Abstract
A clinical study was undertaken to evaluate the associations between the tissue levels of omega-3 (N3), also known as the Omega-3 Index (N3 Index), on various clinical and quality of life outcomes in healthy young adults after heavy eccentric exercise.. To ensure an adequate number of participants with an elevated N3 index would be available for comparison to those with a lower N3 Index, a subgroup of the study participants received N3 dietary supplementation (2.7 g·d-1) for 30 days prior to the performance of the heavy eccentric exercise. The remaining participants received a placebo supplement for the same 30-day period. After 30 days of supplementation, participants performed an eccentric exercise routine and were then measured at baseline (time 0), 24-, 48-, 72-, and 96 hours respectively on the following outcomes; C-reactive protein (CRP) and creatine kinase. Blood lactate levels were analyzed immediately after the exercise. Functional measurements of delayed onset of muscle soreness (DOMS), extension and torque were also analyzed. Quality of life (QOL) was measured by the quantitative questionnaire, the Profile of Mood States Questionnaire (POMS). Safety monitoring and analysis of adverse events was continuous throughout the study. Differences as demonstrated by a reduction in pain following eccentric exercise was experienced at both 72 and 96 hour time points in subjects with a higher N3 Index however there were no differences in extension or strength between the two groups. There was a significant difference in blood lactate levels (p = 0.0309) and improved emotional stability, reflected by the POMS questionnaire, in subjects with a higher N3 Index level. There was a statistically significant difference in CRP levels in subjects with a higher N3 Index level at 24 hours and a trend toward significance over 96 hours. There were no significant differences in creatine kinase levels and no reported adverse events. Subjects with a higher Omega-3 (N3) Index reported less pain related to DOMS following heavy exercise at 72 and 96 hours post-exercise. Reduced pain in the higher N3 Index Group may be due to an increased concentration of omega-3 fatty acids in the muscle cell walls, thus triggering a higher elasticity, flexibility and lower risk of physical damage to muscle tissue during exercise. Serum levels of blood lactate were lower in subjects with a high N3 Index, CRP was reduced at 24 hours and POMS scores were improved in high N3 Index subjects demonstrating better QOL. No serious adverse events were reported further supporting that omega-3 dietary supplementation is safe, bio-available and may improve athletic performance and well being in healthy young adults.
Key Points.
Omega-3 index (N3) is elevated after supplementation versus placebo in healthy young adults
Subjects with higher N3 index demonstrated reduced DOMS after heavy exercise
Subjects with higher N3 index reported better quality of life
Key Words: Omega-3, N3 Index, exercise, delayed onset muscle soreness, quality of life, mood
Introduction
Chronic inflammation has been linked to many diseases including cardiovascular disease, stroke, diabetes mellitus and cancer (Pischon et al., 2011). Epidemiologic studies have demonstrated that populations that consume more fish and less red meat have lower incidences of some inflammatory diseases such as gastric cancer and cardiovascular disease (Huang et al., 2011; InterAct Consortium, 2011). The two most important omega-3 (n-3) polyunsaturated fatty acids are eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA) and it has been suggested that diets high in these fatty acids may benefit mood, behavior and physical performance (Krumbholz et al., 2010; Mozaffarain et al., 2011; Oddy et al., 2011; Poudyal et al., 2011).
