz (according to the correction values provided by testing audiologists); if ABR marked as < a threshold, record at that threshold (eg, < 20 dB = 20 dB) | |
| ASHA | If both air- and bone-conduction thresholds are present, preferentially use air-conduction thresholds |
| Must have preplatinum data for each frequency to which ASHA criteria is applied (ie, do not assume normal preplatinum result) | |
| Brock | If both air- and bone-conduction thresholds are present, preferentially use air-conduction thresholds |
| Must have thresholds for at least two frequencies; at least one of the frequencies must be in the 2,000-8,000 Hz range | |
| For grades 2-4: thresholds must be ≥ 40 dB at all indicated frequencies for that grade; if threshold is ≥ 40 dB at a given frequency, and data are missing for higher frequencies, assume that thresholds for the higher frequencies are also ≥ 40 dB; if the highest frequency tested has a threshold < 40 dB, assume that all frequencies above the highest frequency tested also have thresholds < 40 dB | |
| Chang | If both air- and bone-conduction thresholds are present, preferentially use bone-conduction thresholds (ie, bone-conduction thresholds are preferred data for interpretation of Chang scale; for all other scales, bone conduction is used only to determine presence of air-bone gap) |
| Assignment of grade 0 requires normal thresholds at 1,000, 2,000, and 4,000 Hz; if any of these thresholds are missing, mark as not evaluable | |
| CTCAEv3 | If both air- and bone-conduction thresholds are present, preferentially use air-conduction thresholds |
| If the threshold for a frequency is missing preplatinum, the frequency is considered not evaluable on the postplatinum audiogram unless postplatinum threshold for the frequency is normal (≤ 20 dB) | |
| Grade 2: if the average of two contiguous frequencies is > 25 dB (even if one is normal), the severity grade = 2 | |
| For grade 3 (hearing loss sufficient to indicate therapeutic intervention, including hearing aids [eg, ≥ 20 dB bilateral HL in speech frequencies, ≥ 30 dB unilateral HL, and requiring additional speech-language services]): | |
| —Speech frequencies are defined as 500-4,000 Hz | |
| —≥ 20 dB bilateral HL applies to speech frequencies 500-4,000 Hz | |
| —≥ 30 dB unilateral HL applies to speech frequencies 500-4,000 Hz | |
| —Requiring additional speech-language services is defined as FM trainer, special class/resource teacher, or equivalent intervention, but does not include basic services such as preferential seating or communication strategies | |
| In the absence of any obvious indicator of hearing aid/assistive device use (eg, explicit audiologist notation or aided audiogram), determination regarding whether hearing aids/assistive devices are indicated/in use is based on review of all available audiology notes and is made collaboratively by two investigators based on the overall prescribing pattern determined for cohort; if no data are available, and the indicator is not obvious based on the audiologic report, mark as unknown | |
| Indications for cochlear implant (CTCAEv3 grade 4): thresholds ≥ 80 dB at frequencies between 500 and 4,000 Hz (not correctable with hearing aid) |
Abbreviations: ABR, auditory brainstem response; ASHA, American Speech-Language-Hearing Association; CTCAEv3, Common Terminology Criteria for Adverse Events, version 3; HL, hearing level; nHL, normal hearing level.
Table A4.
Acceptable Date Parameters for Audiologic Testing
| Time Point | Planned per-Protocol Platinum | First Acceptable Date for Audiometric Test | Last Acceptable Date for Audiometric Test |
|---|---|---|---|
| Preplatinum | None | Study enrollment | 1 day before initiation of cycle three |
| Exposure one | Cisplatin 200 mg/m2: induction course one, cycle three, days 0-4 | 4 days after last dose of cisplatin administered during cycle three | 1 day before initiation of cycle five |
| Cisplatin 200 mg/m2: induction course two, cycle five, days 0-4 | 4 days after last dose of cisplatin administered during cycle five | Patients undergoing transplantation: 1 day before initiation of transplantation conditioning regimen Patients without transplantation: 1 day before initiation of topotecan/cyclophosphamide maintenance therapy |
|
| Exposure two | Carboplatin 1,700 mg/m2: delivered over 4 days, beginning 7 days before transplantation and ending 3 days before transplantation | Day after transplantation | Any date after date of transplantation |
NOTE. Exposure one: cisplatin ≤ 400 mg/m2; exposure two: cisplatin 400 mg/m2 and carboplatin 1,700 mg/m2.
