Abstract
The aim of this study was: to describe typical training load (TL) carried out by a professional female futsal team for a period of 5 weeks; and to verify the relationship between TL, stress symptoms, salivary secretory immunoglobulin A (SIgA) levels, and symptoms of upper respiratory infections (URI). Over 45 sessions, the TL of the athletes was monitored daily by means of session-RPE method during the in-season period prior to the main national competition. Stress symptoms were measured weekly by means of the “Daily Analysis of Life Demands in Athletes Questionnaire” (DALDA), SIgA levels, and by symptoms of URI by the “Wisconsin Upper Respiratory Symptom Survey-21” (WURSS). There was a significant increase in TL, monotony, and training strain in week 3, with a concomitant and significant reduction in percentage variation (Δ%) of SIgA concentration and secretion rate (p < 0.05). Additionally, a second order regression model showed a high goodness of fit (R2 = 0.64 - 0.89) between TL and strain with SIgA concentration, secretion rate, and “worse than normal” responses of stress symptoms from the questionnaire. In conclusion, a link between TL and SIgA levels, and stress symptoms in female futsal players was evident in a non linear fashion. There appears to be an optimal range of values of daily TL between ~343 and ~419 AU and strain between ~2639 and 3060 AU, because at levels below and above these values there was an increase in stress symptoms and above ~435 and ~3160 AU to TL and strain there were a decrease in SIgA levels. In contrast, symptoms of URI failed to demonstrate relationship with the variables studied.
Key Points.
There is a dose-response relationship between SIgA levels and stress symptoms with TL.
For the athletes of the present study, values of ~436 AU and ~3161 AU to TL and strain training would be desirable because higher values would decrease responses of SIgA levels.
An optimal range of values of TL between ~336 and ~412 AU to TL and ~2610 and ~3016 AU to strain training would be suggested for this group of athletes, since below and above these values increased responses of stress symptoms were observed.
Key Words: Team sports, mucosal immunity, psychometric measures, overtraining
Introduction
Monitoring training loads (TL) in combination with psychophysiological responses has been suggested as necessary to prevent overreaching and overtraining. The session-rating of perceived exertion (i.e. session-RPE) has been demonstrated to be a simple and practical method for quantifying internal TL in team sports (Alexiou and Coutts, 2008; Foster et al., 2001; Impellizzeri et al., 2004). A way to evaluate the impact of physiological stress on immunity is to analyze the salivary secretory immunoglobulin A (SIgA) level, which is considered to be a marker inversely related to the risk of developing upper respiratory infection (URI) symptoms in athletes (Fahlman and Engels, 2005; Walsh et al., 2011). Some studies have shown that low SIgA levels may reduce resistance to infections and also increase the risk of impaired performance in competitions, which is often regarded as a higher than normal psychophysiological stress level (Gleeson et al., 1999; Tsai et al., 2011).
Additionally, “Daily Analysis of Life Demands in Athletes Questionnaire” (DALDA) (Coutts et al., 2007b; Neville et al., 2008) and the “Wisconsin Upper Respiratory Symptom Survey - 21” (WURSS-21) questionnaire (Moreira et al., 2011a; Rushall, 1990) have previously been used as simple tools to monitor the immune response and prevent excessive psychophysiological stress that may negatively alter athletes’ health and performance. In this regard, Coutts et al., 2007b and Moreira et al. (2011a) have shown that the DALDA questionnaire is a sensitive tool for monitoring individual athletes’ responses to internal TL. Furthermore, significant relationships between increased TL (Foster, 1998) and volume of exercise (Gleeson et al., 2013) with symptoms of URI have been also documented. However, some authors presented inconsistent results between the relationship of TL, stress symptoms, SIgA levels, and URI (Cox et al., 2007; Fahlman and Engels, 2005; Gleeson et al., 2000; Leicht et al., 2012; Neville et al., 2008).
