Abstract
Purpose
To provide an update on recent advances in the management of patients with multiple myeloma who are not eligible for autologous stem-cell transplantation.
Methods
A comprehensive review of the literature on diagnostic criteria is provided, and treatment options and management of adverse events are summarized.
Results
Patients with symptomatic disease and organ damage (ie, hypercalcemia, renal failure, anemia, or bone lesions) require immediate treatment. The International Staging System and chromosomal abnormalities identify high- and standard-risk patients. Proteasome inhibitors, immunomodulatory drugs, corticosteroids, and alkylating agents are the most active agents. The presence of concomitant diseases, frailty, or disability should be assessed and, if present, treated with reduced-dose approaches. Bone disease, renal damage, hematologic toxicities, infections, thromboembolism, and peripheral neuropathy are the most frequent disabling events requiring prompt and active supportive care.
Conclusion
These recommendations will help clinicians ensure the most appropriate care for patients with myeloma in everyday clinical practice.
INTRODUCTION
Multiple myeloma (MM) is a malignant neoplasm that affects primarily elderly patients.1,2 During the past decade, considerable progress has been made in the management of MM, prompting the International Myeloma Working Group (IMWG) to develop these updated guidelines.3–6
METHODS
In 2012, an Update Committee of the IMWG performed a review of key literature, including searches of the Cochrane library, Medline, the Internet, and major meeting reports. Expert consensus was used to propose additional recommendations when published data were insufficient. The Grades of Recommendation, Assessment, Development, and Evaluation system were used to grade recommendations (Appendix Table A1, online only).7 Some of the treatment regimens recommended for consideration are not approved by the regulatory authorities for these indications and hence should not be considered as standard care but rather as reasonable treatment options. In the recommendations, approved regimens are highlighted in bold font.
RECOMMENDATIONS
Diagnosis
The diagnostic process aims to distinguish between monoclonal gammopathy of undetermined significance, asymptomatic (smoldering) MM, symptomatic MM, solitary plasmacytoma, and other plasma cell diseases based on the IMWG criteria (Table 1). Symptomatic MM is defined as the presence of ≥ 10% clonal bone marrow plasma cells and organ damage (hypercalcemia, renal failure, anemia, or bone lesions [CRAB]).8 In addition, the presence of ≥ 60% bone marrow involvement or rapidly climbing paraprotein, regardless of CRAB, are considered by some authors as MM-related symptoms.9
Table 1.
Diagnostic Criteria for Plasma Cell Diseases
| Diagnosis | Diagnostic Criteria |
|---|---|
| MGUS | All three criteria must be met: |
| Serum monoclonal protein (IgG or IgA) < 3 g/100 mL | |
| Clonal bone marrow plasma cells < 10% | |
| Absence of myeloma-related organ damage (CRAB) that can be attributed to plasma-cell proliferative disorder | |
| Smoldering (asymptomatic) MM | Both criteria must be met: |
| Serum monoclonal protein (IgG or IgA) ≥ 3 g/100 mL and/or clonal bone marrow plasma cells ≥ 10% | |
| Absence of myeloma-related organ damage (CRAB) that can be attributed to plasma-cell proliferative disorder | |
| MM (symptomatic) | All three criteria must be met: |
| Clonal bone marrow plasma cells ≥ 10%* | |
| Presence of serum and/or urinary monoclonal protein (except in patients with true nonsecretory MM) | |
| Evidence of myeloma-related organ damage (CRAB) that can be attributed to plasma-cell proliferative disorder, specifically: | |
| Hypercalcemia: serum calcium ≥ 11.5 mg/100 mL | |
| Renal insufficiency: serum creatinine > 1.73 mmol/L | |
| Anemia: normochromic, normocytic with hemoglobin value > 2 g/100 mL below lower limit of normal or hemoglobin value < 10 g/100 mL | |
| Bone lesions: lytic lesions, severe osteopenia, or pathologic fractures | |
| Solitary plasmacytoma | All four criteria must be met: |
| Biopsy-proven solitary lesion of bone or soft tissue with evidence of clonal plasma cells | |
| Normal bone marrow with no evidence of clonal plasma cells | |
| Normal skeletal survey and MRI of spine and pelvis (except for primary solitary lesion) | |
| Absence of myeloma-related organ damage (CRAB) that can be attributed to plasma-cell proliferative disorder | |
| Other plasma-cell diseases | Waldenstrom's macroglobulinemia |
| Systemic AL amyloidosis | |
| Monoclonal Ig deposition disease | |
| POEMS syndrome |
Adapted from Kyle Leukemia 2009.
Abbreviations: AL, amyloid light chain; CRAB, hypercalcemia, renal failure, anemia, or bone lesions; Ig, immunoglobulin; MGUS, monoclonal gammopathy of undetermined significance; MM, multiple myeloma; MRI, magnetic resonance imaging; POEMS, polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, skin changes.
Monoclonal plasma cells usually account for ≥ 10% of all nucleated cells, but they may range from < 5% to almost 100% (International Myeloma Working Group: Br J Haematol 121:749-757, 2003).
The diagnostic work-up should include three subsequent levels of investigation to confirm the diagnosis, assess the prognosis, and establish the appropriate treatment (Table 2). Serum free-light chain (FLC) assay is useful for diagnosis and monitoring of nonsecretory myeloma, when small amounts of monoclonal protein are secreted in the serum and/or urine, and in light chain–only myeloma.10–12 Magnetic resonance imaging (MRI) and positron emission tomography integrated with computed tomography (PET/CT) may be useful in selected circumstances (eg, to detect soft tissue lesions arising from bone lesions, spinal cord compression, and asymptomatic lesions and to evaluate a painful area of the skeleton). MRI is indicated in nonsecretory myeloma for initial assessment and follow-up or to detect occult lesions in asymptomatic MM.13,14
Table 2.
Diagnostic Work-Up for Patients With MM
| Work-Up | Description | General Practice | Clinical Trial |
|---|---|---|---|
| First-level investigations to make diagnosis | |||
| History and physical examination | Always | Always | |
| Blood and urine | Complete blood count and differential; chemistry, including creatinine and calcium; serum protein electrophoresis and immunofixation, quantification of immunoglobulin; 24-hour urine collection for proteinuria, electrophoresis, and immunofixation | Always | Always |
| Serum free light chains | For oligo and nonsecretory MM and light chain only | Always | |
| Bone marrow | Aspirate and trephine biopsy with plasma cells phenotyping | Always | Always |
| Imaging | Skeletal survey | Always | Always |
| Second-level investigations to assess prognosis | |||
| Blood | Albumin, β2-microglobulin, LDH | Always | Always |
| Serum free light chains | Not indicated | Preferred | |
| Cytogenetic | Metaphase karyotype | Preferred | Always |
| FISH | t(4;14), t(11;14), t(14;16), t(14;20), chromosome 13 deletion, 17p13 deletion, and chromosome 1 abnormalities | Preferred | Always |
| Third-level investigations required before starting therapy or enrollment onto clinical trials | |||
| Performance status | Karnofsky performance status and WHO scale | Always | Always |
| Patient status | Assessment of comorbidity, frailty, and disability (cumulative illness rating scale or Charlson score; ADL and IADL score) | Preferred | Always |
| Organ function | Cardiac, pulmonary, hepatic, GI, and renal function | Always | Always |
| Infectious disease | Hepatitis B and C, HIV | Always | Always |
| Additional pretreatment investigations | |||
| Imaging | MRI PET/CT | In selected circumstances | Preferred |
| Prognostic | GEP | Not indicated | Preferred |
Abbreviations: ADL, Activities of Daily Living; FISH, fluorescent in situ hybridization; GEP, gene expression profiling; IADL, Instrumental Activities of Daily Living; LDH, lactate dehydrogenase; MM, multiple myeloma; MRI, magnetic resonance imaging; PET/CT, positron emission tomography/computed tomography.
Recommendations:
Pretreatment Considerations: Definitions of Fit and Unfit Patients
The operative cutoff age of 65 years is no longer sufficient to identify elderly patients. Aging is associated with an increased frequency of comorbidities, frailty, and disability, which have a negative effect on outcome.
Age, comorbidities, and geriatric assessment should be used to define patients' status (very fit, fit, and unfit). Unfit patients are characterized by older age, comorbidity, organ dysfunctions (cardiac, pulmonary, hepatic, GI, renal), and limits in mental/mobility functions. To assess comorbidity, the Charlson index can be used.15 To assess frailty and disability, Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) can be adopted.16 Fit patients should receive full-dose therapy, whereas unfit patients need reduced dose-intensity treatment.
Recommendation:
The assessment of organ function, comorbidities (with the Charlson index), frailty, and disability (defined by ADL and IADL) should be considered to define patients' status (grade C/IV).
Staging and Prognostic Factors
The International Staging System (ISS) is used to assess the prognosis of patients with symptomatic MM (Appendix Table A2, online only).17 ISS stage III is associated with poor prognosis. Chromosomal abnormalities t(4;14), t(14;16), and t(14;20); chromosome 1 abnormalities; and del17p detected by fluorescent in situ hybridization (FISH) are associated with poor prognosis,18–21 whereas the isolated 13q deletion is not considered a high-risk feature. Hyperdiploidy, t(11;14), and t(6;14) are considered standard-risk features. The combination of FISH data with ISS stage improves risk assessment.20 An abnormal κ/λ FLC ratio at diagnosis seems to predict poor prognosis.22 Gene expression profiling (GEP) is emerging as a predictive tool to further refine risk stratification.23,24 The prognostic role of PET/CT has been recently investigated in transplantation-eligible patients,25 although a standardization of this procedure is needed to translate its use into clinical practice. The achievement of complete response (CR) after initial treatment is associated with improved progression-free (PFS) and overall survival (OS).26,27
Recommendations:
The ISS should always be used at diagnosis (grade C/IV).
Chromosomal abnormalities should be detected to predict outcome (grade C/IV).
New prognostic markers (FLC, GEP, and PET/CT) need additional evaluations (grade C/IV).
Indications for Treatment
For asymptomatic patients, close monitoring is suggested every 1 to 3 months. Clinical trials are currently evaluating the role of early therapy with novel agents in high-risk asymptomatic myeloma.28 Conversely, patients with active and symptomatic MM, defined by the presence of CRAB symptoms, require immediate treatment.
Second-line treatment is indicated when there is either a clinical relapse (reoccurrence of CRAB symptoms) or a significant and quick paraprotein increase (doubled monoclonal protein within 2 months, with an increase in the absolute levels of monoclonal protein of ≥ 1g/dL in serum or of ≥ 500 mg per 24 hours in urine confirmed by two consecutive measurements).29 Whether to start treatment in case of biochemical relapse (25% increase in the paraprotein from the lowest response value without CRAB symptoms) is an open issue.
Recommendations:
Asymptomatic patients should be carefully monitored every 1 to 3 months (grade C/IV).
Initial therapy is indicated when CRAB symptoms occur (grade C/IV).
Re-treatment is indicated in case of clinical relapse or if the paraprotein has doubled within 2 months (grade C/IV).
Definition of Response to Therapy
The uniform response criteria were recently revised by the IMWG (Table 3).29,30 The definitions of immunophenotypic CR, molecular CR, and FLC response were introduced to refine the depth of response. MRI and PET/CT have not been incorporated into the response criteria assessment.29
Table 3.