Table A5.
Evaluable Audiometric Tests by Grading Scale by Exposure
| Grading Scale | Evaluable Patients |
Nonevaluable Patients |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure One (n = 66) |
Exposure Two (n = 267) |
Total (n = 333) |
Exposure One (n = 66) |
Exposure Two (n = 267) |
Total (n = 333) |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| ASHA | 33 | 50.0 | 146 | 54.7 | 179 | 53.8 | 33 | 50.0 | 121 | 45.3 | 154 | 46.2 |
| Brock | 63 | 95.5 | 247 | 92.5 | 310 | 93.1 | 3 | 4.5 | 20 | 7.5 | 23 | 6.9 |
| Chang | 62 | 93.9 | 243 | 91.0 | 305 | 91.6 | 4 | 6.1 | 24 | 9.0 | 28 | 8.4 |
| CTCAEv3 | 51 | 77.3 | 242 | 90.6 | 293 | 88.0 | 15 | 22.7 | 25 | 9.4 | 40 | 12.0 |
NOTE. Data for the 333 patients with audiometric data who were evaluable by at least one scale and met specified date parameters.
Abbreviations: ASHA, American Speech-Language-Hearing Association; CTCAEv3, Common Terminology Criteria for Adverse Events, version 3.
Footnotes
Supported in part by Children's Oncology Group Chair's Grant No. U10 CA098543 and Grant No. 5R01 CA137488 from the National Cancer Institute (K.K.).
Presented as a podium presentation at the 47th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2011.
The National Cancer Institute had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00004188.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Wendy Landier, Kristin Knight, Susan G. Kreissman, Mary Lou Schmidt, James G. Gurney, Smita Bhatia
Financial support: Smita Bhatia
Administrative support: Wendy Landier, Wendy B. London, Smita Bhatia
Provision of study materials or patients: Mary Lou Schmidt
Collection and assembly of data: Wendy Landier, Kristin Knight, Ola Thomas, Wendy B. London
Data analysis and interpretation: Wendy Landier, Kristin Knight, F. Lennie Wong, Jin Lee, Ola Thomas, Heeyoung Kim, Mary Lou Schmidt, Lu Chen, Wendy B. London, James G. Gurney, Smita Bhatia
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Brodeur GM, Hogarty MD, Mosse YP, et al. Neuroblastoma. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. Philadelphia, PA: Wolters Kluwer-Lippincott Williams and Wilkins; 2011. pp. 886–922. [Google Scholar]
- 2.Cheung NV, Heller G. Chemotherapy dose intensity correlates strongly with response, median survival, and median progression-free survival in metastatic neuroblastoma. J Clin Oncol. 1991;9:1050–1058. doi: 10.1200/JCO.1991.9.6.1050. [DOI] [PubMed] [Google Scholar]
- 3.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid: Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 4.Berthold F, Boos J, Burdach S, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: A randomised controlled trial. Lancet Oncol. 2005;6:649–658. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 5.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valteau-Couanet D, Michon J, Boneu A, et al. Results of induction chemotherapy in children older than 1 year with a stage 4 neuroblastoma treated with the NB 97 French Society of Pediatric Oncology (SFOP) protocol. J Clin Oncol. 2005;23:532–540. doi: 10.1200/JCO.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 7.Maris JM, Hogarty MD, Bagatell R, et al. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 8.Laverdière C, Cheung NK, Kushner BH, et al. Long-term complications in survivors of advanced stage neuroblastoma. Pediatr Blood Cancer. 2005;45:324–332. doi: 10.1002/pbc.20331. [DOI] [PubMed] [Google Scholar]