It has also been demonstrated that increased consumption of omega-3 fatty acids improves lipid profiles, reduces oxidative stress and reduces inflammation via inhibition of pro- inflammatory mediators such as leukotrienes, prostaglandins and cytokines (Gopinath et al., 2011; Huang et al., 2011). Another manifestation of inflammation is muscle soreness that can occur in both elite athletes and normal human adults. Muscle soreness, sometimes called delayed onset muscle soreness (DOMS), is characterized as a type of soreness that is usually caused by a new or unaccustomed exercise. The exercises that most frequently cause DOMS are primarily exercises of eccentric muscle action i.e.: where a muscle generates tension to control the rate at which it lengthens (Balnave and Thomson, 1993; Bobbert et al., 1986; Wessel and Wan, 1994). The onset of DOMS generally occurs within 24-48 hours following exercise, may be associated with swelling, tenderness and discomfort, and may be experienced up to 1 week following heavy exercise (Bobbert et al., 1986; Wessel and Wan, 1994). While the physiology of DOMS is not completely understood, DOMS is likely related to damage of sarcomeres, ensuing swelling of damaged muscle fibers and subsequent initiation of an inflammatory response (Herbert et al., 2011; Lieber and Friden, 2002; Proske and Morgan, 2001). Strategies that have been tested for their potential to alleviate DOMS include massage therapy, stretching, ultrasound, pharmacologic anti-inflammatory drugs and dietary supplements including omega-3 dietary supplements (Herbert et al., 2011; Hyldahl et al., 2010; Lieber and Friden, 2002; Proske and Morgan, 2001; Rhea et al., 2009). The hypotheses that omega-3 dietary supplementation may reduce DOMS posits that higher systemic omega-3 levels correlate to increased omega-3 concentration in the muscle cell wall thereby increasing elasticity and flexibility and reducing the risk of muscle cell injury during heavy exercise. It has been shown that the omega-3 fatty acid content in omega-3 dietary supplements can increase omega-3 index levels and attenuate inflammatory pathways such as the cyclooxygenase and lipoxygenase pathways resulting in a decreased inflammatory response and reduced pain (Krumbholz et al., 2010; Poudyal et al., 2011). Systemic omega 3- levels are commonly calculated as an omega-3 index (N3 Index). The 0 - 12 range of the N3 Index expresses the amount of EPA and DHA (in weight [%]) present in the tissue cell membrane lipid fraction (Von Schacky, 2010). The Omega-3 Index has been previously characterized as an important risk factor for coronary heart disease (Harris and von Schacky, 2004). Subjects with an N3 Index less than or equal to 4.0 (low N3 index) are considered to have higher cardiovascular risk. Subjects with an N3 index between 4 and 8 are characterized as medium risk, while subjects with an N3 Index of >8 are considered low risk for suffering a fatal cardiovascular event (Harris and von Schacky, 2004; Von Schacky, 2010).
The purpose of the study was to test whether subjects with a higher N3 Index displayed differences in the incidence of DOMS, inflammatory biomarkers and quality of life following vigorous exercise.
Methods
Subjects
Men and women over the age of 18 were allowed to participate in the study if they were otherwise healthy, did not currently take any dietary supplements containing fish oil or omega-3 and were able to read and sign the informed consent in the English language. Excluded from the study were subjects with any known medical conditions, any current medication use for cardiovascular disease including hypertension, hyperlipidemia or past medical history of any coronary disease, current treatment for diabetes mellitus, recent physical injury, excessive consumption of fish (defined as consumption >1 time per week, current drug or alcohol addiction and any reason in the opinion of the investigator not to take part in the study.
Study procedures
This was an Institutional Review Board (IRB) approved study (Ithaca College Institutional Review Board) in 69 human volunteers. Study subjects were randomized 2:1 to take either 6 capsules of an omega-3 dietary supplement per day (KD Pharma, Bexbach, Germany, 2.7g) or 6 capsules of a placebo agent (high oleic sunflower oil) with food 30 days prior to exercise (baseline (0 hour)). Group 1 was subjects who had taken the supplement with an Omega-3 Index above 4.0 (high N3 Index) and Group 2 was subjects who had taken the placebo supplement and had an Omega-3 Index less than 4.0 (low N3 Index). Participants in group 1 who had received omega-3 supplementation of 2.7g EPA+DHA per day for 30 days moved from an initial mean N3 Index of 3.6 ± 1. 66 to 5.4 ± 1.05 (p = 0.0001) as measured at day 0 of the exercise portion of the intervention, indicating good bioavailability of the omega-3 supplement. A second subgroup receiving high oleic acid placebo capsules had an initial mean N3 Index of 3.75 ± 0.95 and after 30 days of placebo supplementation, on day 0 of exercise, a mean value of 3.94 ± 1.08.
