Abstract
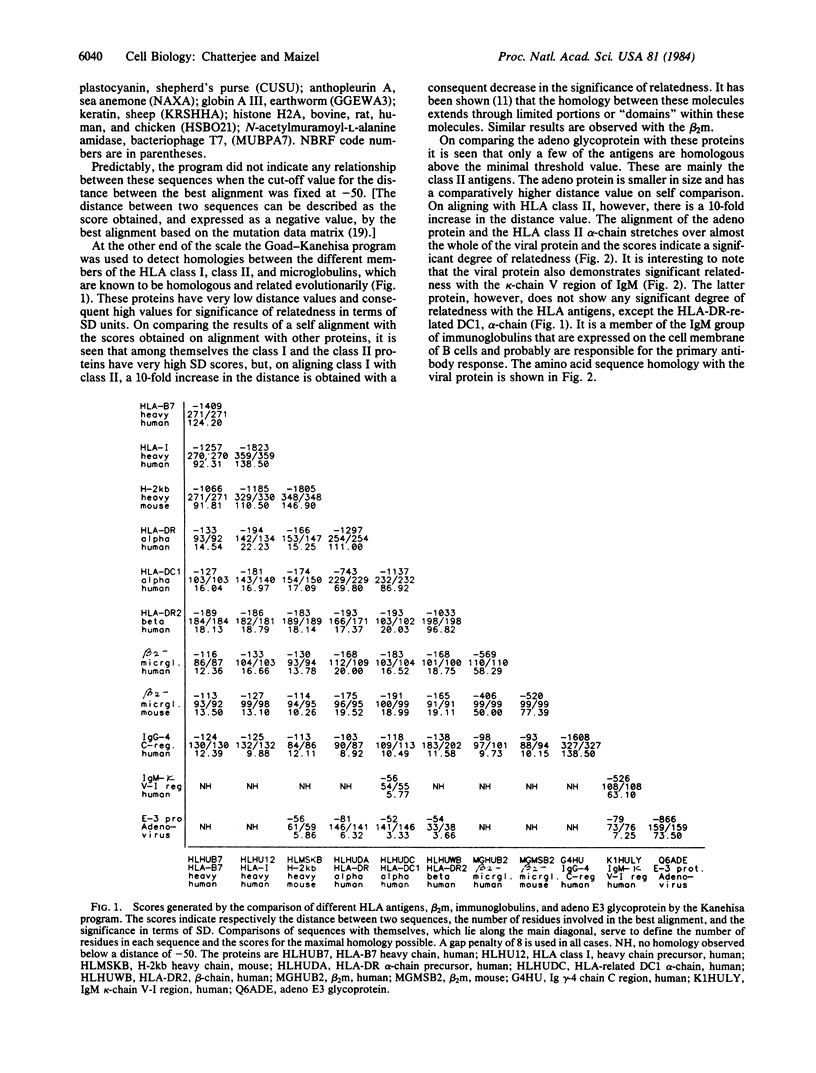
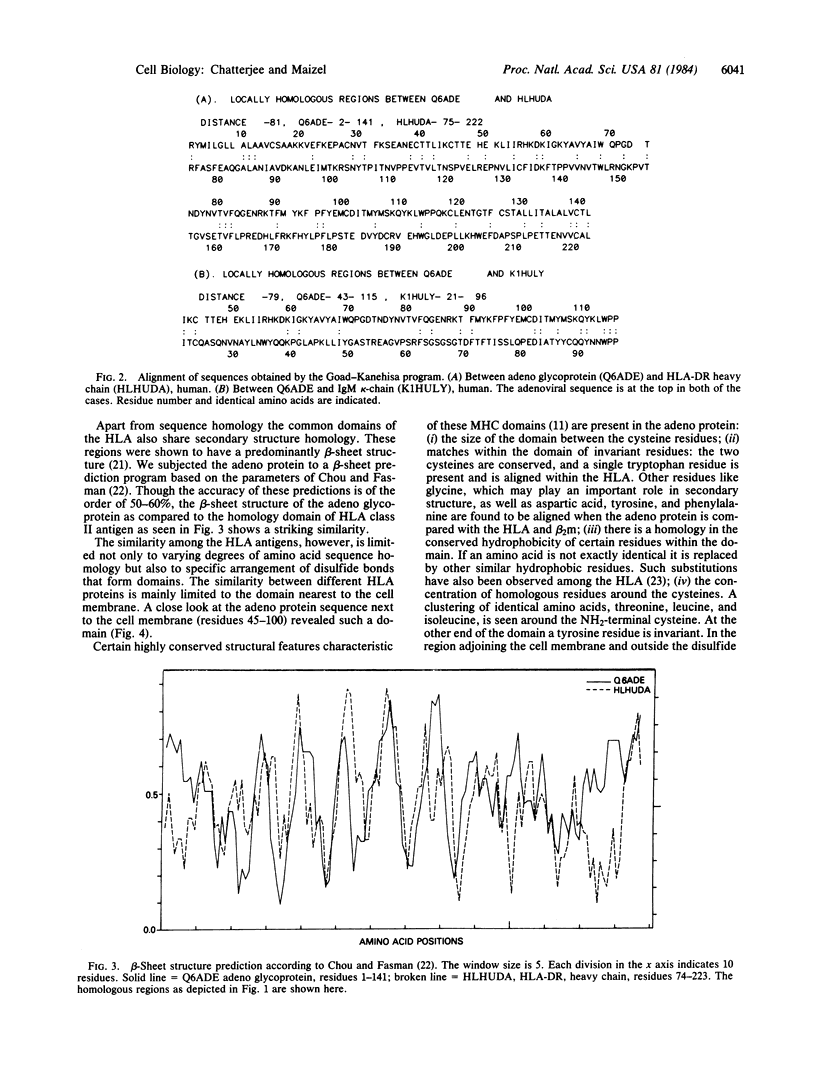
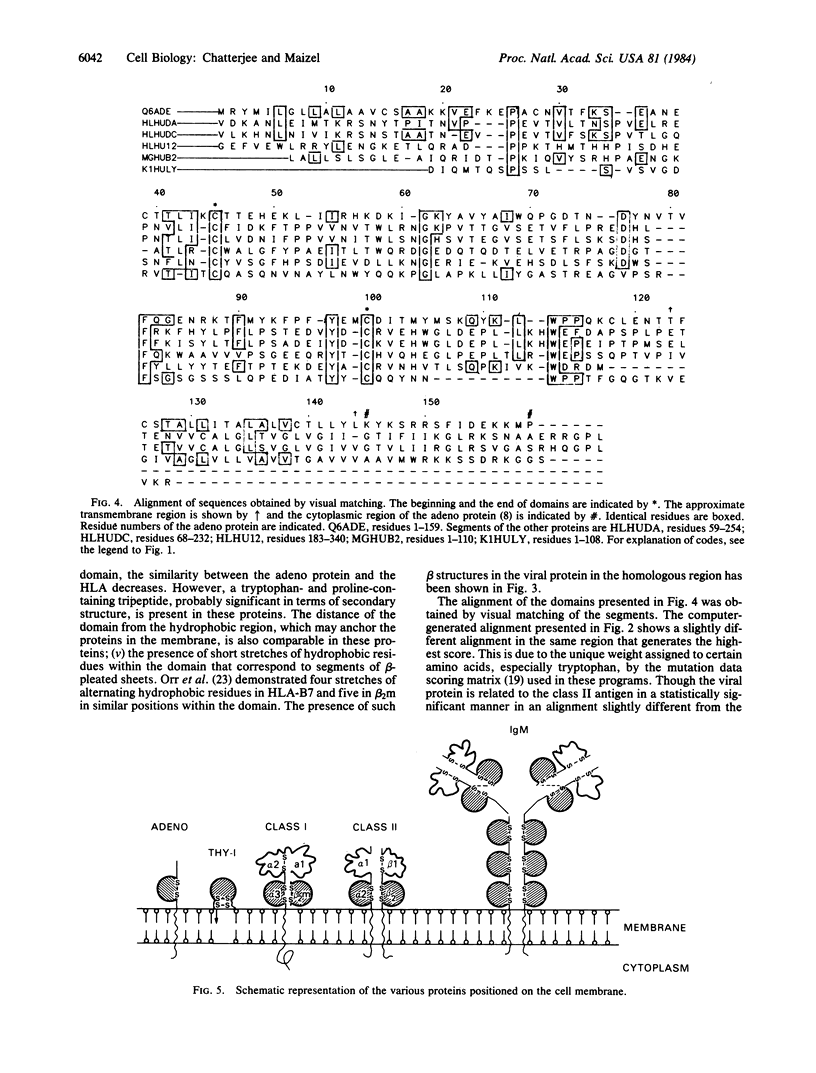
The Mr 19,000 adenovirus glycoproteins coded by the E3 region that is expressed on the cell membrane and presumably binds to HLA class I antigen shows sequence homology to the alpha-chain of human HLA-DR and to the kappa-chain of human IgM. The homology extends to a conserved domain present in all of the major histocompatibility complex antigens, beta 2-microglobulins, and immunoglobulins. Predicted beta-sheet secondary structures are similar between the Mr 19,000 adenovirus protein and these immune system proteins. Evolutionary and functional implications of the homology are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker W. C., Dayhoff M. O. Viral src gene products are related to the catalytic chain of mammalian cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1982 May;79(9):2836–2839. doi: 10.1073/pnas.79.9.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin W. W., Maizel J. V., Jr The polypeptides of adenovirus. VII. Further studies of early polypeptides in vivo and localization of E2 and E2A to the cell plasma membrane. Virology. 1976 Jun;71(2):518–530. doi: 10.1016/0042-6822(76)90378-0. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Novotný J., Sternberg M. J., Campbell D. G., Williams A. F. Analysis of structural similarities between brain Thy-1 antigen and immunoglobulin domains. Evidence for an evolutionary relationship and a hypothesis for its functional significance. Biochem J. 1981 Apr 1;195(1):31–40. doi: 10.1042/bj1950031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Rosenthal K. L., Klein J., Zinkernagel R. M., Singer S. J. Selective and unidirectional membrane redistribution of an H-2 antigen with an antibody-clustered viral antigen: relationship to mechanisms of cytotoxic T-cell interactions. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4603–4607. doi: 10.1073/pnas.76.9.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goad W. B., Kanehisa M. I. Pattern recognition in nucleic acid sequences. I. A general method for finding local homologies and symmetries. Nucleic Acids Res. 1982 Jan 11;10(1):247–263. doi: 10.1093/nar/10.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood L., Steinmetz M., Malissen B. Genes of the major histocompatibility complex of the mouse. Annu Rev Immunol. 1983;1:529–568. doi: 10.1146/annurev.iy.01.040183.002525. [DOI] [PubMed] [Google Scholar]
- Ishibashi M., Maizel J. V., Jr The polypeptides of adenovirus. VI. Early and late glycopolypeptides. Virology. 1974 Apr;58(2):345–361. doi: 10.1016/0042-6822(74)90070-1. [DOI] [PubMed] [Google Scholar]
- Kaufman J. F., Auffray C., Korman A. J., Shackelford D. A., Strominger J. The class II molecules of the human and murine major histocompatibility complex. Cell. 1984 Jan;36(1):1–13. doi: 10.1016/0092-8674(84)90068-0. [DOI] [PubMed] [Google Scholar]
- Kimball E. S., Coligan J. E. Structure of class I major histocompatibility antigens. Contemp Top Mol Immunol. 1983;9:1–63. doi: 10.1007/978-1-4684-4517-6_1. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Auffray C., Schamboeck A., Strominger J. L. The amino acid sequence and gene organization of the heavy chain of the HLA-DR antigen: homology to immunoglobulins. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6013–6017. doi: 10.1073/pnas.79.19.