- 9.Barr RD, Chalmers D, De Pauw S, et al. Health-related quality of life in survivors of Wilms' tumor and advanced neuroblastoma: A cross-sectional study. J Clin Oncol. 2000;18:3280–3287. doi: 10.1200/JCO.2000.18.18.3280. [DOI] [PubMed] [Google Scholar]
- 10.Hobbie WL, Moshang T, Carlson CA, et al. Late effects in survivors of tandem peripheral blood stem cell transplant for high-risk neuroblastoma. Pediatr Blood Cancer. 2008;51:679–683. doi: 10.1002/pbc.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McHaney VA, Thibadoux G, Hayes FA, et al. Hearing loss in children receiving cisplatin chemotherapy. J Pediatr. 1983;102:314–317. doi: 10.1016/s0022-3476(83)80551-4. [DOI] [PubMed] [Google Scholar]
- 12.Schell MJ, McHaney VA, Green AA, et al. Hearing loss in children and young adults receiving cisplatin with or without prior cranial irradiation. J Clin Oncol. 1989;7:754–760. doi: 10.1200/JCO.1989.7.6.754. [DOI] [PubMed] [Google Scholar]
- 13.Heck JE, Ritz B, Hung RJ, et al. The epidemiology of neuroblastoma: A review. Paediatr Perinat Epidemiol. 2009;23:125–143. doi: 10.1111/j.1365-3016.2008.00983.x. [DOI] [PubMed] [Google Scholar]
- 14.Stelmachowicz PG, Pittman AL, Hoover BM, et al. The importance of high-frequency audibility in the speech and language development of children with hearing loss. Arch Otolaryngol Head Neck Surg. 2004;130:556–562. doi: 10.1001/archotol.130.5.556. [DOI] [PubMed] [Google Scholar]
- 15.Wake M, Hughes EK, Poulakis Z, et al. Outcomes of children with mild-profound congenital hearing loss at 7 to 8 years: A population study. Ear Hear. 2004;25:1–8. doi: 10.1097/01.AUD.0000111262.12219.2F. [DOI] [PubMed] [Google Scholar]
- 16.Bess FH, Dodd-Murphy J, Parker RA. Children with minimal sensorineural hearing loss: Prevalence, educational performance, and functional status. Ear Hear. 1998;19:339–354. doi: 10.1097/00003446-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Davis JM, Elfenbein J, Schum R, et al. Effects of mild and moderate hearing impairments on language, educational, and psychosocial behavior of children. J Speech Hear Disord. 1986;51:53–62. doi: 10.1044/jshd.5101.53. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: The influence of age and the cumulative dose. Eur J Cancer. 2004;40:2445–2451. doi: 10.1016/j.ejca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Blakley BW, Myers SF. Patterns of hearing loss resulting from cis-platinum therapy. Otolaryngol Head Neck Surg. 1993;109:385–391. doi: 10.1177/019459989310900302. [DOI] [PubMed] [Google Scholar]
- 20.Brock PR, Bellman SC, Yeomans EC, et al. Cisplatin ototoxicity in children: A practical grading system. Med Pediatr Oncol. 1991;19:295–300. doi: 10.1002/mpo.2950190415. [DOI] [PubMed] [Google Scholar]
- 21.Ilveskoski I, Saarinen UM, Wiklund T, et al. Ototoxicity in children with malignant brain tumors treated with the “8 in 1” chemotherapy protocol. Med Pediatr Oncol. 1996;27:26–31. doi: 10.1002/(SICI)1096-911X(199607)27:1<26::AID-MPO6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 22.Montaguti M, Brandolini C, Ferri GG, et al. Cisplatin and carboplatin-induced ototoxicity in children: Clinical aspects and perspectives for prevention [in Italian] Acta Otorhinolaryngol Ital. 2002;22:14–18. [PubMed] [Google Scholar]
- 23.Skinner R, Pearson AD, Amineddine HA, et al. Ototoxicity of cisplatinum in children and adolescents. Br J Cancer. 1990;61:927–931. doi: 10.1038/bjc.1990.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kushner BH, Budnick A, Kramer K, et al. Ototoxicity from high-dose use of platinum compounds in patients with neuroblastoma. Cancer. 2006;107:417–422. doi: 10.1002/cncr.22004. [DOI] [PubMed] [Google Scholar]