Response Criteria
| Response | Criteria |
|---|---|
| CR | Negative immunofixation of serum and urine, disappearance of any soft tissue plasmacytomas, and < 5% plasma cells in bone marrow; in patients for whom only measurable disease is by serum FLC level, normal FLC ratio of 0.26 to 1.65 in addition to CR criteria is required; two consecutive assessments are needed |
| sCR | CR as defined plus normal FLC ratio and absence of clonal plasma cells by immunohistochemistry or two- to four-color flow cytometry; two consecutive assessments of laboratory parameters are needed |
| Immunophenotypic CR | sCR as defined plus absence of phenotypically aberrant plasma cells (clonal) in bone marrow with minimum of 1 million total bone marrow cells analyzed by multiparametric flow cytometry (with > four colors) |
| Molecular CR | CR as defined plus negative allele-specific oligonucleotide polymerase chain reaction (sensitivity 10−5) |
| VGPR | Serum and urine M component detectable by immunofixation but not on electrophoresis or ≥ 90% reduction in serum M component plus urine M component < 100 mg/24 h; in patients for whom only measurable disease is by serum FLC level, > 90% decrease in difference between involved and uninvolved FLC levels, in addition to VGPR criteria, is required; two consecutive assessments are needed |
| PR | ≥ 50% reduction of serum M protein and reduction in 24-hour urinary M protein by ≥ 90% or to < 200 mg/24 h |
| If serum and urine M protein are not measurable, ≥ 50% decrease in difference between involved and uninvolved FLC levels is required in place of M protein criteria | |
| If serum and urine M protein and serum FLC assay are not measurable, ≥ 50% reduction in bone marrow plasma cells is required in place of M protein, provided baseline percentage was ≥ 30% | |
| In addition, if present at baseline, ≥ 50% reduction in size of soft tissue plasmacytomas is required | |
| Two consecutive assessments are needed; no known evidence of progressive or new bone lesions if radiographic studies were performed | |
| MR for relapsed refractory myeloma only | ≥ 25% but ≤ 49% reduction of serum M protein and reduction in 24-hour urine M protein by 50% to 89% |
| In addition, if present at baseline, 25% to 49% reduction in size of soft tissue plasmacytomas is also required | |
| No increase in size or number of lytic bone lesions (development of compression fracture does not exclude response) | |
| SD | Not meeting criteria for CR, VGPR, PR, or PD; no known evidence of progressive or new bone lesions if radiographic studies were performed |
| PD | Increase of 25% from lowest response value in any of following: |
| Serum M component with absolute increase ≥ 0.5 g/dL; serum M component increases ≥ 1 g/dL are sufficient to define relapse if starting M component is ≥ 5 g/dL and/or; | |
| Urine M component (absolute increase must be ≥ 200 mg/24 h) and/or; | |
| Only in patients without measurable serum and urine M protein levels: difference between involved and uninvolved FLC levels (absolute increase must be > 10 mg/dL); | |
| Only in patients without measurable serum and urine M protein levels and without measurable disease by FLC level, bone marrow plasma cell percentage (absolute percentage must be ≥ 10%) | |
| Development of new or definite increase in size of existing bone lesions or soft tissue plasmacytomas | |
| Development of hypercalcemia that can be attributed solely to plasma cell proliferative disorder | |
| Two consecutive assessments before new therapy are needed |
Recommendation:
The updated IMWG criteria (Table 3) should be used to assess response every 30 to 60 days during treatment (grade C/IV).
Front-Line Therapy
Patients age 65 to 75 years are generally considered ineligible for autologous stem-cell transplantation (ASCT). Because biologic age can differ from chronologic age, this strict range may differ by approximately 5 years.
Different therapeutic approaches may be adopted according to age and patient status (Table 4). For patients age 65 to 70 years in excellent clinical condition (very fit), or younger patients with comorbidities, a reduced dose-intensity ASCT with melphalan 100 mg/m2 (MEL100) can be safely adopted instead of full-dose melphalan 200 mg/m2 (MEL200). For patients age 65 to 75 years in good clinical condition (fit), full-dose conventional chemotherapy is indicated, whereas for frail patients age > 75 years (unfit), or younger patients with comorbidities, reduced dose-intensity therapy is suggested.
Table 4.
Selected Therapeutic Schemas
| Regimen | Schedule | CR (%) | PFS/EFS/TTP | OS |
|---|---|---|---|---|
| Induction regimens | ||||
| MPT | Melphalan: 4 mg/m2 given orally on days 1-7 every 4 weeks for six cycles31 or 0.25 mg/kg on days 1-4 every 6 weeks for 12 cycles32; prednisone: 40 mg/m2 given orally on days 1-7 every 4 weeks for six cycles31 or 2 mg/kg on days 1-4 every 6 weeks for 12 cycles32; thalidomide: 100 mg/day given orally continuously until progression or intolerance31 or 200 mg/day continuously for 12 cycles of 6 weeks32 | 13-16 | Median, 20.3 months33 | Median, 39.3 months33 |
| CTDa | Cyclophosphamide: 500 mg/wk for six to nine cycles every 3 weeks; thalidomide: 100 mg/day increased to 200 mg/day for six to nine cycles every 3 weeks; dexamethasone: 20 mg on days 1-4 and 15-18 for six to nine cycles every 3 weeks34 | 13 | Median, 13 months | Median, 33 months |
| VMP | Bortezomib: 1.3 mg/m2 given as bolus intravenous infusion on days 1, 4, 8, 11, 22, 25, 29, and 32 (cycles one to four) and days 1, 8, 22, and 29 (cycles five to nine) every 6 weeks for nine cycles; melphalan: 9 mg/m2 given orally on days 1-4 every 6 weeks for nine cycles; prednisone: 60 mg/m2 given orally on days 1-4 every 6 weeks for nine cycles35; as alternative, bortezomib: 1.3 mg/m2 on days 1, 8, 15, and 22 every 6 weeks for nine cycles36 | 24-30 | Median, 22-27 months | At 2 years, 85% to 87% |
| VMPT | Bortezomib: 1.3 mg/m2 given as bolus intravenous infusion on days 1, 4, 8, 11, 22, 25, 29, and 32 (cycles one to four) and days 1, 8, 22, and 29 (cycles five to nine) every 6 weeks for nine cycles; melphalan: 9 mg/m2 given orally on days 1-4 every 6 weeks for nine cycles; prednisone: 60 mg/m2 given orally on days 1-4 every 6 weeks for nine cycles; thalidomide: 50 mg/day given orally continuously for nine cycles36 | 38 | Median, 33 months | At 3 years, 86%37 |
| VTP | Bortezomib: 1.3 mg/m2 given as bolus intravenous infusion on days 1, 4, 8, 11, 22, 25, 29, and 32 (cycle one), every 6 weeks, and 1.3 mg/m2 on days 1, 8, 15, and 22 every 5 weeks (cycles two to six); thalidomide: 100 mg/day given orally for six cycles; prednisone: 60 mg/m2 given orally on days 1-4 every 6 weeks for six cycles38 | 28 | Median, 31 months* | At 3 years, 70%* |
| VCD | Bortezomib: 1.3 mg/m2 given as bolus intravenous infusion on days 1, 4, 8, and 11 every 4 weeks for four to 12 cycles; cyclophosphamide: 300 mg/m2 given orally on days 1, 8, 15, and 22 every 4 weeks for four to 12 cycles; dexamethasone: 40 mg/day given orally on days 1-4, 9-12, and 17-20 every 4 weeks for four to 12 cycles39; as alternative, bortezomib: 1.5 mg/m2 given as bolus intravenous infusion on days 1, 8, 15, and 2240 | 39† | — | — |
| VRd | Bortezomib: 1.3 mg/m2 given as bolus intravenous infusion on days 1, 4, 8, and 11 every 3 weeks for eight cycles; lenalidomide: 25 mg given orally on days 1-14 every 3 weeks for eight cycles; dexamethasone: 20 mg given orally on days 1, 2, 4, 5, 8, 9, 11, and 12 every 3 weeks for eight cycles41 | 37 | At 18 months, 75%‡ | At 18 months, 97%‡ |
| Rd | Lenalidomide: 25 mg given orally on days 1-21 every 4 weeks for four cycles§; dexamethasone: 40 mg given orally on days 1, 8, 15, and 22 every 4 weeks for four cycles42 | 4 | Median, 25 months | At 2 years, 87% |
| MPR | Melphalan: 0.18 mg/kg given orally on days 1-4 every 4 weeks for nine cycles; prednisone: 2 mg/kg given orally on days 1-4 every 4 weeks for nine cycles; lenalidomide: 10 mg given orally on days 1-21 every 4 weeks for nine cycles43 | 3 | Median, 14 months | Not reached |
| Maintenance regimens | ||||
| T‖ | Thalidomide: 50 mg given orally, increased to 100 mg if tolerated after 4 weeks, until progression44 | — | Median, 11 months | Median, 38 months |
| R | Lenalidomide: 10 mg given orally on days 1-21 every 4 weeks until progression43 | — | Median, 26 months | — |
| VT | Bortezomib: 1.3 mg/m2 given as bolus intravenous infusion every 2 weeks for 2 years or until progression; thalidomide: 50 mg given orally for 2 years or until progression36,37 | 45 | Median, 27 months | Median, not reached |
| Salvage regimens | ||||
| V | Bortezomib: 1.3 mg/m2 given as bolus intravenous infusion on days 1, 4, 8, and 11 every 3 weeks for eight cycles and on days 1, 8, 15, and 22 every 5 weeks for following three cycles45 | 6 | Median, 6 months | At 1 year, 80% |
| V-Peg | Bortezomib: 1.3 mg/m2 given as bolus intravenous infusion on days 1, 4, 8, and 11 every 3 weeks; peg: 30 mg/m2 on day 4 of each cycle for eight cycles or until progression46 | 4 | Median, 9 months | At 15 months, 76% |
| RD | Lenalidomide: 25 mg given orally on days 1-21; D: 40 mg on days 1-4, 9-12, and 17-20 every 4 weeks for four cycles and on days 1-4 for following cycles until progression47 | 14 | Median, 11 months | Median, 29.6 months |
| Carfilzomib | Carfilzomib: 20 mg/m2 given as 2-10 minute intravenous infusion on days 1, 2, 8, 9, 15, and 16 every 4 weeks (cycle one) and 27 mg/m2 on days 1,2, 8, 9, 15, and 16 every 4 weeks for up to 12 cycles48 | 0.4 | Median, 3.7 months | Median, 15.6 months |
Abbreviations: CR, complete response; CTDa, cyclophosphamide-thalidomide-dexamethasone; D, dexamethasone; EFS, event-free survival; FISH, fluorescent in situ hybridization; MPR, melphalan-prednisone-lenalidomide; MPT, melphalan-prednisone-thalidomide; OS, overall survival; PFS, progression-free survival; R, lenalidomide; Rd, lenalidomide plus low-dose dexamethasone; RD, lenalidomide plus high-dose dexamethasone; TTP, time to progression; V, bortezomib; V-Peg, bortezomib plus pegylated liposomal doxorubicin; VCD, bortezomib, cyclophosphamide, and dexamethasone; VGPR, very good partial response; VMP, bortezomib-melphalan-thalidomide; VMPT, bortezomib-melphalan-prednisone-thalidomide; VTP, bortezomib-thalidomide-prednisone.
