Abstract
Purpose
The objective of this study was to test the efficacy of cognitive-behavioral therapy plus hypnosis (CBTH) to control fatigue in patients with breast cancer undergoing radiotherapy. We hypothesized that patients in the CBTH group receiving radiotherapy would have lower levels of fatigue than patients in an attention control group.
Patients and Methods
Patients (n = 200) were randomly assigned to either the CBTH (n = 100; mean age, 55.59 years) or attention control (n = 100; mean age, 55.97 years) group. Fatigue was measured at four time points (baseline, end of radiotherapy, 4 weeks, and 6 months after radiotherapy). Fatigue was measured using the Functional Assessment of Chronic Illness Therapy (FACIT) –Fatigue subscale and Visual Analog Scales (VASs; Fatigue and Muscle Weakness).
Results
The CBTH group had significantly lower levels of fatigue (FACIT) at the end of radiotherapy (z, 6.73; P < .001), 4-week follow-up (z, 6.98; P < .001), and 6-month follow-up (z, 7.99; P < .001) assessments. Fatigue VAS scores were significantly lower in the CBTH group at the end of treatment (z, 5.81; P < .001) and at the 6-month follow-up (z, 4.56; P < .001), but not at the 4-week follow-up (P < .07). Muscle Weakness VAS scores were significantly lower in the CBTH group at the end of treatment (z, 9.30; P < .001) and at the 6-month follow-up (z, 3.10; P < .02), but not at the 4-week follow-up (P < .13).
Conclusion
The results support CBTH as an evidence-based intervention to control fatigue in patients undergoing radiotherapy for breast cancer. CBTH is noninvasive, has no adverse effects, and its beneficial effects persist long after the last intervention session. CBTH seems to be a candidate for future dissemination and implementation.
INTRODUCTION
In 2013, more than 200,000 American women will be diagnosed with breast cancer (BCa),1 and nearly half will undergo adjuvant radiotherapy.2 Although BCa radiotherapy increases disease-free survival and life expectancy, it is not without adverse consequences, primarily fatigue. Patients receiving radiotherapy rate fatigue as their most prevalent and severe symptom.3 Fatigue increases over the course of radiotherapy,3–5 and off-treatment fatigue occurs among some BCa survivors.6,7 In fact, one study found that up to 40% of patients who received radiotherapy for BCa reported fatigue 1 year after treatment.8 Fatigue has been demonstrated to be the strongest predictor of quality of life in women after radiotherapy,9 and fatigue has pervasive and detrimental effects on numerous aspects of patients' functioning.7,10–12
Fatigue is a multidimensional construct.13 Although cancer treatments are clearly one source of fatigue, psychological factors also contribute.6,8,14–16 Meta-analysis has indicated that psychological interventions, such as cognitive-behavioral therapy (CBT), are efficacious in reducing cancer-related fatigue.17
Meta-analyses (in studies of pain, anxiety, and weight loss) have indicated that adding hypnosis to CBT significantly increases effect sizes relative to CBT alone.18–20 During hypnosis, patients can be given suggestions for reduced fatigue that change patients' expectations for fatigue, which in turn may directly lead to reductions in patients' experiences of fatigue.21 The literature22 has supported the efficacy of hypnosis in controlling cancer treatment–related adverse effects, including fatigue in patients with BCa undergoing surgery.23
Recognizing that the combination of CBT and hypnosis can increase clinical benefit,18 we developed an intervention combining CBT and hypnosis for controlling fatigue during BCa radiotherapy. Initial results5 revealed significant beneficial effects of CBTH on fatigue during radiotherapy, with medium to large effect sizes. Although these findings were encouraging, there were limitations, including a relatively small sample (n = 42), the lack of a professional attention control (AC) group, and that fatigue measurement ended at the conclusion of radiotherapy. It was unknown whether intervention benefits would continue past the acute treatment period.
The objective of this study was to test the efficacy of CBTH to control fatigue in a new randomized controlled trial (RCT) of patients with BCa receiving radiotherapy. We hypothesized that CBTH patients would have lower levels of fatigue than patients in the AC group, and that CBTH effects would persist beyond the end of radiotherapy.