To date, the relationship between session-RPE and corresponding psychophysiological responses has not been sufficiently addressed, especially in female athletes. The clarification of this relationship is important because it is not known if typical TL carried out by professional female team sports players can induce alterations in stress symptoms, SIgA levels, and URI incidence in the same way as their male counterparts. For instance, female athletes may experience more stress in similar TL than male athletes (di Fronso et al., 2013; Kellmann et al., 2001), and consequently they may require special attention from coaches and physical trainers in order to manage TL appropriately on a daily basis.
Thus, the objectives of this study were twofold: 1) to describe typical TL experienced by a professional female futsal team during the 5 weeks before the main national competition by means of the session-RPE method and; 2) to verify the relationship between TL, stress symptoms, SIgA levels and symptoms of URI. We hypothesized that periods of intensified TL would increase stress symptoms, decrease SIgA levels with a corresponding increase in the susceptibility to URI.
Methods
Participants
Thirteen top-level professional female futsal players (mean and SD; age: 22.1 ± 4.2 years; body mass: 60.7 ± 5.9 kg; height: 1.65 ± 0.5 m and BMI 22.3 ± 1.4 kg.m-2) who were second place in the Brazilian National League in 2009 were enrolled in the study. The players had a training experienced of 4-5 years. They signed a written informed consent form. The study was approved by the Institutional Ethics Committee.
Design
Firstly, the players performed an incremental running test to determine the ventilatory threshold (VT), respiratory compensation point (RCP), and maximal oxygen consumption (VO2max). The players were also submitted to an oral examination for detection of clinical signs of periodontal disease, active caries or mucosal lesions. The follow-up study initiated after the State championship played in the first half of the year and was comprised of 5 weeks during the preparation period for a national main championship in Brazil, in the second half of the year. The TL of the players was monitored on a daily basis by means of the session-RPE method for 45 training sessions. Stress symptoms as assessed by DALDA, symptoms of URI as assessed by WURSS-21 and salivary SIgA levels were measured in the afternoon, prior to the last training session of each week. Subsequently, the relationships between TL and training strain (independent variable) with stress symptoms, SIgA, and symptoms of URI responses (dependent variables) were determined to ascertain possible associations.
Procedures: Incremental test
The incremental test on the treadmill (Super ATL - Inbrasport®, Brazil) started at 6 km.h-1. The inclination was kept constant at 1%, and the speed was increased by 1 km.h-1 every minute until voluntary exhaustion. Heart rate (HR) was recorded with a short-range telemetry system (RS800, Polar Electro Oy, Finland). Pulmonary gas exchange was averaged every 20-s using a metabolic cart (Metalyzer 3B, CPX System, Germany). The O2 and CO2 analyzers were calibrated using gases of known concentration and the volume signal was calibrated with a 3 L syringe. The VT and RCP were determined as suggested by Lucia et al., 2003. All the players met at least two of the following criteria at exhaustion to validate the VO2max attainment: a plateau in oxygen consumption, RER values above 1.10, and HR within 5 bpm of age-predicted maximum (Billat et al., 1996).
Training program
The designated training sessions consisted of resistance training (RT), technical-tactical training (TT), and physical training (PT) over a 5 week period. In the typical training sessions (1-3 weeks), RT consisted of 3 sets of 15 reps at 70% of repetition maximum (RM) with rest intervals of 45 s, carried out 4 times a week with a mean duration of ~30-40 minutes per day. The PT was developed together with TT on court, 5 times a week with a mean duration of ~80-120 min per day. In the week 4, RT was decreased to 2 times a week while no RT sessions were performed in the week 5. During the last two weeks, volume and intensity of both TT and PT were maintained relatively constant.
Quantification of internal training load
The internal TL was computed by using the session-RPE method. Approximately 30 minutes following the completion of every training session, the players were asked to rate the intensity of the whole session by means of a modified 10-point RPE scale (Foster et al., 2001). This value of RPE was multiplied by the total duration of the training session. All the players were previously familiarized with the use of the RPE scale. The session-RPE loads were recorded as total weekly and daily average units. Concurrently with the session-RPE, the “strain” and “monotony” were calculated weekly in accordance with Foster, 1998. The monotony was calculated weekly by dividing the weekly mean TL by the standard deviation, while training strain was calculated as the overall weekly TL multiplied by monotony.