- 25.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: Underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23:8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 26.Stohr W, Langer T, Kremers A, et al. Cisplatin-induced ototoxicity in osteosarcoma patients: A report from the late effects surveillance system. Cancer Invest. 2005;23:201–207. doi: 10.1081/cnv-200055951. [DOI] [PubMed] [Google Scholar]
- 27.Lewis MJ, DuBois SG, Fligor B, et al. Ototoxicity in children treated for osteosarcoma. Pediatr Blood Cancer. 2009;52:387–391. doi: 10.1002/pbc.21875. [DOI] [PubMed] [Google Scholar]
- 28.Gupta AA, Capra M, Papaioannou V, et al. Low incidence of ototoxicity with continuous infusion of cisplatin in the treatment of pediatric germ cell tumors. J Pediatr Hematol Oncol. 2006;28:91–94. doi: 10.1097/01.mph.0000199586.98926.8e. [DOI] [PubMed] [Google Scholar]
- 29.Bertolini P, Lassalle M, Mercier G, et al. Platinum compound-related ototoxicity in children: Long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol. 2004;26:649–655. doi: 10.1097/01.mph.0000141348.62532.73. [DOI] [PubMed] [Google Scholar]
- 30.Bates DE, Beaumont SJ, Baylis BW. Ototoxicity induced by gentamicin and furosemide. Ann Pharmacother. 2002;36:446–451. doi: 10.1345/aph.1A216. [DOI] [PubMed] [Google Scholar]
- 31.Ross CJ, Katzov-Eckert H, Dubé MP, et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet. 2009;41:1345–1349. doi: 10.1038/ng.478. [DOI] [PubMed] [Google Scholar]
- 32.Riedemann L, Lanvers C, Deuster D, et al. Megalin genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Pharmacogenomics J. 2008;8:23–28. doi: 10.1038/sj.tpj.6500455. [DOI] [PubMed] [Google Scholar]
- 33.Brock PR, Knight KR, Freyer DR, et al. Platinum-induced ototoxicity in children: A consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol. 2012;30:2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Cancer Institute. Common Terminology Criteria for Adverse Events, Version 3.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 35.Brock PR, Yeomans EC, Bellman SC, et al. Cisplatin therapy in infants: Short and long-term morbidity. Br J Cancer Suppl. 1992;18:S36–S40. [PMC free article] [PubMed] [Google Scholar]
- 36.Pritchard J, Brown J, Shafford E, et al. Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: A successful approach—Results of the first prospective study of the International Society of Pediatric Oncology. J Clin Oncol. 2000;18:3819–3828. doi: 10.1200/JCO.2000.18.22.3819. [DOI] [PubMed] [Google Scholar]
- 37.Parsons SK, Neault MW, Lehmann LE, et al. Severe ototoxicity following carboplatin-containing conditioning regimen for autologous marrow transplantation for neuroblastoma. Bone Marrow Transplant. 1998;22:669–674. doi: 10.1038/sj.bmt.1701391. [DOI] [PubMed] [Google Scholar]
- 38.American Speech-Language-Hearing Association. Guidelines for the audiologic management of individuals receiving cochleotoxic drug therapy. ASHA. 1994;36(suppl 12):11–19. [Google Scholar]
- 39.Chang KW, Chinosornvatana N. Practical grading system for evaluating cisplatin ototoxicity in children. J Clin Oncol. 2010;28:1788–1795. doi: 10.1200/JCO.2009.24.4228. [DOI] [PubMed] [Google Scholar]
- 40.National Cancer Institute. Common Terminology Criteria for Adverse Events, Version 4.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40.