For both patients enrolled in VTP or VMP arms; study detected no significant difference between two treatment arms (VMP v VTP).
Immunofixation-negative CR plus immunofixation-positive CR.
With or without transplantation.
Patients with adverse interphase FISH receiving thalidomide showed no significant PFS benefit and worse OS (P = .009).
After four cycles, patients could discontinue therapy to pursue stem-cell transplantation or continue treatment until disease progression.
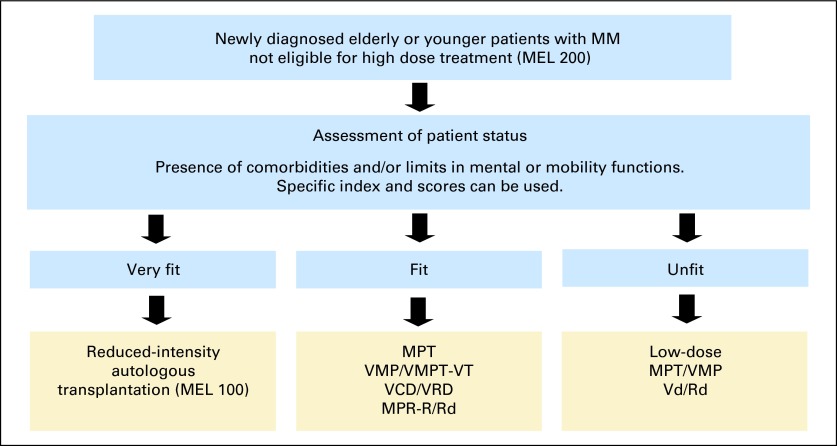
The choice of treatment should take into account patient status (Fig 1), the risk/benefit ratio of each regimen (Table 5), and patient quality of life. Patients with newly diagnosed myeloma should be referred to specialized units to receive appropriate care (Appendix, online only).
Fig 1.
Treatment algorithm for elderly patients with multiple myeloma (MM). MEL 100, melphalan 100 mg/m2; MEL 200, melphalan 200 mg/m2; MPR-R, melphalan-prednisone-lenalidomide followed by lenalidomide; MPT, melphalan-prednisone-thalidomide; Rd, lenalidomide plus low-dose dexamethasone; Vd, bortezomib-dexamethasone; VCD, bortezomib-cyclophosphamide-dexamethasone; VMP, bortezomib-melphalanprednisone; VMPT-VT, bortezomibmelphalan-prednisone-thalidomide followed by bortezomib-thalidomide; VRD, bortezomib-lenalidomide-dexamethasone.
Table 5.
Grade 3 to 4 AEs
| Regimen | Neutropenia (%) | Thrombocytopenia (%) | VTE (%) | Peripheral Neuropathy (%)* | Infection (%) | Fatigue (%) | GI (%) | SPM (%) |
|---|---|---|---|---|---|---|---|---|
| Induction | ||||||||
| MPT31,32,49–53 | 16-48 | 3-14 | 3-12 | 6-23 | 4-28 | 3-8 | 5-11 | NA |
| CTD34 | NA | NA | 16 | 7 | 13 | NA | 4 | NA |
| VMP35 | 40 | 37 | 1 | 22 | 10 | 8 | 17 | 6 |
| VMP weekly54† | 33 | 19 | 3 | 8 | 11 | 4 | 6 | NA |
| VMPT36 | 38 | 22 | 5 | 15 | 13 | 6 | 6 | NA |
| VTP38 | 22 | 12 | 2 | 9 | 1 | NA | 2 | NA |
| VRd41 | 9 | 6 | 5 | 6 | 5 | 3 | 2 | NA |
| Rd42 | 20 | 5 | 12 | 2 | 9 | 9 | NA | NA |
| MPR43 | 66 | 40 | 5 | 0 | 13 | 2 | 5 | 2 |
| Salvage | ||||||||
| V45 | 14 | 30 | 0 | 8 | 13 | 6 | 19 | NA |
| V-Peg46 | 29 | 23 | 1 | 4 | 3 | 6 | 14 | NA |
| RD47 | 41 | 15 | 15 | 2 | 22 | 6 | 10 | NA |
Abbreviations: AE, adverse event; CTD, cyclophosphamide-thalidomide-dexamethasone; MPR, melphalan-prednisone-lenalidomide; NA, not available; Rd, lenalidomide plus low-dose dexamethasone; RD, lenalidomide plus high-dose dexamethasone; SPM, second primary malignancy; V, bortezomib; V-Peg, bortezomib plus pegylated liposomal doxorubicin; VMP, bortezomib-melphalan-thalidomide; VMPT, bortezomib-melphalan-prednisone-thalidomide; VTE, venous thromboembolism; VTP, bortezomib-thalidomide-prednisone.
Sensory neuropathy/motor neuropathy/neuralgia.
Weekly infusion of bortezomib.
Reduced-Intensity Autologous Transplantation
In patients age 65 to 70 years, MEL100 followed by ASCT was superior to standard melphalan-prednisone (MP), improving both event-free survival (28 v 16.4 months) and OS (58 v 37.2 months),55 but in patients age 65 to 75 years, MEL100 was inferior to MP-thalidomide (MPT; PFS, 19.4 v 27.5 months).31 In patients age 65 to 75 years, bortezomib-based induction, tandem MEL100, lenalidomide-prednisone consolidation, and lenalidomide maintenance led to a median PFS of approximately 4 years.56 In selected very fit patients, ASCT remains feasible well beyond the age limit of 65 years. As recommended for patients age < 65 years, bortezomib-based induction and lenalidomide maintenance should be considered for patients undergoing ASCT.57
Recommendation:
Very fit patients age 65 to 75 years, unsuitable for MEL200, may benefit from MEL100 (grade B/IIa).
Thalidomide-Based Regimens
Thalidomide combined with dexamethasone (TD) was superior to high-dose dexamethasone for partial response (63% v 41%)58 and time to progression (TTP; 22.6 v 6.5 months)59 but was more toxic. Similarly, TD was superior to MP for responses, but PFS was similar, and OS was shorter.60
Six randomized studies compared MPT with standard MP. Despite differences in doses and schedules among the trials, better responses and PFS were reported with MPT.31,32,49–53 The effect on OS varied across the studies, and only two trials showed a significant survival benefit.31,52 In a meta-analysis of data from 1,682 patients, MPT improved PFS by 5.4 months and OS by 6.6 months.33 Severe adverse events (AEs), especially nonhematologic, were higher with MPT and negatively affected the prognosis.53 Thalidomide-related AEs included cytopenia, thrombosis, fatigue, and peripheral neuropathy.
Cyclophosphamide-thalidomide-dexamethasone improved responses compared with MP, with similar survival outcomes and higher incidence of AEs.34 Thalidomide doses > 100 mg per day are poorly tolerated and not appropriate for elderly patients. MPT has the advantage of oral administration and reduced hematologic toxicity, but it is associated with an increased risk of peripheral neuropathy, deep-vein thrombosis, and cardiac events. The use of this combination is supported by different phase III trials.
Bortezomib-Based Regimens
In a large phase III trial, the addition of bortezomib to standard MP (VMP) significantly increased CR from 4% to 30%, TTP by approximately 7 months, and OS by 13 months.35,61 Bortezomib-related AEs included primarily neutropenia, thrombocytopenia, and peripheral neuropathy.62 When the twice-per-week bortezomib schedule was decreased to once per week, the rate of grade 3 to 4 peripheral neuropathy was significantly reduced from 28% to 8%, without affecting efficacy.38,54 Recently, subcutaneous bortezomib proved to be as effective as intravenous administration, with a reduced risk of peripheral neuropathy.63
The four-drug combination of bortezomib, melphalan, prednisone, and thalidomide followed by continuous bortezomib-thalidomide (VMPT-VT) demonstrated better responses and a PFS prolongation of 8 months compared with VMP, but the efficacy advantage was mainly reported in fit patients 65 to 75 years of age.36,37 Bortezomib-thalidomide-prednisone (VTP) as induction, followed by VT or bortezomib-prednisone, was not superior to VMP and was associated with more serious AEs and discontinuations.38
Promising results were obtained when cyclophosphamide (VCD)39,40 or lenalidomide (VRD)41 were combined with bortezomib-dexamethasone (VD), producing high-quality responses. Bortezomib, either intravenously or subcutaneously, induces high and rapid responses. Bortezomib does not increase the risk of thromboembolism and may be used in patients with renal failure, but peripheral neuropathy and thrombocytopenia are the main dose-limiting toxicities. The benefits of VMP and VMPT-VT are supported by phase III trials; alternatively, VCD or VRD can be adopted.
Lenalidomide-Based Regimens
The combination lenalidomide plus low-dose dexamethasone (Rd) was better tolerated than lenalidomide plus high-dose dexamethasone (RD), with a significant survival benefit (2-year OS, 87% v 75%). The most common grade ≥ 3 AEs were thrombosis, infections, and fatigue and were more frequent with RD.42
Melphalan-prednisone-lenalidomide followed by lenalidomide (MPR-R) significantly prolonged median PFS by 17 months in comparison with fixed-duration melphalan-prednisone-lenalidomide (MPR) and by 18 months compared with MP. However, this advantage was not confirmed in patients age > 75 years. During induction, the most frequent AEs were hematologic. The incidence rates per 100 patient-years of hematologic second primary malignancies (SPMs) were 1.92, 1.30, and 0.40 in the MPR-R, MPR, and MP groups, respectively, whereas solid SPMs were heterogeneous and balanced across arms.43
Lenalidomide has the advantage of the oral administration and the lack of neurologic toxicity, although myelosuppression is common, and the prevention of venous thromboembolism is recommended. MPR-R is supported by a phase III trial, whereas the evaluation of Rd compared with melphalan-based regimens is ongoing.
Recommendations:
Fit patients should receive full-dose therapy. MPT, VMP, Rd, VMPT-VT, and MPR-R are reasonable therapeutic options (grade A/Ib).
MPT may be preferred for its oral administration and lower cost (grade C/IV).
VMP and VMPT-VT or VCD and VRD may be preferred in patients who need rapid, profound cytoreduction. Once-per-week subcutaneous bortezomib should be considered because of the lower incidence of AEs (grade C/IV).
Rd or MPR-R may be preferred when oral administration and the lack of peripheral neuropathy are major considerations (grade C/IV).
Treatment Options for Unfit Patients
Unfit patients are more susceptible to AEs with subsequent treatment discontinuations that significantly affect dose-intensity and efficacy. In these patients, lower dose-intense therapies are suggested. The three-drug combination MPT has consistently showed a PFS improvement that was less pronounced in patients age > 75 years, whereas VMP was superior to MP in patients age > 75 years.35,52,61 In a randomized study, the outcome was similar between VD, VMP, and VT-dexamethasone, but the discontinuation rate was lower with VD.64 The combination Rd was equally effective in younger and elderly patients. Therefore, two-drug combinations such as corticosteroid plus lenalidomide, thalidomide, or bortezomib should be considered safe treatment options for unfit patients.64–67
Low-dose dexamethasone is mandatory because of the higher toxicity and mortality rates associated with high-dose dexamethasone.42 Lower doses of dexamethasone (10-20 mg/wk) are better tolerated. Thalidomide at 50 mg per day and lenalidomide at 15 mg per day are the preferred doses in this setting.52,68 Subcutaneous once-per-week bortezomib 1 mg/m2 is highly suggested in unfit patients.38,54,63 Because the risk of AEs is higher at the beginning of treatment, therapy may be started at lower doses and subsequently increased after 2 to 4 months if tolerated or if the disease is not adequately controlled.