PATIENTS AND METHODS
Participants
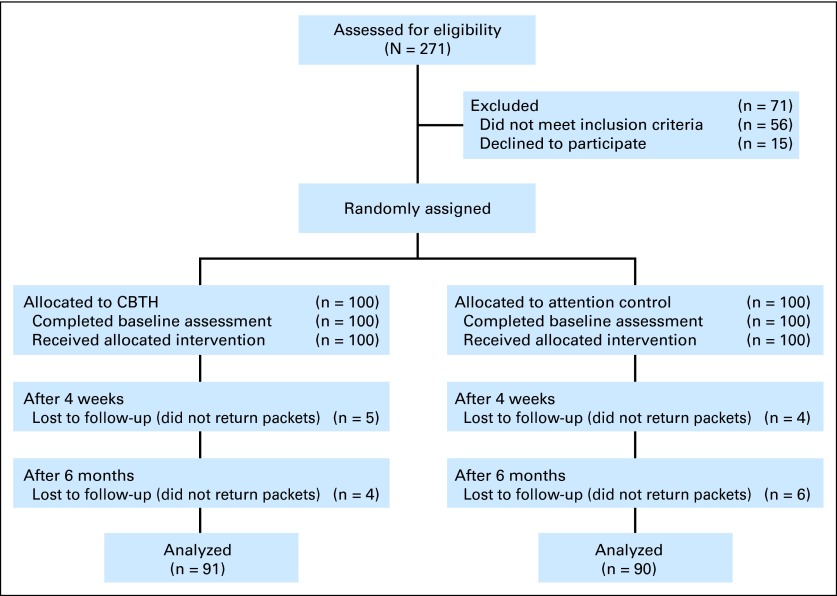
Consecutive patients with BCa receiving radiotherapy were recruited from Mount Sinai Medical Center between January 2006 and July 2011. Eligibility criteria included the following: scheduled for a 6-week course of external-beam radiotherapy; able to speak and read English; older than age 18 years; and willing to be randomly assigned to study treatment groups. Exclusion criteria included: uncontrolled comorbid medical or psychiatric illness (on the basis of chart review); taking medications or having conditions associated with fatigue (eg, chronic fatigue syndrome); or metastatic disease. There were no changes to the criteria after trial commencement. All participants provided written informed consent. The study was approved by our Program for the Protection of Human Subjects (Fig 1).24
Fig 1.
CONSORT diagram. CBTH, cognitive-behavioral therapy plus hypnosis.
Two hundred patients were randomly assigned to either the CBTH (n = 100) or AC (n = 100) group using computer-generated random positive integers.25 Random assignment was performed by one of the authors (G.H.M.) using randomized block lengths, with an average block length of 10. Sample characteristics are presented in Table 1.
Table 1.
Characteristics of Participants by Group
| Characteristic | Group |
Significance | |||
|---|---|---|---|---|---|
| CBTH (n = 100) |
AC (n = 100) |
||||
| No. | % | No. | % | ||
| Age, years | F = 0.06; P < .81 | ||||
| Mean | 55.59 | 55.97 | |||
| SD | 9.69 | 11.89 | |||
| Education | χ2 = 3.05; P < .81 | ||||
| College degree or higher | 66 | 66 | 66 | 66 | |
| Less than college degree | 34 | 34 | 34 | 34 | |
| Marital status | |||||
| Currently married | 53 | 53 | 51 | 51 | |
| Not currently married | 47 | 47 | 49 | 49 | |
| Race | χ2 = 0.95; P < .63 | ||||
| White | 69 | 69 | 66 | 66 | |
| African American | 20 | 20 | 22 | 22 | |
| Other | 11 | 11 | 12 | 12 | |
| Ethnicity | χ2 = 0.28; P < .60 | ||||
| Hispanic | 22 | 22 | 19 | 19 | |
| Non-Hispanic | 78 | 78 | 81 | 81 | |
| Chemotherapy before radiotherapy | χ2 = 0.52; P < .47 | ||||
| Yes | 43 | 43 | 38 | 38 | |
| No | 57 | 57 | 62 | 62 | |
| KPS, % | F = 0.01; P < .92 | ||||
| Mean | 93.95 | 94.05 | |||
| SD | 7.78 | 6.13 | |||
| Adjuvant hormone treatment | χ2 = 0.08; P < .96 | ||||
| Tamoxifen | 30 | 30 | 31 | 31 | |
| Aromatase inhibitors | 44 | 44 | 42 | 42 | |
| None | 26 | 26 | 27 | 27 | |
| Stage | χ2 = 0.26; P < .97 | ||||
| 0 | 29 | 29 | 32 | 32 | |
| I | 39 | 39 | 38 | 38 | |
| II | 21 | 21 | 19 | 19 | |
| III | 11 | 11 | 11 | 11 | |
| Total radiation dose, Gy | F = 0.01; P < .91 | ||||
| Mean | 61.83 | 61.79 | |||
| SD | 2.66 | 2.22 | |||
| Total No. of study sessions | F = 0.45; P < .51 | ||||
| Mean | 12.29 | 12.08 | |||
| SD | 2.55 | 2.01 | |||
| Neuroticism | F = 0.25; P < .63 | ||||
| Mean | 20.82 | 20.31 | |||
| SD | 7.71 | 6.83 | |||
| Treatment credibility | F = 2.30; P < .14 | ||||
| Mean | 42.48 | 40.98 | |||
| SD | 6.34 | 6.52 | |||
Abbreviations: AC, attention control group; CBTH, cognitive-behavioral therapy plus hypnosis; KPS, Karnofsky performance status; SD, standard deviation.