Blood draw was performed at 0, 24-, 48-, 72-, and 96 hours post-exercise for measurement of plasma C-reactive protein (CRP) (mg·ml-1) and creatine kinase (CK) (IU·L-1) by the following procedure. Three milliliters of blood was drawn from the antecubital vein of the non-exercising arm. Blood was collected in a serum separator tube, allowed to sit for 20-60 min, and then centrifuged (IEC Centra- MP4R, Needham Heights, Massachusetts, USA) at 3,500 rpm for 10 min. Serum was then pipetted in a microtainer tube and stored in a refrigerator. At the end of the testing session, tubes were transferred for storage to a freezer at -80°C. At end of data collection, all tubes were transported to an outside laboratory (Human Metabolic Research Laboratory, Cornell University, Ithaca, New York, USA), where a Dimension Xpand Plus automated chemistry analyzer (Siemens Medical Diagnostics Solutions, Newark, Delaware, USA) was used to measure CK and an Immulite 2000 automated analyzer (Siemens Medical Diagnostics Solutions, Newark, Delaware, USA) to measure CRP. Duplicates were run for both tests. Assessment of blood lactate level was also done directly after exercise. Briefly, an alcohol prep pad was used to sterilize a finger on the non-exercising arm immediately after the initial exercise intervention. A sterilized lancet was used to obtain a 0.5μl sample of whole blood, which was analyzed for blood lactate with a Lactate Scout portable lactate analyzer (SensLab GmbH, Leipzig, Germany).
Exercise intervention
We used multiple sets of maximum eccentric forearm extensions performed with the non-dominant arm on a Cybex isokinetic dynamometer (Computer Sports Medicine, Inc. Humac®/Norm™, Model 770, Stoughton, Massachusetts, USA) to induce muscle soreness. Each group did two sets of 30 repetitions. The number of repetitions was based on a pilot study (n = 24) in which DOMS was manipulated into three discrete levels as measured by strength loss and into two levels when assessed by changes in arm circumference at 2 cm, elbow range of motion (ROM), relaxed arm angle (RANG), and unpleasantness of soreness (data not shown).
Assessment of Delayed Onset Muscle Soreness, extension and torque
Functional measurement of DOMS was measured as a visual analog scale (VAS) and recorded as a score from 0-10. This measurement was recorded by the same investigator throughout the study period. Indirect markers of soreness including arm extension (as joint elbow range of motion (ROM)) and torque were also measured. Elbow ROM for the exercising arm was measured with a standardized goniometer for active flexion range. The active flexion ROM was determined as the difference between the actively flexed and extended elbow joint angles. Subjects were tested in the supine position to stabilize the shoulder and upper body. The fulcrum of goniometer was kept at the lateral epicondyle of the humerus, while the stationary arm was parallel to longitudinal axis of humerus and the movement arm was parallel to the longitudinal axis of forearm. Measurement was done from a fully extended position to a fully flexed position (Zainuddin et al., 2005). Placement locations of fulcrum, stationary and movement arm were marked with permanent ink to maintain consistency throughout trials. Three measurements were taken and the mean value was used for analysis.
To measure tourque, subjects completed eccentric elbow extension on a Cybex isokinetic dynamometer (Computer Sports Medicine, Inc. Humac®/Norm™, Model 770, Stoughton, Massachusetts, USA). Prior to any Cybex test, the machine was adjusted according to settings made during the baseline lab visit. In addition, every peak torque (PT) test was preceded by a standardized warm-up that included five sub-maximal and two maximal eccentric triceps contractions at 90°·s-1 After the warm-up, the subjects rested two minutes before completing three maximal eccentric triceps contractions. The average of the three contractions was recorded as PT in Newton- meters. A pilot study performed to test the reliability of the PT protocol yielded a coefficient of variation of 4.3% (n=21) (data not shown).
Questionnaires
The Profile of Mood States (POMS) questionnaire was administered to every subject at each time point. The POMS is a quick well-validated quantitative assessment that is useful in measuring active mood states (McNair 1992).
Safety monitoring
Safety variables as measured by adverse events were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
Statistical analysis
Descriptive statistics are presented as the mean ± standard deviation for normally distributed variables and as the median and frequency for categorical variables. Descriptive statistics and other study outcomes are presented as the mean ± standard deviation to allow for comparison with other studies. Subjects were prospectively grouped according to their baseline N3 Index level and analyzed on the primary endpoint, level of DOMS. Secondary comparisons were done using paired t tests by comparing measurements to times 24-, 48-, 72-, and 96-hours post-exercise or via a one-way analysis of variance (ANOVA) as appropriate. All analyses were performed using STATA (Version 11, College Station, TX) and Microsoft Excel (Microsoft, Redmond, WA).