Recommendation:
Unfit patients should receive reduced-dose MPT or VMP or two-drug combinations with bortezomib or lenalidomide and low-dose dexamethasone (ie, Vd or Rd; grade C/IV).
Maintenance Therapy
Maintenance treatment has consistently prolonged PFS but has inconsistently improved survival.44,69 In a recent meta-analysis, continuous thalidomide improved PFS, with a late OS benefit.44 In another meta-analysis, lenalidomide reduced the risk of progression by 65% in both young and elderly patients.70
In the MRC Myeloma IX trial, the longest PFS was reported in patients treated with thalidomide both at induction and after induction; the shortest PFS was seen in the group treated with MP without thalidomide.44 Continuous thalidomide showed no PFS benefit and worse OS in patients with adverse FISH.
In a prespecified landmark analysis of the MM015 trial, continuous lenalidomide significantly extended PFS from the start of lenalidomide (26 months) as compared with placebo (7 months), regardless of age.43 Similarly, VT prolonged median PFS by approximately 14 months.37 Continuous therapy with VT or bortezomib-prednisone led to a median PFS of 30 months versus 24 months, respectively.71
Drug-related toxicity associated with continuous thalidomide therapy may limit its long-term administration. Lenalidomide is well tolerated, although it is also associated with a higher risk of SPMs. Continuous treatment with bortezomib has the inconvenience of injection administration and a slight increased risk of peripheral neuropathy.
In the future, the impact of maintenance on response and outcome after progression needs to be clarified. Similarly, the optimal duration of maintenance should be defined (for a fixed duration of 2 years or until progression/intolerance).
Recommendations:
The routine use of maintenance in transplantation-ineligible patients is not yet validated.
Thalidomide is an option for standard-risk patients, although its long-term use is limited by the risk of peripheral neuropathy (grade A/Ib).
Lenalidomide is well tolerated but associated with a higher risk of SPMs (grade A/Ib).
Bortezomib can be an effective alternative, with lower risk of peripheral neuropathy than thalidomide (grade B/IIa).
Therapy for Relapsed Disease
When treating patients with relapsed myeloma, duration of response to previous therapy is a fundamental factor to consider. Repeating the same treatment is a valuable option for patients with a durable response lasting more than 20 to 24 months after induction at diagnosis and more than 9 to 12 months after therapy at relapse. In the case of short-term remission duration or progression during initial therapy, an alternative regimen is suggested.
Standard treatments include bortezomib or lenalidomide combined with dexamethasone or bortezomib-pegylated liposomal doxorubicin.45–47,72,73 Rd is highly suggested because it is better tolerated compared with RD.
Re-treatment with bortezomib is a feasible option.74 Re-exposure to immunomodulatory drugs such as lenalidomide after previous thalidomide seems feasible; however, efficacy and survival may be lower.75,76
In case of stable disease without CRAB symptoms, the treatment strategy should not be changed. The asymptomatic status, rather than a response improvement, is the most relevant factor to consider during salvage treatment.77 In case of biochemical relapse, especially during maintenance therapy, increasing the dose of the current drug and subsequently adding another agent is a sensible strategy.
In a recent survey, poor outcome was reported once patients became refractory to both bortezomib and immunomodulatory drugs.78 Ongoing trials are exploring novel agents, such as new proteasome inhibitors (carfilzomib combined with lenalidomide-dexamethasone), anti-CS1 monoclonal antibody (elotuzumab plus lenalidomide-dexamethasone or VD), histone-deacetylase inhibitors (panobinostat and vorinostat), and bendamustine. The US Food and Drug Administration recently approved carfilzomib for progressive MM after at least two prior therapies, including bortezomib and immunomodulatory agents, and pomalidomide in patients relapsed/refractory to lenalidomide.48,79 Thalidomide is preferred for its limited hematologic toxicity; bortezomib is preferred in case of renal failure or previous deep-vein thrombosis; lenalidomide is suggested in case of concomitant peripheral neuropathy. Palliative care is essential when cure is no longer possible (Appendix, online only).
Recommendations:
Repeating the same treatment should be considered after long-lasting remission (20-24 months); an alternative regimen is suggested for patients with shorter remission duration (9 to 12 months; grade C/IV).
VD or bortezomib-pegylated liposomal doxorubicin and lenalidomide-dexamethasone are the treatments of choice (grade A/Ib).
Bone Disease
Bone disease is a highly disabling event that can cause pain, pathologic fractures, spinal cord compression, and hypercalcemia.80 Pain requires pharmacologic analgesia, together with chemotherapy, bisphosphonates, and local interventions.81 Radiotherapy may be useful to prevent further osteolysis at the fracture site; percutaneous vertebroplasty and balloon kyphoplasty are suggested in case of painful spinal fractures.
Oral clodronic acid, intravenous pamidronic acid, and zoledronic acid are the available bisphosphonate treatments.82–84 Zoledronic acid significantly reduced skeletal-related events (SREs) and improved OS compared with sodium clodronate.85,86 Zoledronic acid was as effective as pamidronate in preventing SREs.87,88 No difference was observed between monthly pamidronate at 30 or 90 mg.89 Renal impairment and osteonecrosis of the jaw are infrequent but serious complications of intravenous bisphosphonates.
Recommendations:
Analgesics should be used to treat uncontrolled pain. Low-dose radiation therapy (8 Gy, single fraction) of limited involved fields should be used in case of pain not responding to therapy. Vertebroplasty and kyphoplasty should be considered for painful vertebral collapse (grade C/IV).
Amino-containing bisphosphonates are recommended for the prevention and management of SREs, independently of bone disease status at baseline. Renal function should be carefully monitored, drug doses should be reduced, and dental evaluation should be performed before starting therapy (grade A/Ib). There is insufficient evidence to recommend bisphosphonates in asymptomatic MM.
Renal Failure
Renal failure occurs because of FLC-related damage of proximal tubules, along with hypercalcemia, hyperuricaemia, dehydration, infections, and nephrotoxic drugs. The immediate start of an effective MM treatment is the mainstay to recover renal function. High-dose dexamethasone is a rapid intervention to assure a fall in light chain load.90 Bortezomib can be administered safely, without dose adjustments, and should be preferred in the event of dialysis.91–95 Limited data are present on the role of thalidomide in this setting.96,97 Lenalidomide is active,98,99 but dose reductions are mandatory depending on the creatinine clearance values.100,101 Doxorubicin and cyclophosphamide do not require dose adjustments. Adjusted doses of bisphosphonates are indicated to correct hypercalcemia. Additional studies of the new large-pore hemodialysis membranes to physically remove light chains are awaited.
Recommendations:
High-dose dexamethasone (40 mg per day for 4 days) should be started promptly, along with high fluid intake (≥ 3 L per day of saline solution; grade C/IV).
In case of acute renal failure or for patients requiring dialysis, bortezomib can be safely used without dose modifications (grade C/IV).
In case of chronic renal impairment, thalidomide and lenalidomide can be administered. Appropriate lenalidomide dose reductions are mandatory: 10 mg per day when creatinine clearance is 30 to 50 mL/min; 15 mg every other day when creatinine clearance is < 30 mL/min; 5 mg per day after dialysis when patient requires dialysis (grade C/IV).
Hematologic Toxicity
Myelosuppression is primarily induced by chemotherapy, but patient characteristics, disease stage, type of current and previous treatments, and neutrophil count < 1,000 cells/mL at baseline are additional risk factors of severe neutropenia. Granulocyte colony-stimulating factor (G-CSF) should be used to permit patients to stay on treatment longer.102,103 Anemia can be managed in the short term with transfusions. Erythropoiesis-stimulating agents are indicated during chemotherapy, particularly with renal impairment, when the hemoglobin concentration is < 10 g/dL, and there is no improvement despite response to therapy.104–106 Thrombocytopenia is common with bortezomib, lenalidomide, and alkylating agents, whereas it rarely occurs with thalidomide.103
Recommendations:
G-CSF is recommended to prevent febrile neutropenia in patients at high risk based on age, medical history, disease characteristics, and the expected myelotoxicity of chemotherapy.
When grade 3 to 4 neutropenia occurs during chemotherapy, G-CFS should be added. If neutrophil count restores to > 1,000 cells/mL, therapy can be resumed without dose modifications. If neutrophil count remains < 1,000 cells/mL, treatment should be delayed until neutrophils recovery and resumed at reduced doses (grade C/IV).
Patients with hemoglobin < 10 g/dL during chemotherapy should receive erythropoietin, which should be stopped if an increase of hemoglobin ≥ 1 g/dL after 4 weeks of treatment is not obtained (grade A/Ib). Iron supplementation is recommended if transferrin saturation is inadequate.
If grade 4 thrombocytopenia occurs, treatment should be withheld; it can be resumed when the event resolves to grade 2 (grade C/IV).
Thromboembolism
Myeloma has a high risk of venous thromboembolism (VTE).107 Patient-related risk factors include advanced age, history of VTE or inherited thrombophilia, obesity, comorbidities, central venous catheter in situ, immobility, and surgery. Myeloma-related factors include the diagnosis of myeloma itself, disease burden, and hyperviscosity. Treatment-related factors include the use of thalidomide or lenalidomide, particularly when combined with high-dose steroids or doxorubicin or multiagent chemotherapy, and the concomitant use of erythropoietin.108–110
The role of low–molecular weight heparin (LMWH) in preventing VTE is well recognized; aspirin (ASA) should be used in selected circumstances, and fixed low-dose warfarin has generally been shown to be ineffective.111,112 The American College of Chest Physicians guidelines recommend LMWH or low-dose unfractionated heparin in outpatients with tumors and risk factors for VTE, including thalidomide and lenalidomide therapy.113
Recommendations:
Patients with MM should receive appropriate thromboprophylaxis based on risk factors for the first 4 to 6 months of treatment, until disease control is achieved or as long as the risk of thromboembolism remains high (grade C/IV).
During thalidomide or lenalidomide treatment, ASA should be administered to low-risk patients (with ≤ one risk factor). High-risk patients (with ≥ two risk factors) should receive prophylactic LMWH or dose-adjusted therapeutic warfarin for 4 to 6 months followed by ASA (grade B/IIa)
The dose of LMWH should be adjusted according to renal function (grade C/IV).
For patients who develop VTE, treatment should be temporarily interrupted, and they should receive anticoagulation therapy. When stable anticoagulation is achieved, chemotherapy can be restarted (grade C/IV).
Infections
MM can cause impairment of immune function, with consequent increased risk of infections, particularly during active disease, or treatment with high-dose dexamethasone, myelotoxic agents, or multidrug combinations.114,115 Herpes zoster is a possible complication related to bortezomib administration.35
Recommendations:
For unfit patients with comorbidities and for patients with an increased infection rate, oral antibiotic prophylaxis should be considered for the first 3 months of therapy. Trimethoprim-sulfamethoxazole prophylaxis should be considered at least during the first 2 to 3 months of chemotherapy or steroid administration (grade C/IV)
Antiviral prophylaxis, such as acyclovir or valacyclovir, is recommended against zoster reactivation during bortezomib treatment and for 30 to 60 days after its discontinuation (grade C/IV).