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krangel M. S., Orr H. T., Strominger J. L. Structure, function, and biosynthesis of the major human histocompatibility antigens (HLA-A and HLA-B). Scand J Immunol. 1980;11(6):561–571. doi: 10.1111/j.1365-3083.1980.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Kämpe O., Bellgrau D., Hammerling U., Lind P., Päbo S., Severinsson L., Peterson P. A. Complex formation of class I transplantation antigens and a viral glycoprotein. J Biol Chem. 1983 Sep 10;258(17):10594–10598. [PubMed] [Google Scholar]
- Larhammar D., Gustafsson K., Claesson L., Bill P., Wiman K., Schenning L., Sundelin J., Widmark E., Peterson P. A., Rask L. Alpha chain of HLA-DR transplantation antigens is a member of the same protein superfamily as the immunoglobulins. Cell. 1982 Aug;30(1):153–161. doi: 10.1016/0092-8674(82)90021-6. [DOI] [PubMed] [Google Scholar]
- Lenk R., Storch T., Maizel J. V., Jr Cell architecture during adenovirus infection. Virology. 1980 Aug;105(1):19–34. doi: 10.1016/0042-6822(80)90152-x. [DOI] [PubMed] [Google Scholar]
- Lörincz A. T., Reed S. I. Primary structure homology between the product of yeast cell division control gene CDC28 and vertebrate oncogenes. Nature. 1984 Jan 12;307(5947):183–185. doi: 10.1038/307183a0. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Lenk R. P. Enhanced graphic matrix analysis of nucleic acid and protein sequences. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7665–7669. doi: 10.1073/pnas.78.12.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H. T., Lancet D., Robb R. J., Lopez de Castro J. A., Strominger J. L. The heavy chain of human histocompatibility antigen HLA-B7 contains an immunoglobulin-like region. Nature. 1979 Nov 15;282(5736):266–270. doi: 10.1038/282266a0. [DOI] [PubMed] [Google Scholar]