Results
Sixty-nine male and female college students were enrolled in the study and sixty-three were evaluable. Six subjects were not evaluable because they were lost to follow up. No subjects were lost to any adverse events. The mean ± SD age was 18.6 ± 1.2 (n = 43), 18.9 ± 1.1 (n= 22) in Group 1 (N3 index >4) and 2 (N3 index <4) respectively. The measurement of DOMS (mean worst pain score) at 24 hours was 3.81 ± 3.7 versus 5.32 ± 3.3 in Group 1 versus 2 respectively. At 72 hours and 96 hours there were significant differences between the two groups on DOMS worst pain scores. Group 1 reported mean pain scores of 2.19 ± 1.92 vs 4.36 ± 3.17 (p = 0.031) at 72 hours and 1.63 ± 1.77 vs 3.17 ± 2.75 (p = 0.035) at 96 hours (Group 1 vs 2 respectively) (Figure 1). No significant differences were found between the two groups at any of the time points for extension and torque (Table 1).
Figure 1.
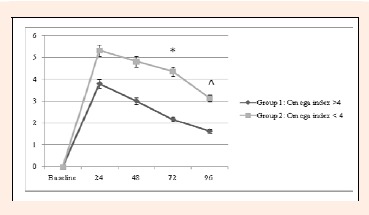
Comparison of DOMS at 24-96 Hours after Exercise between Group 1 and 2. P values are based on two-sample t tests comparing differences in between-group means. * p = 0.031, ^ p = 0.035.
Table 1.
Comparison of between-group mean scores on extension and torque. Data are means (±SD).
| Outcome Measure and Time Point | Group 1 Omega index >4 (n=42) |
Group 2 Omega index < 4 (n=22) |
|---|---|---|
| Extension (expressed as angle) | ||
| 24 hours | 2.94 (5.7) | 2.67 (8.50) |
| 48 hours | 1.6 (6.7) | 1.91 (8.50) |
| 72 hours | 1.8 (6.8) | 1.67 (7.30) |
| 96 hours | 1.12 (6.53) | 1.08 (8.50) |
| Torque (expressed as Nm) | ||
| 24 hours | 50.44 (23.94) | 38.88 (19.74) |
| 48 hours | 48.25 (22.02) | 40.00 (25.01) |
| 72 hours | 49.81 (24.32) | 42.13 (26.81) |
| 96 hours | 52.38 (24.19) | 42.00 (25.57) |
Serum levels of creatine kinase (IU·L-1) were not significantly different between the two groups at any of the time points. Blood lactate level directly after exercise was significantly reduced in Group 1 versus Group 2, 3.56 ± 1.37 mmol·L-1 versus 4.28 ± 2.58 mmol·L-1 (p = 0.0309). C-reactive protein level was statistically significantly different between the two groups at 24 hours, p = 0.001. There was a visible trend for a lower average CRP-levels in Group 1 (high N3 Index level) throughout the remaining time points (Figure 2). Scores on the Profile of Mood States (POMS) demonstrated that there was greater emotional stability in the higher N3 Index Group (Group 1) as compared to the low N3 Index Group (Group 2) suggesting improved QOL in the high N3 Index Group (Figure 3). There were no reported adverse events in either group.
Figure 2.
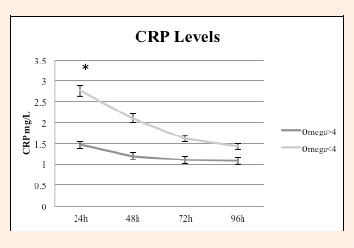
Decreased serum CRP level was shown in Group 1 (N3 Index >4) vs Group 2 (N3 Index <4) at 24 hours, * p = 0.001. There was a non-significant trend in reduced CRP in Group 1 vs 2 from 48-96 H.
Figure 3.