Procedures
To reduce potential bias, blinding procedures were followed for assessment personnel (research assistants), radiation oncologists, radiation therapists, nurses, and front desk staff. Although blinding to group assignment was not formally assessed, the following precautions were taken: study intervention sessions took place in a private room away from clinical staff; no outcome data were collected by clinical staff or study interventionists; and the same interventionists (four doctoral-level clinical psychologists) met with all patients in both the CBTH and AC arms, so study assessment staff were not cued to group assignment by an interventionist's presence. Patients could not be blinded to their group, given that this was a behavioral intervention. Interventionists were given each patient's random assignment by one of the authors (G.H.M.) on the morning of the initial intervention session in a sealed envelope. Eligibility was confirmed before group assignment.
CBTH and AC sessions were delivered to patients individually by interventionists according to the study protocol manual. On patients' radiotherapy simulation day (the first preradiotherapy treatment planning session), baseline questionnaires were administered by an RA.
Consistent across both groups.
In both groups, the initial intervention session (scheduled for patients' radiotherapy verification day) lasted for 30 minutes. During the course of radiotherapy, patients met with an interventionist twice per week; each session lasted 15 minutes. The final intervention session (scheduled for the penultimate day of radiotherapy) lasted for 30 minutes. All patients received standard medical care. At the conclusion of radiotherapy, participants completed a Treatment Credibility Questionnaire.26
CBTH group.
On verification day, patients in the CBTH group received an initial CBTH training session lasing 30 minutes. The CBT segment (15 minutes) had three major components. First, the ABC model of cognitive-behavioral therapy (A, activating events; B, beliefs; C, consequences) was taught.27,28 Patients were taught to identify negative, unhelpful beliefs and the emotional, behavioral, and physical consequences of those beliefs. Second, patients were taught to complete a thought record worksheet that was based on the ABC model.28 Interventionists completed a worksheet together with the patient in this session. Third, patients were taught behavioral strategies (eg, activity scheduling, distraction) to help manage fatigue.
On verification day, patients also received a 15-minute hypnosis session after CBT. First, to allay any potential concerns about hypnosis, possible patient concerns and misconceptions about hypnosis were addressed. Hypnosis then began with relaxing imagery followed by suggestions for reduced distress and reduced fatigue during radiotherapy.5,29 The standardized hypnosis session concluded by providing patients with instructions for how to use hypnosis on their own.30 At the end of the session, each patient was given a CBTH workbook that had been developed by our group (Data Supplement).
In the first weekly session during radiotherapy, a CBT worksheet was reviewed by the patient and therapist, and the ABC model was reinforced (15 minutes). In the second weekly session, a second worksheet was reviewed by the therapist and patient for 10 minutes, and 5 minutes was devoted to hypnosis. Patients did not have to learn or maintain hypnosis on their own over the course of radiotherapy because they received a live session every week. The alternating session content pattern was repeated throughout the course of radiotherapy. On the penultimate day of radiotherapy, the 15-minute CBT component of the session reviewed themes that had emerged over the course of treatment and provided relapse prevention strategies. The second 15-minute period was devoted to hypnosis, including suggestions for increased well-being and reduced fatigue after the conclusion of radiotherapy.
AC group.
An AC group was used to control for attention, notably the potential effects of simply interacting with an empathic interventionist. AC participants met with an interventionist for the same amount of time as CBTH participants. AC procedures were based on manualized approaches.23,31 For AC participants, the interventionist did not lead the patient in imagery, relaxation, evaluation of thought processes, or even simple discussion. Rather, the interventionist allowed the patient to direct the flow of the conversation and provided supportive/empathic comments.
All interventionists had advanced training in CBT and hypnosis, underwent didactic and practical training, and completed at least five practice interventions with healthy volunteers under the direct supervision of one of the authors (J.B.S.). Patient permission was obtained to audiotape sessions, and 20% of audiotapes were randomly reviewed by one of the authors (G.H.M.) to ensure treatment fidelity using a standardized fidelity checklist. There were no significant effects of interventionist on outcome variables (Ps > .52).
Outcomes
Patients completed self-report outcome measures at baseline (simulation day), at the end of radiotherapy (penultimate day of radiotherapy), and at 4 weeks and 6 months after radiotherapy. At 4 weeks and 6 months, patients returned questionnaires using prepaid and preaddressed envelopes. Patients were reminded by RAs over the phone to return follow-up questionnaires. There were no changes to trial outcomes after study commencement.
Primary fatigue measure.
The 13-item Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F) subscale was used to measure fatigue.32 The FACIT-F has demonstrated excellent internal consistency (α = .93 to .95), good test-retest reliability (α = .90), and validity in patients with BCa.32 For ease of interpretation, the FACIT-F was scored such that higher scores indicated greater fatigue. In the present sample, baseline α was .94.
Secondary fatigue measures.