- Persson H., Jörnvall H., Zabielski J. Multiple mRNA species for the precursor to an adenovirus-encoded glycoprotein: identification and structure of the signal sequence. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6349–6353. doi: 10.1073/pnas.77.11.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Philipson L. Regulation of adenovirus gene expression. Curr Top Microbiol Immunol. 1982;97:157–203. doi: 10.1007/978-3-642-68318-3_4. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Kaufman J. F., Korman A. J., Strominger J. L. HLA-DR antigens: structure, separation of subpopulations, gene cloning and function. Immunol Rev. 1982;66:133–187. doi: 10.1111/j.1600-065x.1982.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Signäs C., Katze M. G., Persson H., Philipson L. An adenovirus glycoprotein binds heavy chains of class I transplantation antigens from man and mouse. Nature. 1982 Sep 9;299(5879):175–178. doi: 10.1038/299175a0. [DOI] [PubMed] [Google Scholar]
- Storch T. G., Maizel J. V., Jr The early proteins of the nondefective Ad2-SV40 hybrid viruses: the 19K glycoprotein is coded by Ad2 early region 3. Virology. 1980 May;103(1):54–67. doi: 10.1016/0042-6822(80)90125-7. [DOI] [PubMed] [Google Scholar]
- Walter G., Maizel J. V., Jr The polypeptides of adenovirus. IV. Detection of early and late virus-induced polypeptides and their distribution in subcellular fractions. Virology. 1974 Feb;57(2):402–408. doi: 10.1016/0042-6822(74)90180-9. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Gagnon J. Neuronal cell Thy-1 glycoprotein: homology with immunoglobulin. Science. 1982 May 14;216(4547):696–703. doi: 10.1126/science.6177036. [DOI] [PubMed] [Google Scholar]