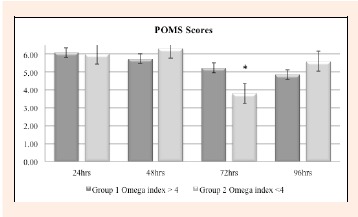
Profile of Mood States (POMS) Scores. Change in the group mean quality of life scores as measured by the POMS questionnaire from baseline to 24-, 48-, 72- and 96 hours for the two groups demonstrated significance at 72 hours. * p < 0.05
Discussion
Our study demonstrates that omega-3 supplementation resulted in an increased omega-3 fatty acid tissue level (N3 Index) in healthy human subjects. Subjects with a N3 Index > 4 also demonstrated reduced delayed onset muscle soreness (DOMS) at 72 and 96 hours post-exercise. These findings support the hypothesis that higher omega-3 tissue levels may have a protective effect on muscle cells during exercise and may act by reducing the inflammatory response and subsequent DOMS. Secondary results showed that subjects with a higher N3 Index had reduced CRP levels at 24 hours, reduced blood lactate levels directly after exercise and reported improved overall quality of life as measured by a more stable mood at 72 hours. There were no changes in creatine kinase levels or differences in extension or torque between the two groups. Supplementation with the omega-3 dietary supplement was also safe and well tolerated.
Delayed onset muscle soreness (DOMS) is characterized as a painful condition that is commonly experienced after new or unaccustomed exercises. It is characterized as a benign medical condition that can easily subside with rest however it can cause considerable discomfort in both normal adults and trained athletes. A large clinical trial recently demonstrated that peak DOMS can last up to one-week post exercise and can also impede athletic performance (Jamtyedt et al., 2010). Many different treatments for DOMS have been studied and include massage therapy, stretching, transcutaneous electrical stimulation, ultrasound and the administration of a variety of anti- inflammatory drugs such as prednisone and dietary supplements such as vitamin C (Cheung 2003; Connolly, 2003; Deneger, 1992; Torres, 2012).
The physiologic mechanism of action of DOMS appears to be multifold but is largely related to inflammation, pain and muscle damage to the myofibrils, sarcolemma and sarcoplasmic reticulum (Clarkson, 1999). The mechanism by which this occurs includes activation of a variety of cytokines and enzymes that also attract neutrophils in order to coordinate a healing response. However these functions are also thought to activate pain receptors and can also be responsible for destruction of normal healthy tissue (O’Conner, 1999; Weiss, 1989).
Dietary supplementation of omega-3 has become more common and is being prospectively tested for its effect on a variety of conditions including mood and behavioral disorders, physical performance and metabolic conditions (Bloomer et al., 2009; Huang et al., 2011; Lenn et al., 2002; Oddy et al., 2011; Poudyal et al., 2011; Tartibian et al., 2009). One of the main connections between omega-3 fatty acids and inflammation is the effect of N3 fatty acids on eicosanoids, primary mediators of inflammation. Eicosanoids are generated from arachadonic acid and are largely involved in modulation of the inflammatory response (Sammuleson 1991). Physiologically, increased consumption of N3 fatty acids such as EPA and DHA have been shown to result in increased levels of EPA and DHA in human inflammatory cells resulting in decreased production of a variety of eicosanoids including thromboxane, leukotrines and prostaglandins (Heally 2000). Moreover studies have also shown that various anti-inflammatory supplements including DHA and tocopherols attenuate inflammatory markers of cell damage (Phillips, 2003).
Our study demonstrated that subjects with higher N3 levels due to omega-3 supplementation experienced less DOMS at 72- and 96- hours post-exercise. This is consistent with a recent study of pomegranate juice supplementation that demonstrated reduced DOMS in subjects at 48- and 72-hours post exercise (Trombold et al., 2011). A hypothesis for the reduction in DOMS may be that an increase in polyunsaturated fatty acids in the muscle cell membrane improves the elasticity of cells thereby reducing the degree of muscle damage during heavy eccentric exercise.
Our study also demonstrated that young adults with a higher N3 Index reported a better quality of life as measured by quantitative questionnaire, the Profile of Mood States (POMS). This finding is consistent with recent research which observed a correlation between low N3 levels and depression in adolescents and another study that showed that omega-3 supplementation improved anxiety levels and reduced inflammation in healthy students (Kiecolt-Glaser et al., 2011; Swenne et al., 2011). While the mechanism is still unclear it has been suggested that omega 3 supplementation may attenuate inflammatory conditions that have been linked with mood disorders such as metabolic syndrome (Lopresti, 2013).