- 41.Neuwelt EA, Brock P. Critical need for international consensus on ototoxicity assessment criteria. J Clin Oncol. 2010;28:1630–1632. doi: 10.1200/JCO.2009.26.7872. [DOI] [PubMed] [Google Scholar]
- 42.Katzenstein HM, Chang KW, Krailo M, et al. Amifostine does not prevent platinum-induced hearing loss associated with the treatment of children with hepatoblastoma: A report of the Intergroup Hepatoblastoma Study P9645 as a part of the Children's Oncology Group. Cancer. 2009;115:5828–5835. doi: 10.1002/cncr.24667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kreissman SG, Seeger RC, Matthay KK, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): A randomised phase 3 trial. Lancet Oncol. 2013;14:999–1008. doi: 10.1016/S1470-2045(13)70309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 45.Zhang H, Xia Y, Chen R, et al. On modeling longitudinal binomial responses: Implications from two dueling paradigms. J Appl Stat. 2011;38:2373–2390. [Google Scholar]
- 46.Breslow NE, Clayton DG. Approximate inference in generalized linear mixed models. J Am Stat Assoc. 1993;88:9–25. [Google Scholar]
- 47.Siegel S. Nonparametric Statistics for the Behavioral Sciences. New York, NY: McGraw-Hill; 1956. [Google Scholar]
- 48.Cox DR. Analysis of Binary Data. London, United Kingdom: Chapman and Hall; 1969. [Google Scholar]
- 49.Womer RB, Pritchard J, Barratt TM. Renal toxicity of cisplatin in children. J Pediatr. 1985;106:659–663. doi: 10.1016/s0022-3476(85)80098-6. [DOI] [PubMed] [Google Scholar]
- 50.Schacht J, Talaska AE, Rybak LP. Cisplatin and aminoglycoside antibiotics: Hearing loss and its prevention. Anat Rec (Hoboken) 2012;295:1837–1850. doi: 10.1002/ar.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Punnett A, Bliss B, Dupuis LL, et al. Ototoxicity following pediatric hematopoietic stem cell transplantation: A prospective cohort study. Pediatr Blood Cancer. 2004;42:598–603. doi: 10.1002/pbc.20036. [DOI] [PubMed] [Google Scholar]
- 52.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castleberry RP, Cantor AB, Green AA, et al. Phase II investigational window using carboplatin, iproplatin, ifosfamide, and epirubicin in children with untreated disseminated neuroblastoma: A Pediatric Oncology Group study. J Clin Oncol. 1994;12:1616–1620. doi: 10.1200/JCO.1994.12.8.1616. [DOI] [PubMed] [Google Scholar]
- 54.Kushner BH, LaQuaglia MP, Bonilla MA, et al. Highly effective induction therapy for stage 4 neuroblastoma in children over 1 year of age. J Clin Oncol. 1994;12:2607–2613. doi: 10.1200/JCO.1994.12.12.2607. [DOI] [PubMed] [Google Scholar]
- 55.Ladenstein RL, Poetschger U, Luksch R, et al. Busulphan-melphalan as a myeloablative therapy (MAT) for high-risk neuroblastoma: Results from the HR-NBL1/SIOPEN trial. J Clin Oncol. 2011;29(suppl):5s. abstr 2. [Google Scholar]
- 56.Ladenstein R, Valteau-Couanet D, Brock P, et al. Randomized trial of prophylactic granulocyte colony-stimulating factor during rapid COJEC induction in pediatric patients with high-risk neuroblastoma: The European HR-NBL1/SIOPEN study. J Clin Oncol. 2010;28:3516–3524. doi: 10.1200/JCO.2009.27.3524. [DOI] [PubMed] [Google Scholar]
- 57.Ding D, Allman BL, Salvi R. Review: Ototoxic characteristics of platinum antitumor drugs. Anat Rec (Hoboken) 2012;295:1851–1867. doi: 10.1002/ar.22577. [DOI] [PubMed] [Google Scholar]
- 58.Horwitz AR, Dubno JR, Ahlstrom JB. Recognition of low-pass-filtered consonants in noise with normal and impaired high-frequency hearing. J Acoust Soc Am. 2002;111:409–416. doi: 10.1121/1.1427357. [DOI] [PubMed] [Google Scholar]
- 59.Berg AL, Spitzer JB, Garvin JH., Jr Ototoxic impact of cisplatin in pediatric oncology patients. Laryngoscope. 1999;109:1806–1814. doi: 10.1097/00005537-199911000-00016. [DOI] [PubMed] [Google Scholar]
- 60.Grewal S, Merchant T, Reymond R, et al. Auditory late effects of childhood cancer therapy: A report from the Children's Oncology Group. Pediatrics. 2010;125:e938–e950. doi: 10.1542/peds.2009-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gurney JG, Tersak JM, Ness KK, et al. Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: A report from the Children's Oncology Group. Pediatrics. 2007;120:e1229–e1236. doi: 10.1542/peds.2007-0178. [DOI] [PubMed] [Google Scholar]
- 62.Chang KW. Clinically accurate assessment and grading of ototoxicity. Laryngoscope. 2011;121:2649–2657. doi: 10.1002/lary.22376. [DOI] [PubMed] [Google Scholar]