Two 100-mm visual analog scales (VASs) were used to assess fatigue and muscle weakness.5,23,33 The fatigue VAS stated, “RIGHT NOW how fatigued do you feel?” The line was anchored by “Not at all fatigued,” and “As fatigued as I could be.” A VAS to assess muscle weakness, another aspect of fatigue,13 was used in an identical manner (“RIGHT NOW how much muscle weakness do you have?” The line was anchored by “No muscle weakness at all,” and “As much muscle weakness as there could be”).
Possible covariates.
On the basis of previous research,5,34,35 possible covariates of fatigue included neuroticism and chemotherapy history. The NEO Neuroticism Subscale (NEO-N)36 was used to assess neuroticism. The NEO-N36 is a widely used, well-validated, self-report questionnaire with 12 items and good internal consistency (α = .86; present sample, α = .85). Chemotherapy history was assessed via medical charts and scored dichotomously (yes/no). Self-reported demographic data were also collected, and relevant medical history variables (eg, adjuvant hormonal therapy, Karnofsky performance status37) were abstracted from medical records.
Sample Size
Sample size was based on published effect sizes for CBTH on fatigue in this population (d, 0.59 to 0.82)5 and the ability to detect the influence of up to six potential covariates. With power set at 0.80, two-tailed α set at .05, and using a repeated-measures design, the minimum total sample size was calculated to be 175 participants.38 Intent-to-treat procedures were followed. Specifically, the mixed-effects regression approach that was employed used all available observations to estimate effects at each time point. Effects were computed using full information maximum likelihood estimation, which provides unbiased estimates when data are missing at random. Mixed-model approaches are consistent with the intent-to-treat principle in that all available patients are used to estimate effects. This approach provides tests that have greater statistical power and lower bias than other methods of handling missing observations in RCTs.39
Statistical Analyses
To test the efficacy of CBTH on fatigue, we fit a series of multilevel models, wherein observations were nested within individuals and the model intercept was a random effect.40 Given the uneven spacing of measurements and our expectation that treatment effects would not be a linear function of time, we treated assessment point as a categorical independent variable. Thus, our primary analyses tested whether there were significant group differences between CBTH and AC at baseline, end of radiotherapy, 4-week follow-up, and 6-month follow-up. To control for multiple statistical comparisons, we applied a family-wise error correction that maintained an overall α level of .05 for each outcome model.41 Adjusted P values are reported on the basis of this single-step multiple comparison correction.
Pretreatment comparisons of sample characteristics were conducted using analysis of variance and χ2 tests. All statistical tests were two-tailed. It was planned that any factor differing between the groups would be included as a covariate.
RESULTS
Groups did not differ with respect to any medical or demographic characteristics, number of study sessions, neuroticism, or treatment credibility (Table 1).
Effects of CBTH on Fatigue
There were significant main effects of group [χ2(1) = 58.20; P < .001] and time [χ2(3) = 80.54; P < .001] on FACIT-Fatigue. These main effects were qualified by a time × group interaction [χ2(3) = 59.86; P < .001]. We then estimated the simple main effects of group on FACIT-Fatigue at each time point, controlling for family-wise error (Fig 2A). The CBTH group had significantly less fatigue at all three outcome assessment points: end of treatment (z, 6.73; adjusted P < .001; d, 0.83; 95% CI, 0.54 to 1.11), 4-week follow-up (z, 6.98; adjusted P < .001; d, 0.92; 95% CI, 0.63 to 1.21), and 6-month follow-up (z, 7.99; adjusted P < .001; d, 1.69; 95% CI, 1.37 to 2.01). There was no significant difference in fatigue at baseline (z, 0.92; adjusted P = 0.82; d, 0.11; 95% CI, −0.16 to 0.39).
Fig 2.
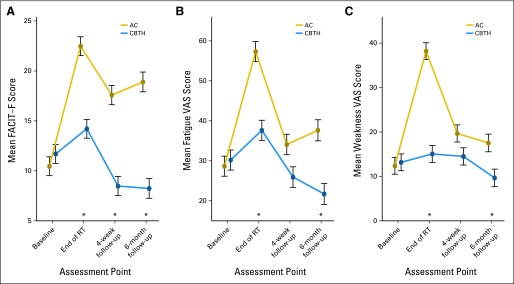
The effects over time of cognitive-behavioral therapy plus hypnosis (CBTH) on fatigue in patients with breast cancer undergoing radiotherapy (RT). Asterisks indicate adjusted P < .05, controlling for family-wise error (multiple comparisons). (A) CBTH effects on mean FACIT-F, (B) CBTH effects on mean Fatigue VAS, (C) CBTH effects on mean Muscle Weakness VAS. AC, attention control group; FACIT-F, Functional Assessment of Chronic Illness Therapy–Fatigue; VAS, Visual Analog Scale.