Stress symptoms
The DALDA (Rushall, 1990) was administered to measure weekly stress sources/symptoms. The DALDA questionnaire is divided into two parts, namely Part A and Part B, which represent the sources of life stress and symptoms of stress, respectively. Each subject was required to complete the DALDA prior the last training session of each week at the same time of the day. The players marked every question as being either “worse than the normal”, “normal” or “better than the normal”. This questionnaire was filled out at the end of every week of training and number of responses labeled as “worse than normal” was retained for analysis (Moreira et al., 2011b). We considered for analysis only part B of the questionnaire, in accordance with Coutts et al., 2007c.
Salivary secretory immunoglobulin A (SIgA)
Enzyme-linked immunosorbent assay (ELISA) was used for analyses of SIgA levels. The samples were collected at rest. The baseline values were determined from saliva samples collected at rest one week before the start of the training period. Saliva samples were collected prior the last training session of each week at the same time of the day. Unstimulated whole saliva samples were collected after individuals had rinsed their mouth twice with water. Participants were asked to spit saliva into sterile tubes for a period of 5 min. Saliva samples were centrifuged at 12,000 rpm for 10 min, and the supernatants were stored at -20 ºC until use. Saliva flow rate was determined by the volume of secreted saliva per minute (ml.min-1).
Total levels of salivary SIgA were determined using microtiter plates (Costar 3590, Corning, NY, USA) and a commercial kit (Human IgA ELISA Quantification set, E80-102, Bethyl laboratories, Montgomery, USA) according to the manufacturer’s instructions. After being coated with primary antibody and blocking plates, saliva samples were diluted at 1:1000 and incubated for 1 h at room temperature. After washing, plates were incubated with anti IgA peroxidase conjugated antibody. For the determination of SIgA concentration (µg·ml-1), absorbance values at 450 nm were plotted against the standard curve obtained for the serial dilutions of a known concentration of purified human IgA. The SIgA secretion rate was expressed by the amount of IgA secreted per minute (µg·ml-1).
Upper respiratory infection (URI)
The WURSS-21 (Barrett et al., 2005) was used to compute symptoms of URI prior to the last training session of each week at the same time of the day. It was assumed that the responses to WURSS-21 would be a reasonable marker of URI and functional impairment, in agreement with previous study of Spence et al., 2007. This questionnaire includes 10 items assessing symptoms, with nine items assessing functional impairments and one item assessing global severity and global change (‘How sick do you feel today?’ and ‘compared to yesterday, I feel that my cold is … ’). All the items are responded to using a Likert scale of severity, ranging from 0-7. The total number of occurrences regardless of severity level was retained for analysis.
Statistical analyses
The distribution of data was analyzed by the Shapiro-Wilk test. The sphericity of data was analyzed by Mauchly’s test with Greenhouse-Geisser correction. All variables are presented as mean ± standard deviation (SD). In addition, saliva flow rate and SIgA levels were also presented as percentage variation (Δ%) taking into account the relative change of each week investigated compared to the baseline period [i.e (week 1 value - baseline value)/baseline value*100]. All the variables over the five weeks were compared by means of repeated measures analysis of variance (ANOVA). Post-hoc analyses were carried out using Fisher’s least squares difference (LSD) test. The relationship between the TL and strain with SIgA levels, symptoms of URI and stress symptoms were estimated from a second-order regression as used by Manzi et al., 2009 based on mean values of 8 subjects to each week. Differences were considered significant if p < 0.05. SPSS (version 17.0 for Windows; Chicago, IL) was used for all statistical calculations.