Patients with MM should be treated promptly with broad-spectrum antibiotics in case of fever or suspected infections (grade C/IV).
Peripheral Neuropathy
Peripheral neuropathy can be caused by the disease itself or by thalidomide and bortezomib therapy. Because treatment-emergent peripheral neuropathy is related to the duration of drug exposure and is cumulative,116,117 early reduction or temporary discontinuation of the drug should be adopted.118,119 Subcutaneous and weekly bortezomib infusions significantly reduced peripheral neuropathy, without considerably affecting outcome.116 Neuropathic pain is often poorly responsive to standard analgesia, but gabapentin and opioid drugs may improve symptoms.120–122
Recommendations:
Close monitoring of patients receiving bortezomib and thalidomide is highly recommended. Patients should be informed about the risk of peripheral neuropathy and instructed to promptly seek medical advice when symptoms emerge. When grade 1 peripheral neuropathy with pain or grade ≥ 2 occur, treatment should be interrupted until resolution of symptoms and reinitiated at lower doses (grade C/IV).
Prompt thalidomide dose reductions (from 100 to 50 mg per day) are essential to avoid irreversible damage (grade C/IV).
Once-per-week bortezomib at a dose of 1.3 mg/m2 should be reduced to 1.0 mg/m2 and subsequently to 0.7 mg/m2 per week (grade C/IV).
Appendix
International Myeloma Working Group:
Niels Abildgaard, Syddansk Universitet, Odense, Denmark; Rafat Abonour, Indiana University School of Medicine, Indianapolis, IN; Ray Alexanian, MD Anderson, Houston, TX; Melissa Alsina, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL; Kenneth C. Anderson, Dana-Farber Cancer Institute, Boston, MA; Michel Attal, Purpan Hospital, Toulouse, France; Hervé Avet-Loiseau, Institute de Biologie, Nantes, France; Ashraf Badros, University of Maryland, Baltimore, MD; Dalsu Baris, National Cancer Institute, Bethesda, MD; Bart Barlogie, Myeloma Institute for Research and Therapy, University of Arkansas for Medical Sciences, Little Rock, AR; Régis Bataille, Institute de Biologie, Nantes, France; Meral Beksaç, Ankara University, Ankara, Turkey; Andrew Belch, Cross Cancer Institute, Edmonton, Alberta, Canada; Dina Ben-Yehuda, Hadassah University Hospital, Hadassah, Israel; Bill Bensinger, Fred Hutchinson Cancer Center, Seattle, WA; P. Leif Bergsagel, Mayo Clinic Scottsdale, Scottsdale, AZ; Jenny Bird, Bristol Hematology and Oncology Center, Bristol, United Kingdom; Joan Bladé, Hospital Clinica, Barcelona, Spain; Mario Boccadoro, University of Torino, Torino, Italy; Jo Caers, Centre Hospitalier Universitaire de Liège, Liège, Belgium; Asher Chanan-Khan, Mayo Clinic, Jacksonville, FL; Wen Ming Chen, MM Research Center of Beijing, Beijing, People's Republic of China; Marta Chesi, Mayo Clinic Scottsdale, Scottsdale, AZ; Tony Child, Leeds General Hospital, Leeds, United Kingdom; James Chim, Department of Medicine, Queen Mary Hospital, Hong Kong, Special Administrative Region, People's Republic of China; Wee-Joo Chng, National University Health System, Singapore, Singapore; Ray Comenzo, Tufts Medical School, Boston, MA; John Crowley, Cancer Research and Biostatistics, Seattle, WA; William Dalton, H. Lee Moffitt, Tampa, FL; Faith Davies, Royal Marsden Hospital, London, United Kingdom; Javier de la Rubia, Hospital Universitario La Fe, Valencia, Spain; Cármino de Souza, Univeridade de Campinas, Caminas, Brazil; Michel Delforge, University Hospital Gasthuisberg, Leuven, Belgium; Meletios Dimopoulos, University of Athens School of Medicine, Athens, Greece; Angela Dispenzieri, Mayo Clinic, Rochester, MN; Johannes Drach, University of Vienna, Vienna, Austria; Matthew Drake, Mayo Clinic Rochester, Rochester, MN; Brian G.M. Durie, Cedars-Sinai Samuel Oschin Cancer Center, Los Angeles, CA; Hermann Einsele, Universitätsklinik Würzburg, Würzburg, Germany; Theirry Facon, Centre Hospitalier Regional Universitaire de Lille, Lille, France; Dorotea Fantl, Socieded Argentinade Hematolgia, Buenos Aires, Argentina; Jean-Paul Fermand, Hopitaux de Paris, Paris, France; Carlos Fernández de Larrea, Hospital Clínic de Barcelona, Barcelona, Spain; Rafael Fonseca, Mayo Clinic Arizona, Scottsdale, AZ; Gösta Gahrton, Karolinska Institute for Medicine, Huddinge, Sweden; Ramón García-Sanz, University Hospital of Salamanca, Salamanca, Spain; Christina Gasparetto, Duke University Medical Center, Durham, NC; Morie Gertz, Mayo Clinic, Rochester, MN; Irene Ghobrial, Dana-Farber Cancer Institute, Boston, MA; John Gibson, Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; Peter Gimsing, University of Copenhagen, Copenhagen, Denmark; Sergio Giralt, Memorial Sloan-Kettering Cancer Center, New York, NY; Hartmut Goldschmidt, University Hospital Heidelberg, Heidelberg, Germany; Philip Greipp, Mayo Clinic, Rochester, MN; Roman Hajek, Brno University, Brno, Czech Republic; Izhar Hardan, Tel Aviv University, Tel Aviv, Israel; Parameswaran Hari, Medical College of Wisconsin, Milwaukee, WI; Hiroyuki Hata, Kumamoto University Hospital, Kumamoto, Japan; Yutaka Hattori, Keio University School of Medicine, Tokyo, Japan; Tom Heffner, Emory University, Atlanta, GA; Joy Ho, Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; Antje Hoering, Cancer Research and Biostatistics, Seattle, WA; Jian Hou, Shanghai Chang Zheng Hospital, Shanghai, People's Republic of China; Vania Hungria, Clinica San Germano, Sao Paolo, Brazil; Shinsuke Ida, Nagoya City University Medical School, Nagoya, Japan; Peter Jacobs, Constantiaberg Medi-Clinic, Plumstead, South Africa; Sundar Jagannath, Mt. Sinai Cancer Institute, New York, NY; Hans Johnsen, Aalborg Hospital Science and Innovation Center, Aalborg, Denmark; Douglas Joshua, Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; Artur Jurczyszyn, Myeloma Treatment Foundation, Poland; Jonathan Kaufman, Emory Clinic, Atlanta, GA; Michio Kawano, Yamaguchi University, Ube, Japan; Eva Kovacs, Cancer Immunology Research-Life, Birsfelden, Switzerland; Amrita Krishnan, City of Hope, Duarte, CA; Sigurdur Kristinsson, Karolinska University Hospital and Karolinska Institutet, Stockholm, Sweden; Nicolaus Kröger, University Hospital Hamburg, Hamburg, Germany; Shaji Kumar, Department of Hematology, Mayo Clinic, MN; Robert A. Kyle, Department of Laboratory Medicine and Pathology, Mayo Clinic, MN; Chara Kyriacou, Northwick Park Hospital, London, United Kingdom; Martha Lacy, Mayo Clinic Rochester, Rochester, MN; Juan José Lahuerta, Grupo Español di Mieloma, Hospital Universitario 12 de Octubre, Madrid, Spain; Ola Landgren, National Cancer Institute, Bethesda, MD; Jacob Laubach, Dana-Farber Cancer Institute, Boston, MA; Garderet Laurent, Hôpital Saint Antoine, Paris, France; Fernando Leal da Costa, Instituto Portugues De Oncologia, Lisbon, Portugal; Jae Hoon Lee, Gachon University Gil Hospital, Incheon, Korea; Merav Leiba, Sheba Medical Center, Tel Hashomer, Israel; Xavier LeLeu, Hospital Huriez, Centre Hospitalier Régional Universitaire, Lille, France; Suzanne Lentzsch, University of Pittsburgh, Pittsburgh, PA; Henk Lokhorst, University Medical Center Utrecht, Utrecht, the Netherlands; Sagar Lonial, Emory University Medical School, Atlanta, GA; Heinz Ludwig, Wilhelminenspital der Stat Wien, Vienna, Austria; Anuj Mahindra, Dana-Farber Cancer Institute, Massachusetts General Hospital, Boston, MA; Angelo Maiolino, Rua fonte da Saudade, Rio de Janeiro, Brazil; María Mateos, University of Salamanca, Salamanca, Spain; Amitabha Mazumder, New York University Comprehensive Cancer Center, New York, NY; Philip McCarthy, Roswell Park Cancer Center, Buffalo, NY; Jayesh Mehta, Northwestern University, Chicago, IL; Ulf-Henrik Mellqvist, Sahlgrenska University Hospital, Gothenburg, Sweden; GiamPaolo Merlini, University of Pavia, Pavia, Italy; Joseph Mikhael, Mayo Clinic Arizona, Scottsdale, AZ; Philippe Moreau, University Hospital, Nantes, France; Gareth Morgan, Royal Marsden Hospital, London, United Kingdom; Nikhil Munshi, Diane Farber Cancer Institute, Boston, MA; Hareth Nahi, Karolinska University Hospital, Stockholm, Sweden; Ruben Niesvizky, Weill Cornell Medical College, New York, NY; Amara Nouel, Hospital Rutz y Paez, Bolivar, Venezuela; Yana Novis, Hospital SÍrio Libanês, Bela Vista, Brazil; Enrique Ocio, Salamanca, Spain; Robert Orlowski, MD Anderson Cancer Center, Houston, TX; Antonio Palumbo, Cathedra Ematologia, Torino, Italy; Santiago Pavlovsky, Fundaleu, Buenos Aires, Argentina; Linda Pilarski, University of Alberta, Edmonton, Alberta, Canada; Raymond Powles, Leukemia and Myeloma, Wimbledon, United Kingdom; Noopur Raje, Massachusetts General Hospital, Boston, MA; S. Vincent Rajkumar, Mayo Clinic, Rochester, MN; Donna Reece, Princess Margaret Hospital, Toronto, Ontario, Canada; Tony Reiman, Saint John Regional Hospital, Saint John, New Brunswick, Canada; Paul G. Richardson, Dana-Farber Cancer Institute, Boston, MA; Angelina Rodríguez Morales, Bonco Metro Politano de Sangre, Caracas, Venezuela; Kenneth R. Romeril, Wellington Hospital, Wellington, New Zealand; David Roodman, University of Pittsburgh School of Medicine, Pittsburgh, PA; Laura Rosiñol, Hospital Clinic, Barcelona, Spain; Stephen Russell, Mayo Clinic, Rochester, MN; Jesús San Miguel, University of Salamanca, Salamanca, Spain; Rik Schots, Universitair Ziekenhuis Brussel, Brussels, Belgium; Sabina Sevcikova, Masaryk University, Brno, Czech Republic; Orhan Sezer, Universität Hamburg, Hamburg, Germany; Jatin J. Shah, MD Anderson Cancer Institute, Houston, TX; John Shaughnessy, Myeloma Institute for Research and Therapy, University of Arkansas for Medical Sciences, Little Rock, AR; Kazuyuki Shimizu, Aichi Gakuin University, Nagoya, Japan; Chaim Shustik, McGill University, Montreal, Quebec, Canada; David Siegel, Hackensack, Cancer Center, Hackensack, NJ; Seema Singhal, Northwestern University, Chicago, IL; Pieter Sonneveld, Erasmus Medical Centre, Rotterdam, the Netherlands; Andrew Spencer, Alfred Hospital, Melbourne, Victoria, Australia; Edward Stadtmauer, University of Pennsylvania, Philadelphia, PA; Keith Stewart, Mayo Clinic Arizona, Scottsdale, AZ; Evangelos Terpos, University of Athens School of Medicine, Athens, Greece; Patrizia Tosi, Italian Cooperative Group, Istituto di Ematologia Seragnoli, Bologna, Italy; Guido Tricot, Huntsman Cancer Institute, Salt Lake City, UT; Ingemar Turesson, Skåne University Hospital, Malmo, Sweden; Saad Usmani, Myeloma Institute for Research and Therapy, University of Arkansas for Medical Sciences, Little Rock, AR; Ben Van Camp, Vrije Universiteit Brussels, Brussels, Belgium; Brian Van Ness, University of Minnesota, Minneapolis, MN; Ivan Van Riet, Brussels Vrija University, Brussels, Belgium; Isabelle Vande Broek, Vrije Universiteit Brussels, Brussels, Belgium; Karin Vanderkerken, Vrije University Brussels VUB, Brussels, Belgium; Robert Vescio, Cedars-Sinai Cancer Center, Los Angeles, CA; David Vesole, Hackensack Cancer Center, Hackensack, NJ; Peter Voorhees, University of North Carolina, Chapel Hill, NC; Anders Waage, University Hospital, Trondheim, Norway; Michael Wang, MD Anderson, Houston, TX; Donna Weber, MD Anderson, Houston, TX; Jan Westin, Sahlgrenska University Hospital, Gothenburg, Sweden; Keith Wheatley, University of Birmingham, Birmingham, United Kingdom; Elena Zamagni, University of Bologna, Bologna, Italy; Jeffrey Zonder, Karmanos Cancer Institute, Detroit, MI; and Sonja Zweegman, Vrije Universiteit University Medical Center, Amsterdam, the Netherlands.