There were significant main effects of group [χ2(1) = 21.65; P < .001] and time [χ2(3) = 91.33; P < .001] on the Fatigue VAS. Main effects were qualified by a time × group interaction [χ2(3) = 25.48; P < .001]. Fatigue VAS scores were significantly lower in the CBTH group at the end of treatment (z, 5.81; adjusted P < .001; d, 0.70; 95% CI, 0.41 to 0.98) and the 6-month follow-up (z, 4.56; P < .001; d, 0.69; 95% CI, 0.40 to 0.97). Fatigue VAS in the CBTH group at the 4-week follow-up was not significantly lower than in the AC group (z, 2.38; adjusted P < .07; d, 0.36; 95% CI, 0.08 to 0.64; Fig 2B), although marginal. There was no significant group difference in Fatigue VAS at baseline (z, 0.03; adjusted P = 1.0; d, 0.06; 95% CI, −0.22 to 0.34).
There were significant main effects of group [χ2(1) = 25.40; P < .001] and time [χ2(3) = 87.21; P < .001] on Muscle Weakness VAS (Fig 2C). These main effects were qualified by a time × group interaction [χ2(3) = 56.84; P < .001]. Muscle Weakness VAS scores were significantly lower in the CBTH group at the end of radiotherapy (z, 9.30; adjusted P < .001; d, 1.08; 95% CI, 0.78 to 1.37) and at the 6-month follow-up (z, 3.10; adjusted P < .02; d, 0.62; 95% CI, 0.33 to 0.90). Muscle Weakness in the CBTH group at the 4-week follow-up was not significantly lower than in the AC group (z, 2.12; adjusted P < .13; d, 0.31; 95% CI, 0.03 to 0.59). There was no significant group difference in Muscle Weakness VAS at baseline (z, 0.17; adjusted P = 1.0; d, 0.03; 95% CI, −0.24 to 0.31).
Effects of Baseline Fatigue
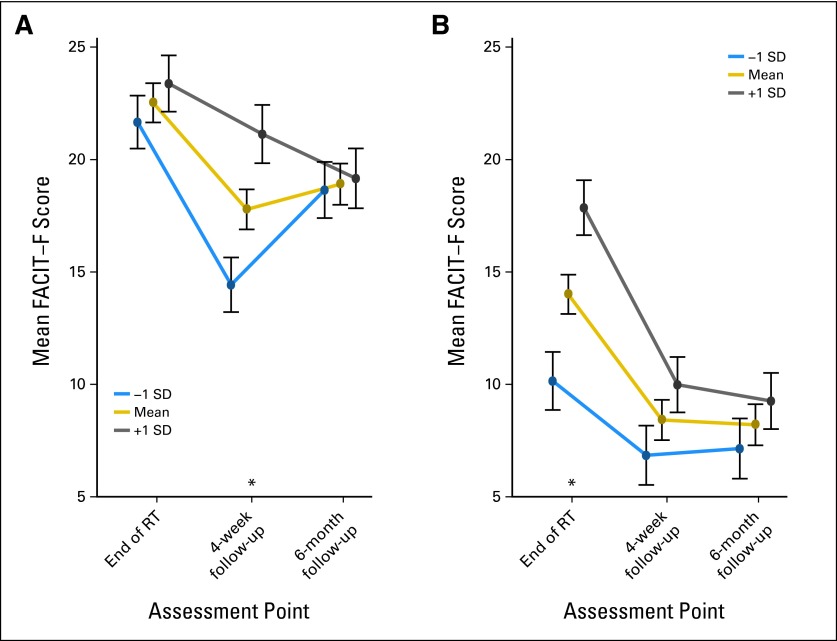
To explore whether baseline fatigue moderated the efficacy of CBTH, we tested whether there was a group × baseline fatigue × time interaction, using FACIT-F scores (our most sensitive measure) and correcting for multiple comparisons.41 We found a significant three-way interaction [χ2(2) = 8.97; P = .01] driven by the differences in the timing of the baseline fatigue moderation effect (Figs 3A and 3B). However, the main effect of group remained highly significant after controlling for baseline fatigue [χ2(1) = 122.84; P < .001].
Fig 3.
Effects of baseline fatigue levels on intervention effects over time in (A) attention control group and (B) cognitive-behavioral therapy plus hypnosis group. Asterisks indicate adjusted P < .05, controlling for family-wise error (multiple comparisons). FACIT-F, Functional Assessment of Chronic Illness Therapy–Fatigue; RT, radiotherapy; SD, standard deviation.