Results
The physiological characteristics of the 13 players are shown in Table 1. Five did not complete the study. One contracted an oral infection, 2 suffered injuries during the observation period and 2 players were called to the Brazilian national team. Hence, the results for TL, stress symptoms, SIgA levels and symptoms of URI were obtained from 8 players.
Table 1.
Mean and standard deviation of physiological variables (n = 13).
| Variables | Mean (SD) |
|---|---|
| VO2max (ml.kg-1.min-1) | 53.1 (7.0) |
| VO2 at VT (ml.kg-1.min-1) | 40.9 (8.1) |
| VO2 at RCP (ml.kg-1.min-1) | 47.6 (6.3) |
| HR at VT (beats.min-1) | 162 (19) |
| HR at RCP (beats.min-1) | 181 (9) |
| HRmax (beats.min-1) | 190 (6) |
aximum oxygen uptake (VO2max), ventilatory threshold (VT), respiratory compensation point (RCP), heart rate at ventilatory threshold (HR at VT), heart rate at respiratory compensation point (HR at RCP), maximum heart rate (HRmax).
The main effects on average TL, overall TL and training strain across the 5 weeks of observation were significant (F = 29.064, df = 4, p < 0.001; F = 29,963, df = 4, p < 0.001; F = 23.298, df = 4, p < 0.001 ). The average (Figure 1A) and overall (Figure 1B) TL were significantly higher in week 3 than in weeks 1, 4 and 5. During the two weeks (4 and 5) preceding the competition, the TL was significantly reduced compared with weeks 1, 2 and 3. The training strain (Figure 1D) was also higher in week 3 compared with weeks 1, 2, 4 and 5 (p < 0.05).
Figure 1.
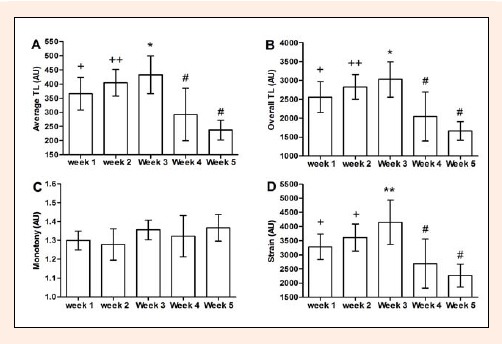
(A) Average weekly training load (TL), (B) overall TL, (C) monotony, and (D) strain training over 5 weeks of monitoring (n = 8). + different from weeks 3, 4 and 5 (p < 0.05). ++ different from weeks 4 and 5 (p < 0.05). * different from weeks 1, 4 and 5 (p < 0.05). # different from week 1, 2 and 3 (p < 0.05). ** different from week 3 to weeks 1, 2, 4 and 5 (p < 0.05).
During the weeks 4 and 5 the values were significantly reduced compared with weeks 1, 2 and 3. The monotony of training (Figure 1C) was not significantly altered across the weeks.
The SIgA levels were highly variable between subjects across the weeks. The greatest variation was in the baseline, with values of 66% and 111% regarding the concentration and secretion rate of SIgA, respectively. The SIgA concentration showed tendency of change across the weeks (F = 2.05, p = 0.070). However, neither SIgA concentration, SIgA secretion rate nor saliva flow rates were significantly altered during the training period (Table 2). However, when values were normalized for the individual’s mean baseline values, the Δ% of SIgA concentration significantly changed across the weeks (F = 5.81, p = 0.002). The Δ% of SIgA concentration was lower in week 3 in relation to weeks 2 and 4 (Table 2)
Table 2.