Facilities and Services
To provide high-quality care, it is strongly advisable that patients with myeloma are diagnosed and treated in clinical hematology units, with specific expertise in the management of multiple myeloma (MM). The hematology team should coordinate and share the care of patients, assuring a good communication flow between the general practitioner and other specialists involved in the management of complications. Accurate information should be provided to patients and their caregivers so they are able to make sensible decisions.
Recommendation:
The general practitioner should refer patients to specialized units with MM experts to offer the most appropriate care (grade C/IV).
Palliative Care
Palliative care aims to optimize the comfort, function, and social support of patients and their families, when cure is no longer achievable (WHO definition of palliative care is available at http://www.who.int/cancer/palliative/definition/en/). In the absence of effective antimyeloma treatments, counseling for patients and families provided by a palliative specialist is suggested. To relieve the disabling myeloma-related symptoms, low doses of cyclophosphamide, corticosteroids, or thalidomide may be used. Treatment of pain should start with nonopioid analgesic agents, but weak or stronger opioid analgesics should be introduced when previous agents are ineffective (WHO Expert Committee: World Health Organ Tech Rep Ser 804:1-75, 1990).
Recommendation:
Terminal care should include a multidisciplinary approach aimed at alleviating symptoms and addressing patient desires (grade C/IV).
Table A1.
Levels of Evidence and Grade of Recommendations
| Level of Evidence | Source of Evidence | Grade of Recommendation | Rationale for Recommendation |
|---|---|---|---|
| Ia | Meta-analyses of randomized controlled trials | A | At least one randomized controlled trial of good quality and consistency addressing specific recommendation |
| Ib | At least one randomized controlled trial | ||
| IIa | At least one well-designed, nonrandomized study, including phase II trials and case-control trials | B | Well-conducted studies but no randomized controlled trials on topic of recommendation |
| IIb | At least one other type of well-designed, quasi-experimental study (ie, studies without planned intervention, including observational studies) | ||
| III | Well-designed, nonexperimental descriptive studies; meta-analyses or randomized controlled trials or phase II studies only published in abstract form | ||
| IV | Expert committee reports or opinions and/or clinical experience of respected authorities | C | Expert committee reports and/or clinical experience of respected authorities |
Table A2.
International Staging System
| Stage | Description |
|---|---|
| I | Serum β2-microglobulin < 3.5 mg/L and serum albumin ≥ 3.5 g/dL |
| II | Serum β2-microglobulin < 3.5 mg/L and serum albumin < 3.5 g/dL or serum β2-microglobulin 3.5 to < 5.5 mg/L |
| III | Serum β2-microglobulin ≥ 5.5 mg/L |
Footnotes
Written on behalf of the International Myeloma Working Group.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Antonio Palumbo, Amgen (C), Bristol-Myers Squibb (C), Celgene (C), Janssen-Cilag (C), Millennium Pharmaceuticals (C), Onyx Pharmaceuticals (C); Jesus F. San Miguel, Janssen-Cilag (C), Millennium Pharmaceuticals (C), Celgene (C), Onyx Pharmaceuticals (C), Novartis (C); Ruben Niesvizky, Onyx Pharmaceuticals (C), Millennium Pharmaceuticals (C), Celgene (C); Roman Hajek, Merck (C), Celgene (C); Hermann Einsele, Celgene (C), Janssen-Cilag (C); Kenneth C. Anderson, Celgene (C), sanofi-aventis (C), Gilead (C), Onyx Pharmaceuticals (C); Meletios A. Dimopoulos, Celgene (C), Ortho Biotech (C); Paul G. Richardson, Celgene (C), Millennium Pharmaceuticals (C), Johnson & Johnson (C), Bristol-Myers Squibb (C), Novartis (C); Michele Cavo, Novartis (C), Celgene (C), Janssen-Cilag (C), Millennium Pharmaceuticals (C); Andrew Spencer, Novartis (C), Janssen-Cilag (C), Celgene (C); A. Keith Stewart, Celgene (C), Onyx Pharmaceuticals (C), Millennium Pharmaceuticals (C); Kazuyuki Shimizu, Fujimoto Pharma (C); Sagar Lonial, Bristol-Myers Squibb (C), Celgene (C), Onyx Pharmaceuticals (C), Millennium Pharmaceuticals (C), Janssen-Cilag (C); Philippe Moreau, Celgene (C), Millennium Pharmaceuticals (C), Onyx Pharmaceuticals (C), Janssen-Cilag (C); Robert Z. Orlowski, Celgene (C), Bristol-Myers Squibb (C), Abbott Laboratories (C), Genentech (C), Onyx Pharmaceuticals (C), Millennium Pharmaceuticals (C), Array Biopharma (C), Merck (C) Stock Ownership: Kenneth C. Anderson, Acetylon, Oncoprep Honoraria: Antonio Palumbo, Celgene, Bristol-Myers Squibb, Amgen, Janssen-Cilag, Millennium Pharmaceuticals, Onyx Pharmaceuticals; Jesus F. San Miguel, Janssen-Cilag, Millennium Pharmaceuticals, Celgene, Onyx Pharmaceuticals, Novartis; Alessandra Larocca, Celgene, Janssen-Cilag; Ruben Niesvizky, Onyx Pharmaceuticals, Millennium Pharmaceuticals, Celgene; Roman Hajek, Merck, Celgene, Janssen-Cilag; Hermann Einsele, Celgene, Janssen-Cilag; Meletios A. Dimopoulos, Celgene, Ortho Biotech; Michele Cavo, Celgene, Janssen; A. Keith Stewart, Celgene, Millennium Pharmaceuticals, Onyx Pharmaceuticals; Kazuyuki Shimizu, Takeda-Millennium, Daiichi-Sankyo, Fujimoto-Pharma; Sagar Lonial, Celgene, Onyx Pharmaceuticals, Millennium Pharmaceuticals, Bristol-Myers Squibb; Brian G.M. Durie, Celgene; Philippe Moreau, Celgene, Millennium Pharmaceuticals, Onyx Pharmaceuticals, Janssen-Cilag Research Funding: Ruben Niesvizky, Onyx Pharmaceuticals, Millennium Pharmaceuticals, Celgene; Hermann Einsele, Celgene, Janssen-Cilag; Meletios A. Dimopoulos, Celgene; Kazuyuki Shimizu, Daiichi Sankyo; Pieter Sonneveld, Celgene, Janssen-Cilag, Onyx Pharmaceuticals; Robert Z. Orlowski, Celgene, Millennium Pharmaceuticals, Onyx Pharmaceuticals, Bristol-Myers Squibb Expert Testimony: Kazuyuki Shimizu, Amgen (U) Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Antonio Palumbo, S. Vincent Rajkumar, Pieter Sonneveld
Collection and assembly of data: Antonio Palumbo, Alessandra Larocca
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Altekruse SF, Kosary CL, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2007, National Cancer Institute. http://seer.cancer.gov/csr/1975_2007/
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 3.Fonseca R, Bergsagel PL, Drach J, et al. International Myeloma Working Group molecular classification of multiple myeloma: Spotlight review. Leukemia. 2009;23:2210–2221. doi: 10.1038/leu.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palumbo A, Sezer O, Kyle R, et al. International Myeloma Working Group guidelines for the management of multiple myeloma patients ineligible for standard high-dose chemotherapy with autologous stem cell transplantation. Leukemia. 2009;23:1716–1730. doi: 10.1038/leu.2009.122. [DOI] [PubMed] [Google Scholar]
- 5.Bird JM, Owen RG, D'Sa S, et al. Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol. 2011;154:32–75. doi: 10.1111/j.1365-2141.2011.08573.x. [DOI] [PubMed] [Google Scholar]
- 6.Snowden JA, Ahmedzai SH, Ashcroft J, et al. Guidelines for supportive care in multiple myeloma 2011. Br J Haematol. 2011;154:76–103. doi: 10.1111/j.1365-2141.2011.08574.x. [DOI] [PubMed] [Google Scholar]
- 7.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490–1498. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajkumar SV, Larson D, Kyle RA. Diagnosis of smoldering multiple myeloma. N Engl J Med. 2011;365:474–475. doi: 10.1056/NEJMc1106428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradwell AR, Carr-Smith HD, Mead GP, et al. Serum test for assessment of patients with Bence Jones myeloma. Lancet. 2003;361:489–491. doi: 10.1016/S0140-6736(03)12457-9. [DOI] [PubMed] [Google Scholar]
- 11.Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: A retrospective population-based cohort study. Lancet. 2010;375:1721–1728. doi: 10.1016/S0140-6736(10)60482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23:215–224. doi: 10.1038/leu.2008.307. [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos M, Kyle R, Fermand JP, et al. Consensus recommendations for standard investigative workup: Report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117:4701–4705. doi: 10.1182/blood-2010-10-299529. [DOI] [PubMed] [Google Scholar]
- 14.Zamagni E, Nanni C, Patriarca F, et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica. 2007;92:50–55. doi: 10.3324/haematol.10554. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Palumbo A, Bringhen S, Ludwig H, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: A report of the European Myeloma Network (EMN) Blood. 2011;118:4519–4529. doi: 10.1182/blood-2011-06-358812. [DOI] [PubMed] [Google Scholar]
- 17.Greipp PR, San Miguel J, Durie BG, et al. International Staging System for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 18.Ross F, Avet-Loiseau H, Ameye G, et al. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Haematologica. 2012;97:1272–1277. doi: 10.3324/haematol.2011.056176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munshi NC, Anderson KC, Bergsagel PL, et al. Consensus recommendations for risk stratification in multiple myeloma: Report of the International Myeloma Workshop Consensus Panel 2. Blood. 2011;117:4696–4700. doi: 10.1182/blood-2010-10-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avet-Loiseau H, Durie BG, Cavo M, et al. Combining fluorescent in situ hybridization (FISH) data with ISS staging improves risk assessment in myeloma: An International Myeloma Working Group (IMWG) collaborative project. Leukemia. 2013;27:711–717. doi: 10.1038/leu.2012.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajkumar SV. Multiple myeloma: 2012 update on diagnosis, risk-stratification, and management. Am J Hematol. 2012;87:78–88. doi: 10.1002/ajh.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snozek CL, Katzmann JA, Kyle RA, et al. Prognostic value of the serum free light chain ratio in newly diagnosed myeloma: Proposed incorporation into the international staging system. Leukemia. 2008;22:1933–1937. doi: 10.1038/leu.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulligan G, Mitsiades C, Bryant B, et al. Gene expression profiling and correlation with outcome in clinical trials of the proteasome inhibitor bortezomib. Blood. 2007;109:3177–3188. doi: 10.1182/blood-2006-09-044974. [DOI] [PubMed] [Google Scholar]
- 24.Shaughnessy JD, Jr, Zhan F, Burington BE, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- 25.Zamagni E, Patriarca F, Nanni C, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011;118:5989–5995. doi: 10.1182/blood-2011-06-361386. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Lopez J, Blade J, Mateos MV, et al. Long-term prognostic significance of response in multiple myeloma after stem cell transplantation. Blood. 2011;118:529–534. doi: 10.1182/blood-2011-01-332320. [DOI] [PubMed] [Google Scholar]