DISCUSSION
This RCT demonstrated that CBTH was efficacious for controlling fatigue in patients with BCa who underwent radiotherapy for up to 6 months after radiotherapy. Beneficial CBTH effects were independent of participants' medical and demographic characteristics, suggesting the generalizability of CBTH effects. Results are consistent with literature that supports combining CBT with hypnosis5,18–20 and literature that supports the benefits of CBT for controlling cancer-related fatigue.17,42,43 Effect sizes reported here (d, 0.62 to 1.69) are greater than those reported for CBT alone (d, 0.43) or exercise (d, 0.38).17 Additionally, significant between-group differences were greater than minimally important difference thresholds for the FACIT-F (3 to 4 points),44 and for fatigue VASs (8 to 11 points).45
There are several potential explanations for the large clinical benefit of CBTH. First, CBTH includes hypnosis, unlike previous CBT interventions. CBT in the present study was based on Rational Emotive Behavior Therapy (REBT),27,28 rather than other CBT approaches. An important difference between REBT and most other forms of CBT is REBT's focus on addressing core irrational beliefs, which are seen as the proximal causes of dysfunctional feelings and behaviors.46 In addition, CBTH was conducted face-to-face in the radiotherapy clinic, which may have reduced patient burden. CBTH was also conducted twice per week, whereas many other CBT approaches involve once-per-week sessions. CBTH was intended to be preventive, so the intervention began before radiotherapy. And lastly, interventionists were doctoral-level clinical psychologists.
Interestingly, there were no group differences using the VASs at the 4-week follow-up; most likely because of the greater measurement error typically associated with single-item measures (VASs) relative to multi-item approaches (FACIT-F).47 The present results are consistent with recommendations that studies investigating fatigue as a primary end point use multi-item assessment approaches.13
Fatigue levels in this study were equivalent to those previously published for patients with BCa receiving radiation.5 AC fatigue levels indicate that patients were experiencing clinically relevant levels of fatigue on the basis of published norms48,49 and support the view that fatigue during radiotherapy is problematic for patients and an appropriate target for clinical interventions.
Baseline fatigue levels did have differential effects by group over time (Fig 3). Baseline fatigue levels moderated group effects at the end of radiotherapy in the CBTH group, and at the 4-week follow-up in the AC group. This finding may be a result of the decreasing influence of baseline fatigue in the CBTH group, perhaps reflecting the increased influence of the intervention (eg, patients becoming more facile with the techniques). In the AC group, there may have been a ceiling effect at the end of radiotherapy, such that all patients, regardless of baseline fatigue, had higher levels of fatigue at that time. However, baseline fatigue did not account for intervention effects on fatigue.
Results suggest four future directions to address study limitations. First, investigation of the contributions of individual intervention components (REBT, hypnosis) to CBTH effects is needed. We chose to test effects of an intervention that combined multiple cognitive-behavioral components and hypnosis. We prioritized establishing clinical effects of the CBTH package over examining unique contributions of intervention elements. Consequently, we cannot determine the required active ingredients, so to speak, of CBTH. In subsequent work, we plan to experimentally investigate the relative contributions of intervention components. Second, it will be important to investigate potential mediators of intervention effects suggested by the literature on REBT and hypnosis16,21 (REBT: irrational beliefs; hypnosis: expectancies). We speculate that CBTH works through changing irrational beliefs (eg, catastrophizing) to more rational alternatives, by changing expectancies for fatigue, and by reducing emotional distress.21 Third, psychologists may not be available at all institutions. Future research should test the delivery of CBTH by other professionals (eg, nurses, physicians) or technologies (Web or smartphone). Fourth, investigations of cost-effectiveness seem warranted as a step toward future dissemination.
In conclusion, the results should encourage additional investigation of CBTH as an evidence-based intervention to control fatigue in patients with BCa undergoing radiotherapy. CBTH is noninvasive, had no adverse effects, and had beneficial effects long after the last intervention session and the end of radiotherapy. CBTH thus seems to be a promising candidate for future dissemination.
Supplementary Material
Acknowledgment
We thank the radiation oncology clinic staff and research study team, as well as all of the study participants for so graciously sharing their experiences.