Mean and standard deviation of saliva flow rate and SIgA levels and percentage variation (Δ%), upper respiratory illness symptoms and stress symptoms (n = 8). Data are means (±SD).
| Baseline | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | |
|---|---|---|---|---|---|---|
| Saliva flow rate (ml·min-1) | .84 (.26) | .91 (.18) | .81 (.17 | .81 (.27) | .78 (.32) | .83 (.46) |
| Δ% saliva flow rate | - | 12.8 (32.3) | .99 (28.50) | 3.49 (41.90) | −2.60 (44.7) | −.75 (51.3) |
| SIgA concentration (µg·ml-1) | 52.2 (32.1) | 55.5 (32.3) | 78.2 (30.3) | 38.8 (16.6) | 69.3 (34.9) | 59.8 (31.4) |
| Δ% SIgA concentration | - | 14.3 (34.1) | 67.0 (37.2) | −15.0 (27.8) †† | 97.1 (90.7) | 35.8 (73.5) |
| SIgA secretion rate (µg·ml-1) | 52.2 (59.4) | 49.0 (27.1) | 61.0 (21.9) | 30.3 (13.7) | 56.3 (35.3) | 49.0 (27.3) |
| Δ% SIgA secretion rate | - | 31.7 (68.4) | 69.1 (74.7) | −10.7 (54.3) | 60.0 (127.6) | 54.6 (135.7) |
| URI – total reports | - | - | - | 17 | 3 | - |
| URINo. of affected individuals | - | - | - | 2 | 2 | - |
| URI episodes (max and min) | - | - | - | 0 -10 | 0 – 2 | - |
|
Stress symptoms Stress symptoms (max and min) |
- | 2.4 (2.2) (0 – 6) |
2.6 (3.4) (0 – 7) |
3.1 (4.5 ) (0 – 11) |
2.3 (3.9 ) (0 – 7) |
2.6 (3.7) (0 – 10) |
†† p < 0.05, in relation to weeks 2 and 4.
Two players reported 17 URI symptom items in week 3 while other 2 reported 3 symptom items in week 4. There were no significant differences in weekly stress symptoms, assessed by DALDA scores across the training period (p > 0.05) (Table 2).
A second order regression model showed a high goodness of fit (R2 = 0.64 - 0.89) between TL and strain with SIgA concentration and secretion rate, and with the “worse than normal” responses for stress symptoms in the DALDA questionnaire (Figures 2 A, B and C).
Figure 2.
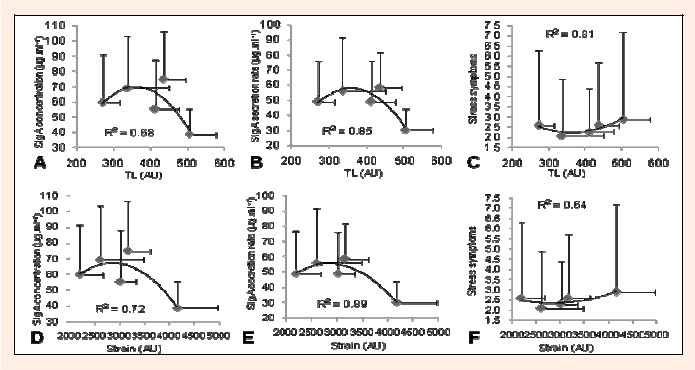
Weekly dose-response relationships based on mean values from 8 players (weeks 1-5) of training load (TL) and strain, with SIgA concentration (A and D), SIgA secretion rate (B and E), and “worse than normal” responses of stress symptoms (C and F) (n = 8).
Discussion
To the best of our knowledge, this is the first study describing the typical TL and the psychophysiological stress experienced by a female professional futsal team during a 5-week training mesocycle. This study demonstrates that increased TL, monotony and training strain may be associated with alterations in SIgA levels and stress symptoms in a non-linear fashion, therefore suggesting a non-linear dose-response relationship. Nevertheless, our results failed to demonstrate any correlation among these variables.
The team coaches programmed the taper strategy to reduce training loads in the last 2 weeks (weeks 4 and 5) of training period before the competition, decreasing volume and maintaining training intensity of the sessions. In the week 4, RT frequency was decreased to 2 times per week, while no RT was performed in the week 5. Technical tactical training volume and intensity were maintained constant during this period. Internal TL varied in accordance to the planned external training loads (Figure 1). The strategy of reducing TL prior to the competition is a common practice among strength and conditioning coaches as a recovery period because low TL may result in a transient improvement in performance due to supercompensation (Coutts et al., 2007b; 2007c).