- 27.Gay F, Larocca A, Wijermans P, et al. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: Analysis of 1175 patients. Blood. 2011;117:3025–3031. doi: 10.1182/blood-2010-09-307645. [DOI] [PubMed] [Google Scholar]
- 28.Mateos MV, López-Corral L, Hernández M, et al. Smoldering multiple myeloma (SMM) at high-risk of progression to symptomatic disease: A phase III, randomized, multicenter trial based on lenalidomide-dexamethasone (Len-Dex) as induction therapy followed by maintenance therapy with len alone vs no treatment. Blood. 2011:118. (abstr 991) [Google Scholar]
- 29.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bladé J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation: Myeloma Subcommittee of the EBMT—European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 30a.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 31.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): A randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 32.Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: Randomised controlled trial. Lancet. 2006;367:825–831. doi: 10.1016/S0140-6736(06)68338-4. [DOI] [PubMed] [Google Scholar]
- 33.Fayers PM, Palumbo A, Hulin C, et al. Thalidomide for previously untreated elderly patients with multiple myeloma: Meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood. 2011;118:1239–1247. doi: 10.1182/blood-2011-03-341669. [DOI] [PubMed] [Google Scholar]
- 34.Morgan GJ, Davies FE, Gregory WM, et al. Cyclophosphamide, thalidomide, and dexamethasone (CTD) as initial therapy for patients with multiple myeloma unsuitable for autologous transplantation. Blood. 2011;118:1231–1238. doi: 10.1182/blood-2011-02-338665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 36.Palumbo A, Bringhen S, Rossi D, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: A randomized controlled trial. J Clin Oncol. 2010;28:5101–5109. doi: 10.1200/JCO.2010.29.8216. [DOI] [PubMed] [Google Scholar]
- 37.Palumbo A, Bringhen S, Rossi D, et al. Overall survival benefit for bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide (VMPT-VT) versus bortezomib-melphalan-prednisone (VMP) in newly diagnosed multiple myeloma patients. Blood. 2012:120. doi: 10.1182/blood-2011-05-353995. (abstr 200) [DOI] [PubMed] [Google Scholar]
- 38.Mateos MV, Oriol A, Martínez-López J, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: A randomised trial. Lancet Oncol. 2010;11:934–941. doi: 10.1016/S1470-2045(10)70187-X. [DOI] [PubMed] [Google Scholar]
- 39.Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: High response rates in a phase II clinical trial. Leukemia. 2009;23:1337–1341. doi: 10.1038/leu.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reeder CB, Reece DE, Kukreti V, et al. Once- versus twice-weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood. 2010;115:3416–3417. doi: 10.1182/blood-2010-02-271676. [DOI] [PubMed] [Google Scholar]
- 41.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366:1759–1769. doi: 10.1056/NEJMoa1112704. [DOI] [PubMed] [Google Scholar]
- 44.Morgan GJ, Gregory WM, Davies FE, et al. The role of maintenance thalidomide therapy in multiple myeloma: MRC Myeloma IX results and meta-analysis. Blood. 2012;119:7–15. doi: 10.1182/blood-2011-06-357038. [DOI] [PubMed] [Google Scholar]
- 45.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 46.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: Combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 47.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 48.Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waage A, Gimsing P, Fayers P, et al. Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood. 2010;116:1405–1412. doi: 10.1182/blood-2009-08-237974. [DOI] [PubMed] [Google Scholar]
- 50.Beksac M, Haznedar R, Firatli-Tuglular T, et al. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: Results of a randomized trial from the Turkish Myeloma Study Group. Eur J Haematol. 2011;86:16–22. doi: 10.1111/j.1600-0609.2010.01524.x. [DOI] [PubMed] [Google Scholar]
- 51.Wijermans P, Schaafsma M, Termorshuizen F, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: The HOVON 49 Study. J Clin Oncol. 2010;28:3160–3166. doi: 10.1200/JCO.2009.26.1610. [DOI] [PubMed] [Google Scholar]
- 52.Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27:3664–3670. doi: 10.1200/JCO.2008.21.0948. [DOI] [PubMed] [Google Scholar]
- 53.Palumbo A, Waage A, Hulin C, et al. Safety of thalidomide in newly diagnosed elderly myeloma patients: A meta-analysis of data from individual patients in six randomized trials. Haematologica. 2013;98:87–94. doi: 10.3324/haematol.2012.067058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bringhen S, Larocca A, Rossi D, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116:4745–4753. doi: 10.1182/blood-2010-07-294983. [DOI] [PubMed] [Google Scholar]
- 55.Palumbo A, Bringhen S, Petrucci MT, et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: Results of a randomized controlled trial. Blood. 2004;104:3052–3057. doi: 10.1182/blood-2004-02-0408. [DOI] [PubMed] [Google Scholar]
- 56.Palumbo A, Gay F, Falco P, et al. Bortezomib as induction before autologous transplantation, followed by lenalidomide as consolidation-maintenance in untreated multiple myeloma patients. J Clin Oncol. 2010;28:800–807. doi: 10.1200/JCO.2009.22.7561. [DOI] [PubMed] [Google Scholar]
- 57.Cavo M, Rajkumar SV, Palumbo A, et al. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood. 2011;117:6063–6073. doi: 10.1182/blood-2011-02-297325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajkumar SV, Blood E, Vesole D, et al. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: A clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 59.Rajkumar SV, Rosiñol L, Hussein M, et al. A multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone versus dexamethasone as initial therapy for newly diagnosed multiple myeloma. J Clin Oncol. 2008;26:2171–2177. doi: 10.1200/JCO.2007.14.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ludwig H, Hajek R, Tóthová E, et al. Thalidomide-dexamethasone compared with melphalan-prednisolone in elderly patients with multiple myeloma. Blood. 2009;113:3435–3442. doi: 10.1182/blood-2008-07-169565. [DOI] [PubMed] [Google Scholar]
- 61.San Miguel JF, Schlag R, Khuageva NK, et al. Continued overall survival benefit after 5 years' follow-up with bortezomib-melphalan-prednisone (VMP) versus melphalan-prednisone (MP) in patients with previously untreated multiple myeloma, and no increased risk of second primary malignancies: Final results of the phase 3 VISTA trial. Blood. 2011:118. (abstr 476) [Google Scholar]
- 62.Mateos MV, Richardson PG, Schlag R, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: Updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28:2259–2266. doi: 10.1200/JCO.2009.26.0638. [DOI] [PubMed] [Google Scholar]
- 63.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: A randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 64.Niesvizky R, Flinn IW, Rifkin R, et al. Efficacy and safety of three bortezomib-based combinations in elderly, newly diagnosed multiple myeloma patients: Results from all randomized patients in the community-based, phase 3b UPFRONT study. Blood. 2011:118. (abstr 478) [Google Scholar]
- 65.Vesole DH, Jacobus S, Rajkumar SV, et al. Lenalidomide plus low-dose dexamethasone (Ld): Superior one and two year survival regardless of age compared to lenalidomide plus high-dose dexamethasone (LD) Blood. 2010:116. (abstr 308) [Google Scholar]
- 66.Kropff M, Richardson PG, Schlag R, et al. Similar results in patients aged ≥ 75 vs < 75 with VMP in frontline MM and bortezomib in relapsed MM. Clin Lymphoma Myeloma. 2009:9. (abstr 512) [Google Scholar]
- 67.Jacobus S, Callander N, Siegel D, et al. Outcome of elderly patients 70 years and older with newly diagnosed myeloma in the ECOG randomized trial of lenalidomide/high-dose dexamethasone (RD) versus lenalidomide/low-dose dexamethasone (Rd) Haematologica. 2010;(suppl 2):95. abstr 0370. [Google Scholar]
- 68.Quach H, Fernyhough L, Henderson R, et al. Lower-dose lenalidomide and dexamethasone reduces toxicity without compromising efficacy in patients with relapsed/refractory myeloma, who are aged ≥ 60 years or have renal impairment: Planned interim results of a prospective multicentre phase II trial. Blood. 2010:116. (abstr 1961) [Google Scholar]
- 69.Ludwig H, Adam Z, Tóthová E, et al. Thalidomide maintenance treatment increases progression-free but not overall survival in elderly patients with myeloma. Haematologica. 2010;95:1548–1554. doi: 10.3324/haematol.2009.020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ludwig H, Durie BG, McCarthy P, et al. IMWG consensus on maintenance therapy in multiple myeloma. Blood. 2012;119:3003–3015. doi: 10.1182/blood-2011-11-374249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mateos MV, Oriol A, Teruel AI, et al. Maintenance therapy with bortezomib plus thalidomide (VT) or bortezomib plus prednisone (VP) in elderly myeloma patients included in the GEM2005MAS65 Spanish randomized trial. Blood. 2011:118. doi: 10.1182/blood-2012-05-427815. (abstr 477) [DOI] [PubMed] [Google Scholar]
- 72.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 73.Touzeau C, Blin N, Clavert A, et al. Efficacy of lenalidomide plus dexamethasone in patients older than 75 years with relapsed multiple myeloma. Leuk Lymphoma. 2012;53:1318–1320. doi: 10.3109/10428194.2011.654116. [DOI] [PubMed] [Google Scholar]
- 74.Petrucci T, Blau I, Corradini P, et al. Efficacy and safety of retreatment with bortezomib in patients with multiple myeloma: Interim results from retrieve, a prospective international phase 2 study. Haematologica. 2010;(suppl 2):95. abstr 0377. [Google Scholar]
- 75.Dimopoulos MA, Kastritis E, Christoulas D, et al. Treatment of patients with relapsed/refractory multiple myeloma with lenalidomide and dexamethasone with or without bortezomib: Prospective evaluation of the impact of cytogenetic abnormalities and of previous therapies. Leukemia. 2010;24:1769–1778. doi: 10.1038/leu.2010.175. [DOI] [PubMed] [Google Scholar]
- 76.Wang M, Dimopoulos MA, Chen C, et al. Lenalidomide plus dexamethasone is more effective than dexamethasone alone in patients with relapsed or refractory multiple myeloma regardless of prior thalidomide exposure. Blood. 2008;112:4445–4451. doi: 10.1182/blood-2008-02-141614. [DOI] [PubMed] [Google Scholar]
- 77.Mohty B, El-Cheikh J, Yakoub-Agha I, et al. Treatment strategies in relapsed and refractory multiple myeloma: a focus on drug sequencing and 'retreatment' approaches in the era of novel agents. Leukemia. 2012;26:73–85. doi: 10.1038/leu.2011.310. [DOI] [PubMed] [Google Scholar]
- 78.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter international myeloma working group study. Leukemia. 2012;26:1153. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.US Food and Drug Administration. Drug approvals and databases: Pomalidomide. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm339286.htm.