Footnotes
Supported by National Cancer Institute Grants No. CA131473, CA159530, CA081137, CA166042, and CA129094, and by American Cancer Society Grant No. RSGPB-04-213-01-CPPB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, National Institutes of Health, or American Cancer Society.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Guy H. Montgomery, Daniel David, Maria Kangas, Dana H. Bovbjerg, Julie B. Schnur
Collection and assembly of data: Guy H. Montgomery, Sheryl Green, Madalina Sucala, Julie B. Schnur
Data analysis and interpretation: Guy H. Montgomery, Daniel David, Michael N. Hallquist
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Data Base. Chicago, IL: The Commission on Cancer of the American College of Surgeons; 2012. Radiation Therapy of Breast Cancer Diagnoses in 2000 to 2009. [Google Scholar]
- 3.Hickok JT, Morrow GR, Roscoe JA, et al. Occurrence, severity, and longitudinal course of twelve common symptoms in 1129 consecutive patients during radiotherapy for cancer. J Pain Symptom Manage. 2005;30:433–442. doi: 10.1016/j.jpainsymman.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Knobf MT, Sun Y. A longitudinal study of symptoms and self-care activities in women treated with primary radiotherapy for breast cancer. Cancer Nurs. 2005;28:210–218. doi: 10.1097/00002820-200505000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery GH, Kangas M, David D, et al. Fatigue during breast cancer radiotherapy: An initial randomized study of CBT plus hypnosis. Health Psychol. 2009;28:317–322. doi: 10.1037/a0013582. [DOI] [PubMed] [Google Scholar]
- 6.Andrykowski MA, Donovan KA, Laronga C, et al. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer. 2010;116:5740–5748. doi: 10.1002/cncr.25294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt ME, Chang-Claude J, Vrieling A, et al. Fatigue and quality of life in breast cancer survivors: Temporal courses and long-term pattern. J Cancer Surviv. 2012;6:11–19. doi: 10.1007/s11764-011-0197-3. [DOI] [PubMed] [Google Scholar]
- 8.Noal S, Levy C, Hardouin A, et al. One-year longitudinal study of fatigue, cognitive functions, and quality of life after adjuvant radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys. 2011;81:795–803. doi: 10.1016/j.ijrobp.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 9.Lee TS, Kilbreath SL, Refshauge KM, et al. Quality of life of women treated with radiotherapy for breast cancer. Support Care Cancer. 2008;16:399–405. doi: 10.1007/s00520-007-0328-6. [DOI] [PubMed] [Google Scholar]
- 10.Jereczek-Fossa BA, Marsiglia HR, Orecchia R. Radiotherapy-related fatigue. Crit Rev Oncol Hematol. 2002;41:317–325. doi: 10.1016/s1040-8428(01)00143-3. [DOI] [PubMed] [Google Scholar]
- 11.Spelten ER, Verbeek JH, Uitterhoeve AL, et al. Cancer, fatigue and the return of patients to work: A prospective cohort study. Eur J Cancer. 2003;39:1562–1567. doi: 10.1016/s0959-8049(03)00364-2. [DOI] [PubMed] [Google Scholar]
- 12.Alcântara-Silva TR, Freitas-Junior R, Freitas NM, et al. Fatigue related to radiotherapy for breast and/or gynaecological cancer: A systematic review. J Clin Nurs. 2013;22:2679–2686. doi: 10.1111/jocn.12236. [DOI] [PubMed] [Google Scholar]
- 13.Barsevick AM, Cleeland CS, Manning DC, et al. ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. J Pain Symptom Manage. 2010;39:1086–1099. doi: 10.1016/j.jpainsymman.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobsen PB, Andrykowski MA, Thors CL. Relationship of catastrophizing to fatigue among women receiving treatment for breast cancer. J Consult Clin Psychol. 2004;72:355–361. doi: 10.1037/0022-006X.72.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montgomery GH, Bovbjerg DH. Presurgery distress and specific response expectancies predict postsurgery outcomes in surgery patients confronting breast cancer. Health Psychol. 2004;23:381–387. doi: 10.1037/0278-6133.23.4.381. [DOI] [PubMed] [Google Scholar]
- 16.Sucala M, Schnur JB, Brackman EH, et al. The role of specific and core dysfunctional beliefs in breast cancer radiotherapy patients' fatigue. J Health Psychol. doi: 10.1177/1359105313482166. [epub ahead of print on April 30, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: A systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull. 2008;134:700–741. doi: 10.1037/a0012825. [DOI] [PubMed] [Google Scholar]
- 18.Kirsch I, Montgomery G, Sapirstein G. Hypnosis as an adjunct to cognitive-behavioral psychotherapy: A meta-analysis. J Consult Clin Psychol. 1995;63:214–220. doi: 10.1037//0022-006x.63.2.214. [DOI] [PubMed] [Google Scholar]
- 19.Castel A, Cascón R, Padrol A, et al. Multicomponent cognitive-behavioral group therapy with hypnosis for the treatment of fibromyalgia: Long-term outcome. J Pain. 2012;13:255–265. doi: 10.1016/j.jpain.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Bryant RA, Moulds ML, Guthrie RM, et al. The additive benefit of hypnosis and cognitive-behavioral therapy in treating acute stress disorder. J Consult Clin Psychol. 2005;73:334–340. doi: 10.1037/0022-006X.73.2.334. [DOI] [PubMed] [Google Scholar]