In the present study there was a reduction of about 45% of TL during the taper period. Coutts et al., 2007a observed that large reduction (~45%) of internal TL during taper period in male rugby players induced an increase in the testosterone/cortisol (T/C) ratio and glutamine/glutamate (Gln/Glu) ratio and decreased plasma glutamate and creatine kinase (CK) activity concomitant with positive endurance and power performance changes. The tapering period also allows athletes to recover from psychophysiological distress or illness. In agreement with the findings of Papacosta et al., 2013 that reported changes in SIgA levels during the tapering period in judo athletes, the present study also presented a significant positive change in Δ% of SIgA concentration in the same period of training, suggesting that this tapering strategy is a suitable approach to allow some degree of immune function recovery.
The effectiveness of the training programs depends on the successful manipulation of the total training volume and intensity. High scores of monotony and training strain are a result of low TL variability, which in turn has been suggested to be related to the onset of overtraining, when combined with high TL (Foster, 1998). In the present study, the third week presented the highest overall weekly TL (3057 AU), monotony (1.6 AU) and training strain (4186 AU), as noted in Figure 1A, B, C and D1, respectively. Foster et al. (1998) found that for top-level speed skaters the incidence of banal infections, which is thought to be a marker of the early stages of overtraining, was higher in the weeks at which accumulated TL, monotony and strain exceeded approximately 4400, 2.2, and 6000 AU, respectively. However, to determine the relationships between TL and infection risk or to determine a secure TL threshold for individual training is still a matter of debate, instigating further investigations in sports sciences.
The perceived TL can be influenced by innate characteristics, quantity and the nature of external TL and fitness level (Impellizzeri et al., 2004; Milanez et al., 2011). In male futsal, for example, players with a higher aerobic fitness reported lower TL values compared with their less fit counterparts, despite undergoing similar external TL (Milanez et al., 2011). Furthermore, gender differences would be also influencing psycophysiological response to TL in individual and team sports (Kellmann et al., 2001; di Fronso et al., 2013). For instance, Kellmann et al., 2001, suggested that female rowers would experience higher levels of stress and lower levels of recovery than males when exposed to similar TL. Further, di Fronso et al., 2013 found lower scores of physical recovery, sleep quality, and self efficacy in female basketball players when compared to males. Consequently, female athletes may require more attention from coaches and physical trainers during the training monitoring process. However, further evidence is necessary in this area for a better understanding of the role of aerobic fitness, competitive experience and immunological responses in stress tolerance to training and competitive loads in female athletes.
Previous studies evaluated the salivary SIgA levels in order to monitor psychophysiological stress in response to TL in similar training periods (Fahlman and Engels, 2005; Leicht et al., 2012) but few of them quantified the TL by the session-RPE method (Moreira et al., 2009; 2011a). In the present study, the significant reduction in Δ% SIgA concentration was found in week 3 in response to increased TL, monotony and strain. Our results are in agreement with previous studies in the literature (Leicht et al., 2012; Moreira et al., 2011b). For instance, Moreira et al. (2011b) found a significant decrease in the SIgA secretion rate after a period of 4 weeks of training in basketball players. Subsequently, Leicht et al., 2012 described a negative relationship between TL and SIgA levels in tetraplegic wheelchair rugby players. In this respect, the goodness of fit (R2 ranged from 0.68 to 0.89) found in the present study would suggest a non-linear dose-response relationship between SIgA with TL and strain. That is, for this group of players, values of TL and strain ~435 and ~3160 AU respectively would be desirable because higher values would decrease SIgA levels (Figure 2A, B, D and E). These results provide important information for coaches and sport scientists regarding the utilization of SIgA as useful markers of physiological stress and the “optimal” TL to potentially minimize the risk of URI.