- 80.Terpos E, Dimopoulos MA. Myeloma bone disease: Pathophysiology and management. Ann Oncol. 2005;16:1223–1231. doi: 10.1093/annonc/mdi235. [DOI] [PubMed] [Google Scholar]
- 81.Ahmedzai SH, Boland J. The total challenge of cancer pain in supportive and palliative care. Curr Opin Support Palliat Care. 2007;1:3–5. doi: 10.1097/SPC.0b013e328151c401. [DOI] [PubMed] [Google Scholar]
- 82.Terpos E, Sezer O, Croucher PI, et al. The use of bisphosphonates in multiple myeloma: Recommendations of an expert panel on behalf of the European Myeloma Network. Ann Oncol. 2009;20:1303–1317. doi: 10.1093/annonc/mdn796. [DOI] [PubMed] [Google Scholar]
- 83.Kyle RA, Yee GC, Somerfield MR, et al. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25:2464–2472. doi: 10.1200/JCO.2007.12.1269. [DOI] [PubMed] [Google Scholar]
- 84.Lacy MQ, Dispenzieri A, Gertz MA, et al. Mayo Clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clin Proc. 2006;81:1047–1053. doi: 10.4065/81.8.1047. [DOI] [PubMed] [Google Scholar]
- 85.Morgan GJ, Davies FE, Gregory WM, et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): A randomised controlled trial. Lancet. 2010;376:1989–1999. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morgan GJ, Child JA, Gregory WM, et al. Effects of zoledronic acid versus clodronic acid on skeletal morbidity in patients with newly diagnosed multiple myeloma (MRC Myeloma IX): Secondary outcomes from a randomised controlled trial. Lancet Oncol. 2011;12:743–752. doi: 10.1016/S1470-2045(11)70157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berenson JR. Advances in the biology and treatment of myeloma bone disease. Am J Health Syst Pharm. 2001;58(suppl 3):S16–S20. doi: 10.1093/ajhp/58.suppl_3.S16. [DOI] [PubMed] [Google Scholar]
- 88.Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: A randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735–1744. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 89.Gimsing P, Carlson K, Turesson I, et al. Effect of pamidronate 30 mg versus 90 mg on physical function in patients with newly diagnosed multiple myeloma (Nordic Myeloma Study Group): A double-blind, randomised controlled trial. Lancet Oncol. 2010;11:973–982. doi: 10.1016/S1470-2045(10)70198-4. [DOI] [PubMed] [Google Scholar]
- 90.Alexanian R, Dimopoulos MA, Delasalle K, et al. Primary dexamethasone treatment of multiple myeloma. Blood. 1992;80:887–890. [PubMed] [Google Scholar]
- 91.Jagannath S, Barlogie B, Berenson JR, et al. Bortezomib in recurrent and/or refractory multiple myeloma: Initial clinical experience in patients with impared renal function. Cancer. 2005;103:1195–1200. doi: 10.1002/cncr.20888. [DOI] [PubMed] [Google Scholar]
- 92.San Miguel JF, Richardson PG, Sonneveld P, et al. Efficacy and safety of bortezomib in patients with renal impairment: Results from the APEX phase 3 study. Leukemia. 2008;22:842–849. doi: 10.1038/sj.leu.2405087. [DOI] [PubMed] [Google Scholar]
- 93.Dimopoulos MA, Richardson PG, Schlag R, et al. VMP (bortezomib, melphalan, and prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: Cohort analysis of the phase III VISTA study. J Clin Oncol. 2009;27:6086–6093. doi: 10.1200/JCO.2009.22.2232. [DOI] [PubMed] [Google Scholar]
- 94.Morabito F, Gentile M, Mazzone C, et al. Safety and efficacy of bortezomib-melphalan-prednisone-thalidomide followed by bortezomib-thalidomide maintenance (VMPT-VT) versus bortezomib-melphalan-prednisone (VMP) in untreated multiple myeloma patients with renal impairment. Blood. 2011;118:5759–5766. doi: 10.1182/blood-2011-05-353995. [DOI] [PubMed] [Google Scholar]
- 95.Dimopoulos MA, Terpos E, Chanan-Khan A, et al. Renal impairment in patients with multiple myeloma: A consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol. 2010;28:4976–4984. doi: 10.1200/JCO.2010.30.8791. [DOI] [PubMed] [Google Scholar]
- 96.Eriksson T, Höglund P, Turesson I, et al. Pharmacokinetics of thalidomide in patients with impaired renal function and while on and off dialysis. J Pharm Pharmacol. 2003;55:1701–1706. doi: 10.1211/0022357022241. [DOI] [PubMed] [Google Scholar]
- 97.Tosi P, Zamagni E, Cellini C, et al. Thalidomide alone or in combination with dexamethasone in patients with advanced, relapsed or refractory multiple myeloma and renal failure. Eur J Haematol. 2004;73:98–103. doi: 10.1111/j.1600-0609.2004.00272.x. [DOI] [PubMed] [Google Scholar]
- 98.Dimopoulos M, Alegre A, Stadtmauer EA, et al. The efficacy and safety of lenalidomide plus dexamethasone in relapsed and/or refractory multiple myeloma patients with impaired renal function. Cancer. 2010;116:3807–3814. doi: 10.1002/cncr.25139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ludwig H, Zojer N. Renal recovery with lenalidomide in a patient with bortezomib-resistant multiple myeloma. Nat Rev Clin Oncol. 2010;7:289–294. doi: 10.1038/nrclinonc.2010.31. [DOI] [PubMed] [Google Scholar]
- 100.European Medicines Agency. Revlimid summary of product characteristics. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000717/human_med_001034.jsp&mid=WC0b01ac058001d124.
- 101.Celgene. Revlimid package insert. http://www.revlimid.com/
- 102.Palumbo A, Bladé J, Boccadoro M, et al. How to manage neutropenia in multiple myeloma. Clin Lymphoma Myeloma Leuk. 2012;12:5–11. doi: 10.1016/j.clml.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 103.Gay F, Palumbo A. Management of older patients with multiple myeloma. Blood Rev. 2011;25:65–73. doi: 10.1016/j.blre.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 104.Birgegård G, Gascón P, Ludwig H. Evaluation of anaemia in patients with multiple myeloma and lymphoma: Findings of the European Cancer Anaemia Survey. Eur J Haematol. 2006;77:378–386. doi: 10.1111/j.1600-0609.2006.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rizzo JD, Brouwers M, Hurley P, et al. American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol. 2010;28:4996–5010. doi: 10.1200/JCO.2010.29.2201. [DOI] [PubMed] [Google Scholar]
- 106.Bohlius J, Schmidlin K, Brillant C, et al. Erythropoietin or Darbepoetin for patients with cancer: Meta-analysis based on individual patient data. Cochrane Database Syst Rev. 2009;3:CD007303. doi: 10.1002/14651858.CD007303.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Srkalovic G, Cameron MG, Rybicki L, et al. Monoclonal gammopathy of undetermined significance and multiple myeloma are associated with an increased incidence of venothromboembolic disease. Cancer. 2004;101:558–566. doi: 10.1002/cncr.20405. [DOI] [PubMed] [Google Scholar]
- 108.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414–423. doi: 10.1038/sj.leu.2405062. [DOI] [PubMed] [Google Scholar]
- 109.Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zonder JA, Barlogie B, Durie BG, et al. Thrombotic complications in patients with newly diagnosed multiple myeloma treated with lenalidomide and dexamethasone: Benefit of aspirin prophylaxis. Blood. 2006;108:403. doi: 10.1182/blood-2006-01-0154. [DOI] [PubMed] [Google Scholar]
- 111.Palumbo A, Cavo M, Bringhen S, et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: A phase III, open-label, randomized trial. J Clin Oncol. 2011;29:986–993. doi: 10.1200/JCO.2010.31.6844. [DOI] [PubMed] [Google Scholar]
- 112.Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood. 2012;119:933–939. doi: 10.1182/blood-2011-03-344333. [DOI] [PubMed] [Google Scholar]
- 113.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed—American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e419S–e494S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nucci M, Anaissie E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis. 2009;49:1211–1225. doi: 10.1086/605664. [DOI] [PubMed] [Google Scholar]
- 115.Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: Analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002—Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23:9219–9226. doi: 10.1200/JCO.2005.03.2086. [DOI] [PubMed] [Google Scholar]
- 116.Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Expert Opin Drug Saf. 2004;3:535–546. doi: 10.1517/14740338.3.6.535. [DOI] [PubMed] [Google Scholar]
- 117.Richardson PG, Briemberg H, Jagannath S, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113–3120. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- 118.Mileshkin L, Prince HM. The troublesome toxicity of peripheral neuropathy with thalidomide. Leuk Lymphoma. 2006;47:2276–2279. doi: 10.1080/10428190600948303. [DOI] [PubMed] [Google Scholar]
- 119.Richardson PG, Delforge M, Beksac M, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012;26:595–608. doi: 10.1038/leu.2011.346. [DOI] [PubMed] [Google Scholar]
- 120.Caraceni A, Zecca E, Bonezzi C, et al. Gabapentin for neuropathic cancer pain: A randomized controlled trial from the Gabapentin Cancer Pain Study Group. J Clin Oncol. 2004;22:2909–2917. doi: 10.1200/JCO.2004.08.141. [DOI] [PubMed] [Google Scholar]
- 121.Keskinbora K, Pekel AF, Aydinli I. Gabapentin and an opioid combination versus opioid alone for the management of neuropathic cancer pain: A randomized open trial. J Pain Symptom Manage. 2007;34:183–189. doi: 10.1016/j.jpainsymman.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 122.Ho TW, Backonja M, Ma J, et al. Efficient assessment of neuropathic pain drugs in patients with small fiber sensory neuropathies. Pain. 2009;141:19–24. doi: 10.1016/j.pain.2008.07.013. [DOI] [PubMed] [Google Scholar]