Limitations of our study include its small sample size and possible selection bias to those who agreed to participate in this type of study given the time requirement and need to perform exercise. For measured parameters such as CRP levels, there was a statistically significant difference at 24 hours and a trend towards continued decreased observed between the high and low N3 Index groups and these trends may warrant further investigation. Overall, the small degree of separation between the high and low N3 Index may have made it difficult to detect statistically significant differences between groups. Future studies may provide more conclusive data should the high and low N3 Index be defined at greater extremes along the Omega-3 Index spectrum of values.
Conclusion
In conclusion, dietary supplementation with an omega-3 supplement lead to a higher N3 Index level and decreased incidence of DOMS in healthy college aged subjects. Moreover, supplementation was safe and tolerable. More research is needed in order to elucidate how omega-3 fatty acid supplementation and a higher N3 Index may affect inflammatory changes and other physical manifestations following heavy exercise in adults.
Acknowledgements
The authors would like to thank KD-Pharma Bexbach (Germany) for the supply of the placebo capsules and the omega-3 supplement “pur3” (in the US known as “Recoup90”) containing over 90% long chain Omega-3 fatty acid ethyl ester.
Biographies
Peter LEMBKE
Employment
KD Pharma, Bexbach, Germany
Degree
PhD
Research interests
Omega-3, dietary supplements, inflammation
Jillian CAPODICE
Employment
Nutraceutical Medical Research, NY, NY, USA
Degree
MS, OM, LAC, AP
Research interests
complementary medicine, dietary supplements, herbal medicine, traditional oriental medicine
E-mail: jcapodice@nutraceuticalmedicalresearch.com
Kathleen HEBERT
Employment
Nutraceutical Medical Research, NY, NY, USA
Degree
BS
Research interests
Human nutrition
Thomas SWENSON
Employment
Ithaca College, Ithica, NY, USA
Degree
PhD
Research interests
Inflammation
References
- Alison M.R., Nicholson L.J., Lin W.R. (2011) Chronic inflammation and hepatocellular carcinoma. Recent Results Cancer Research 185, 135-148 [DOI] [PubMed] [Google Scholar]
- Balnave C.D., Thomson M.W. (1993) Effect of training on eccentric exercise-induced muscle damage. Journal of Applied Physiology 75, 1545-1551 [DOI] [PubMed] [Google Scholar]
- Bloomer R.J., Larson D.E., Fisher-Wellman K.H., Galpin A.J., Schilling BK. (2009) Effect of eicosapentaenoic and docosahexaenoic acid on resting and exercise-induced inflammatory and oxidative stress biomarkers: a randomized, placebo controlled, cross-over study. Lipids in Health and Disease 19(8), 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobbert M.F., Hollander A.P., Huijing P.A. (1986) Factors in delayed onset muscle sorenesss of man. Medicine and Science in Sports and Exercise 18, 75-81 [PubMed] [Google Scholar]
- Cheung K., Hume P., Maxwell L. (2003) Delayed onset muscle soreness: treatment strategies and performance factors. Sports Medicine 33(2), 145-164 [DOI] [PubMed] [Google Scholar]
- Clarkson P.M., Sayers S.P. (1999) Etiology of exercise-induced muscle damage. Canadian Journal of Applied Physiology 24, 234-248 [DOI] [PubMed] [Google Scholar]
- Connolly D.A., Lauzon C., Agnew J., Dunn M., Reed B. (2003) The effects of vitamin C supplementation on symptoms of delayed onset muscle soreness. The Journal of Sports Medicine and Physical Fitness 46, 462-467 [PubMed] [Google Scholar]
- Denegar C.R., Perrin D.H. (1992) Effect of transcutaneous electrical nerve stimulation, cold, and a combination treatment on pain, decreased range of motion, and strength loss associated with delayed onset muscle soreness. Journal of Athletic Training 27(3), 200-206 [PMC free article] [PubMed] [Google Scholar]