Impairment of salivary SIgA secretion in response to TL and psychophysiological stress before or during the URI symptom items has been suggested by other authors as a symptom of overreaching/overtraining (Gleeson et al., 2011; Neville et al., 2008; Tsai et al., 2011). It is assumed that increases in the symptom items of intense and rigorous training periods may lead to the formation of the “open-window” of immunosuppression and increase the risk of URI (Koch et al., 2007; Nieman, 1997). Fahlman and Engels, 2005 observed, over a 12 month training period, that college football players had a greater risk of contracting infections when SIgA secretion was below 40 µg·min-1. Gleeson et al., 1999 observed, over a 7 month training period, that SIgA concentration values ≤ 40 µg·ml-1 were associated with an increased number of URI symptom items over a training season in elite swimmers. In the present study, mean SIgA concentration and SIgA secretion rate of the team reached risk levels (in week 3) as suggested by Gleeson et al., 1999 and Fahlman and Engels, 2005 respectively, but the large increase of URI symptom items in the week 3 was not significant.
Our results are in agreement with previous studies that reported no significant relationships between SIgA and symptoms of URI in different sports like tennis, female soccer, elite tetraplegic rugby and basketball (Leicht et al., 2012; Novas et al., 2002; Novas et al., 2003). Although Novas et al., 2002 found a relationship between the increase in energy expenditure and URI symptoms, these authors did not find a relationship between URI symptoms and SIgA levels in female tennis players (Novas et al., 2003). Thus, the relationship between SIgA levels and URI is still not clear as there are contradictory results in the literature (Fahlman and Engels, 2005; Gleeson et al., 1999; Leicht et al., 2012; Novas et al., 2002; Novas et al., 2003; Vardiman et al., 2011). Hence, such a relationship must be considered with caution because factors other than salivary antibody levels may contribute to infection development (Diamond et al., 2008). Some of those studies found increased symptoms of URI concomitant with a decrease in SIgA levels, although a non-linear relationship among these variables is expected (Fahlman and Engels, 2005; Gleeson et al., 1999).
DALDA questionnaire also has been suggested to be useful for monitoring psychophysiological stress in response to TL (Coutts and Reaburn, 2008; Moreira et al., 2011b). This tool has been shown to be sensitive to TL (Coutts and Reaburn, 2008; Moreira et al., 2011b) as well as to bodily reactions to training stress (Coutts and Reaburn, 2008). For instance, Nicholls et al., 2009 found that DALDA was able to discriminate between different periods such as rest, training and pre- and post-match days. In this previous study, professional rugby players reported greater stress on training days, when compared to rest period and match days. In the present study we found a goodness of fit (R2 ranged from 0.64 to 0.81) between an increased number of “worse than normal” scores with TL and strain. To the best of our knowledge, this is the first study reporting such as relationships. Therefore, it could be suggested that the DALDA questionnaire is sensitive for monitoring psychophysiological stress in response to the variations of TL. For this group of players an optimal range of values between 343 and 419 UA to TL and 2639 and 3060 AU to strain training would be suggested, since below and above these values increased responses of stress symptoms were observed (see Figure 2 C and F). Higher levels of stress symptoms at low TL values coincided with the period immediately preceding the competition. Hence, they may have been mainly caused by anxiety and psychological stress rather than by TL.
The main limitation of the present investigation is the small sample size, but some players were injured while others were called to the National Brazilian team and could not complete the study. Additionally, the goalkeepers were excluded from the study because their training routine and the TL experienced are quite different from the outfield players. Furthermore, the training program period investigated was relatively shorter than those used in previous studies that found some relationship between SIgA and URI (Fahlman and Engels, 2005; Gleeson et al., 1999). Additionally, URI symptoms could have been more reliable if diagnosed by a medical doctor rather than using the questionnaire.
In summary, the present study demonstrated an interesting link between TL, monotony and training strain with SIgA levels and stress symptoms. However, although two players reported altogether 17 URI symptoms in week 3 concurrent with an increase in TL, monotony, strain training, stress symptoms and a decrease in SIgA concentration, this increase was not statistically significant. However, a non-linear dose-response relationship between TL and strain with SIgA and stress symptoms was detected in the present study.