- 21.Montgomery GH, Hallquist MN, Schnur JB, et al. Mediators of a brief hypnosis intervention to control side effects in breast surgery patients: Response expectancies and emotional distress. J Consult Clin Psychol. 2010;78:80–88. doi: 10.1037/a0017392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montgomery GH, Schnur JB, Kravits K. Hypnosis for cancer care: Over 200 years young. CA Cancer J Clin. 2013;63:31–44. doi: 10.3322/caac.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montgomery GH, Bovbjerg DH, Schnur JB, et al. A randomized clinical trial of a brief hypnosis intervention to control side effects in breast surgery patients. J Natl Cancer Inst. 2007;99:1304–1312. doi: 10.1093/jnci/djm106. [DOI] [PubMed] [Google Scholar]
- 24.Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Obstet Gynecol. 2010;115:1063–1070. doi: 10.1097/AOG.0b013e3181d9d421. [DOI] [PubMed] [Google Scholar]
- 25.SAS Institute. SAS Proprietary Software 9.2. Cary, NC: SAS Institute; 2009. [Google Scholar]
- 26.Borkovec TD, Nau SD. Credibility of analogue therapy rationales. J Behav Ther Exp Psychol. 1972;3:117–126. [Google Scholar]
- 27.Ellis A. Reason and Emotion in Psychotherapy. Secaucus, NJ: Birch Lane; 1994. [Google Scholar]
- 28.Walen S, Digiuseppe R, Dryden W. A Practitioner's Guide to Rational Emotive Therapy. ed 2. New York, NY: Oxford University Press; 1992. pp. 3–377. [Google Scholar]
- 29.Pizzo PA, Lynn SJ, Rhue JW, Kirsch I, editors. Handbook of Clinical Hypnosis. ed 2. Washington, DC: American Psychological Association; 2010. [Google Scholar]
- 30.Enqvist B, Fischer K. Preoperative hypnotic techniques reduce consumption of analgesics after surgical removal of third mandibular molars: A brief communication. Int J Clin Exp Hypn. 1997;45:102–108. doi: 10.1080/00207149708416112. [DOI] [PubMed] [Google Scholar]
- 31.Lang EV, Benotsch EG, Fick LJ, et al. Adjunctive non-pharmacological analgesia for invasive medical procedures: A randomised trial. Lancet. 2000;355:1486–1490. doi: 10.1016/S0140-6736(00)02162-0. [DOI] [PubMed] [Google Scholar]
- 32.Yellen SB, Cella DF, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 33.Geinitz H, Zimmermann FB, Thamm R, et al. Fatigue in patients with adjuvant radiation therapy for breast cancer: Long-term follow-up. J Cancer Res Clin Oncol. 2004;130:327–333. doi: 10.1007/s00432-003-0540-9. [DOI] [PubMed] [Google Scholar]
- 34.Michielsen HJ, Van der Steeg AF, Roukema JA, et al. Personality and fatigue in patients with benign or malignant breast disease. Support Care Cancer. 2007;15:1067–1073. doi: 10.1007/s00520-007-0222-2. [DOI] [PubMed] [Google Scholar]
- 35.Donovan KA, Jacobsen PB, Andrykowski MA, et al. Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Manage. 2004;28:373–380. doi: 10.1016/j.jpainsymman.2004.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costa PT, Jr, McCrae RR. Lutz, FL: Psychological Assessment Resources; 1992. Revised NEO Personality Inventory (NEO PI-R) and NEO Five-Factor Inventory (NEO-FFI) Professional Manual. [Google Scholar]
- 37.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: Pizzo PA, MacLeod CM, editors. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949. pp. 199–205. [Google Scholar]
- 38.Faul F, Erdfelder E, Lang AG, et al. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 39.Touloumi G, Babiker AG, Pocock SJ, et al. Impact of missing data due to drop-outs on estimators for rates of change in longitudinal studies: A simulation study. Stat Med. 2001;20:3715–3728. doi: 10.1002/sim.1114. [DOI] [PubMed] [Google Scholar]
- 40.Snijders TAB, Bosker R. Thousand Oaks, CA: Sage Publications; 1999. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. [Google Scholar]
- 41.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 42.Goedendorp MM, Peters ME, Gielissen MF, et al. Is increasing physical activity necessary to diminish fatigue during cancer treatment? Comparing cognitive behavior therapy and a brief nursing intervention with usual care in a multicenter randomized controlled trial. Oncologist. 2010;15:1122–1132. doi: 10.1634/theoncologist.2010-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gielissen MF, Verhagen S, Witjes F, et al. Effects of cognitive behavior therapy in severely fatigued disease-free cancer patients compared with patients waiting for cognitive behavior therapy: A randomized controlled trial. J Clin Oncol. 2006;24:4882–4887. doi: 10.1200/JCO.2006.06.8270. [DOI] [PubMed] [Google Scholar]
- 44.FACIT.org. The Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F) Scale: Summary of development and validation—June 27, 2007 Update. www.facit.org/FACITOrg/Questionnaires.
- 45.Khanna D, Pope JE, Khanna PP, et al. The minimally important difference for the fatigue visual analog scale in patients with rheumatoid arthritis followed in an academic clinical practice. J Rheumatol. 2008;35:2339–2343. doi: 10.3899/jrheum.080375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pizzo PA, David D, Lynn SJ, Ellis A, editors. New York, NY: Oxford University Press; 2010. Rational and Irrational Beliefs: Research, Theory, and Clinical Practice. [Google Scholar]
- 47.Peter JP. Reliability: A review of psychometric basics and recent marketing practices. J Mark Res. 1979;16:6–17. [Google Scholar]
- 48.FACIT.org. Population norms. www.facit.org/FACITOrg/Questionnaires.
- 49.Cella D, Lai JS, Chang CH, et al. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.