- Gopinath B., Buyken A.E., Flood V.M., Empson M., Rochtchina E., Mitchell P. (2011) Consumption of polyunsaturated fatty acids, fish, and nuts and risk of inflammatory disease mortality. American Journal of Clinical Nutrition 93, 1073-1079 [DOI] [PubMed] [Google Scholar]
- Harris W.S., von Schacky C. (2004) The Omega-3 Index: A New Risk Factor for Death from CHD? Preventive Medicine 39, 212-220 [DOI] [PubMed] [Google Scholar]
- Healy D.A., Wallace F.A., Miles E.A., Calder P.C., Newsholme P. (2000) The effect of low to moderate amounts of dietary fish oil on neutrophil lipid composition and function. Lipids 35, 763-768 [DOI] [PubMed] [Google Scholar]
- Herbert R.D., de Noronha M., Kamper S.J. (2011) Stretching to prevent or reduce muscle soreness after exercise. Cochrane Database Systematic Reviews, 7, CD004577. [DOI] [PubMed] [Google Scholar]
- Huang J., Frohlich J., Ignaszewski A.P. (2011) The impact of dietary changes and dietary supplements on lipid profile. Canadian Journal of Cardiology 27, 488-505 [DOI] [PubMed] [Google Scholar]
- Hyldahl R.D, Keadle J., Rouzier P.A., Pearl D., Clarkson P.M. (2010) Effects of ibuprofen topical gel on muscle soreness. Medicine and Science in Sports and Exercise 42, 614-621 [DOI] [PubMed] [Google Scholar]
- InterAct Consortium, Romaquera D., Guevara M., Norat T., Langenberg C., Forouhi N.G., Sharp S., Slimanj N., Schulze M.B., Buiisse B., Buckland G., Molina-Montes E., Sanchez M.J., Moreno-Jribas M.C., Bendinelli B., Grioni S., van der Schouw Y.T., Arriola L., Beulens J.W., Boeing H., Clavel-Chapelon F., Cottet V., Crowe F.L., de Lauzon-Guillan B., Franks P.W., Gonzalez C., Hallmans G., Kaaks R., Key T.J., Khaw K., Nilsson P., Ovetyad K., Palla L., Palli D., Panico S., Quiros J.R., Rolandsson O., Romieu I., Sacerdote C., Spilkerman A.M., Teucher B., Tionneland A., Tormo M.J., Tumino R., van der A.D., Feskens E.J., Wareham N.J. (2011) Mediterranean diet and type 2 diabetes risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study: the InterAct project. Diabetes Care 34, 1913-1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamtvedt G., Herbert R.D., Flottorp S., Odgaard-Jensen J., Havelsrud K., Barratt A. (2010). A pragmatic randomised trial of stretching before and after physical activity to prevent injury and soreness. British Journal of Sports Medicine 44, 1002-1009 [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J.K., Belury M.A., Andridge R., Malarkey W.B., Glaser R. (2011) Omega-3 supplementation lowers inflammation and anxiety in medical students: A randomized controlled trial. Brain, Behavior and Immunity 25, 1725-1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkaya H., Wicha M.S. (2011) Inflammation and autophagy conspire to promote tumor growth. Cell Cycle 10(16), 2623-2624 [DOI] [PubMed] [Google Scholar]
- Krumbholz R., Lembke P., Schirra N. Patent: Novel use of Omega-3 Fatty Acids. (EP2222292.A2), publ. 01-09-2010. [Google Scholar]
- Lenn J., Uhl T., Mattacola C., Boissonneault G., Yates J., Ibrahim W., Bruckner G. (2002) The effects of fish oil and isoflavones on delayed onset muscle soreness. Medicine and Science in Sports and Exercise 34, 1605-1613 [DOI] [PubMed] [Google Scholar]
- Lieber R.L., Friden J. (2002) Morphologic and mechanical basis of delayed-onset muscle soreness. Journal of the American Academy of Orthopaedic Surgeons 10, 67-73 [PubMed] [Google Scholar]
- Lopresti A.L., Drummond P.D. (2013) Obesity and psychiatric disorders: Commonalities in dysregulated biological pathways and their implications for treatment. Progress in Neuro-Psychopharmacology & Biological Psychiatry 14(45C), 92-99 [DOI] [PubMed] [Google Scholar]
- McNair D.M., Lorr M., Droppleman L.F. (1992) EDITS manual profile of mood states. San Diego: Educational and Industrial Testing Service [Google Scholar]
- Mozaffarian D., Lemaitre R.N., King I.B., Song X., Spiegelman D., Sacks F.M., Rimm E.B., Siscovick D.S. (2011) Circulating Long-Chain {omega}-3 Fatty Acids and Incidence of Congestive Heart Failure in Older Adults: The Cardiovascular Health Study: A Cohort Study. Annals of Internal Medicine 155, 160-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Conner P.J., Cook D.B. (1999) Exercise and pain: the neurobiology, measurement, and laboratory study of pain in relation to exercise in humans. Exercise and Sport Science Reviews 27, 119-166 [PubMed] [Google Scholar]
- Oddy W.H., Hickling S., Smith M.A., O’Sullivan T., Robinson M., H de Klerk N., Beilin L.J., Mori T., Syrette J.R., Zubrick S.R., Silburn S. (2011) Dietary intake of omega-3 fatty acids and risk of depressive symptoms in adolescents. Depress Anxiety 28, 582-588 [DOI] [PubMed] [Google Scholar]
- Phillips T., Childs A.C., Dreon D.M., Phinney S., Leeuwenburgh C. (2003) A dietary supplement attenuates IL-6 and CRP after eccentric exercise in untrained males. Medicine and Science in Sports and Exercise 35(12), 2032-2037 [DOI] [PubMed] [Google Scholar]
- Pischon T., Hu F.B., Girman C.J., Rifai N., Manson J.E., Rexrode K.M., Rimm E.B. (2011) Plasma total and high molecular weight adiponectin levels and risk of coronary heart disease in women. Atherosclerosis 219, 322-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudyal H., Panchal S.K., Diwan V., Brown L. (2011) Omega-3 fatty acids and metabolic syndrome: Effects and emerging mechanisms of action. Progress in Lipid Research 50, 372-387 [DOI] [PubMed] [Google Scholar]
- Proske U., Morgan D.L. (2001) Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. Journal of Physiology 537, 333-345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea M.R., Bunker D., Marín P.J., Lunt K. (2009) Effect of iTonic whole-body vibration on delayed-onset muscle soreness among untrained individuals. The Journal of Strength and Conditioning Research 6, 1677-1682 [DOI] [PubMed] [Google Scholar]
- Samuelsson B. (1991) Arachidonic acid metabolism: role in inflammation. Rheumatology 50(Suppl 1), 3-6 [PubMed] [Google Scholar]
- Swenne I., Rosling A., Tengblad S., Vessby B. (2011) Omega-3 polyunsaturated essential fatty acids are associated with depression in adolescents with eating disorders and weight loss. Acta Paediatrica 100, 1610-1615 [DOI] [PubMed] [Google Scholar]
- Tartibian B., Maleki B.H., Abbasi A. (2009) The effects of ingestion of omega-3 fatty acids on perceived pain and external symptoms of delayed onset muscle soreness in untrained men. Clinical Journal of Sport Medicine 19, 115-119 [DOI] [PubMed] [Google Scholar]
- Torres R., Ribeiro F., Alberto Duarte J., Cabri J.M. (2012) Evidence of the physiotherapeutic interventions used currently after exercise-induced muscle damage: systematic review and meta-analysis. Physical Therapy in Sport 13(2), 101-114 [DOI] [PubMed] [Google Scholar]
- Trombold J.R., Reinfeld A.S., Casler J.R., Coyle E.F. (2011) The effect of pomegranate juice supplementation on strength and soreness after eccentric exercise. Journal of Strength Conditioning Research 25, 1782-1788 [DOI] [PubMed] [Google Scholar]
- Von Schacky C. (2010) Omega-3 Index and Cardiovascular Disease Prevention: principle and rationale. Lipid Technology 22(7), 151-174 [Google Scholar]
- Weiss S.J. (1989) Tissue destruction by neutrophils. New England Journal of Medicine 320, 365-376 [DOI] [PubMed] [Google Scholar]
- Wessel J., Wan A. (1994) Effect of stretching on the intensity of delayed-onset muscle soreness. Clinical Journal of Sport Medicine 4, 82-87 [Google Scholar]
- Zainuddin Z., Newton M., Sacco P., Nosaka K. (2005) Effects of massage on delayed-onset muscle soreness, swelling, and recovery of muscle function. Journal of Athletic Training 40(3), 174-180 [PMC free article] [PubMed] [Google Scholar]