Conclusion
The present study confirms the need for coaches and physical trainers to monitor TL, monotony and strain in combination with psychophysiological responses during periods of training before an important competition. The study demonstrated that increased TL, monotony and training strain may be associated with SIgA levels and stress symptoms. In the present study a significant increase in SIgA levels was observed during the tapering period, suggesting that this is a suitable approach to allow immune function recovery. Furthermore, for this group of players, there appears to be an optimal range of values of daily TL between ~343 and ~419 AU and strain between ~2639 and 3060, because at levels below and above these values there was an increase in stress symptoms and above of ~435 and ~3160 AU to TL and strain there were a decrease in SIgA levels. These results provide important information regarding the utilization of SIgA and stress symptoms derived from the DALDA questionnaire as useful markers of training stress. On the other hand, URI symptom items were not directly related to the variation in SIgA and responses to the DALDA questionnaire.
Acknowledgements
The authors would like to thank Dr. Alexandre Moreira by important contribution to this paper.
Biographies

Vinicius Flavio MILANEZ
Employment
Universidade Estadual de Londrina, Londrina, Brasil.
Degree
MSc
Research interests
Physiological adaptations to training
E-mail: viniciunesp@hotmail.com
Solange de Paula RAMOS
Employment
Universidade Estadual de Londrina, Londrina, Brasil.
Degree
PhD
Research interests
Regeneration, adaptation and tissue repair to training
E-mail: ramossolange@yahoo.com.br

Nilo M. OKUNO
Employment
Universidade Estadual de Ponta Grossa, Ponta Grossa, Brasil.
Degree
PhD
Research interests
Physiological adaptations to training
E-mail: nilookuno@yahoo.com.br

Daniel A. BOULLOSA
Employment
Universidade Católica de Brasília, Brasilia, Brasil.
Degree
PhD
Research interests
Physiological and psychological adaptations to training
E-mail: d_boullosa@yahoo.es

Fabio Y. NAKAMURA
Employment
Universidade Estadual de Londrina, Londrina, Brasil.
Degree
PhD
Research interests
Physiological adaptations to training
E-mail: fabioy_nakamura@yahoo.com.br
References
- Alexiou H., Coutts A.J. (2008) A comparison of methods used for quantifying internal training load in women soccer players. International Journal of Sports Physiology and Performance 3, 320-330 [DOI] [PubMed] [Google Scholar]
- Barrett B., Brown R., Mundt M., Safdar N., Dye L., Maberry R., Alt J. (2005) The wisconsin upper respiratory symptom survey is responsive, reliable, and valid. J Clin Epidemiol 58, 609-617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billat V.L., Hill D.W., Pinoteau J., Petit B., Koralsztein J.P. (1996) Effect of protocol on determination of velocity at VO2 max and on its time to exhaustion. Archives of Physiology and Biochemistry 104, 313-321 [DOI] [PubMed] [Google Scholar]
- Coutts A.J., Reaburn P. (2008) Monitoring changes in rugby league players’ perceived stress and recovery during intensified training. Perceptual and Motor Skills 106, 904-916 [DOI] [PubMed] [Google Scholar]
- Coutts A.J., Reaburn P., Piva T.J., Rowsell G.J. (2007a) Monitoring for overreaching in rugby league players. European Journal of Applied Physiology 99, 313-324 [DOI] [PubMed] [Google Scholar]
- Coutts A.J., Slattery K.M., Wallace L.K. (2007b) Practical tests for monitoring performance, fatigue and recovery in triathletes. Journal of Science and Medicine in Sport 10, 372-381 [DOI] [PubMed] [Google Scholar]
- Coutts A.J., Wallace L.K., Slattery K.M. (2007c) Monitoring changes in performance, physiology, biochemistry, and psychology during overreaching and recovery in triathletes. International Journal of SportsMedicine 28, 125-134 [DOI] [PubMed] [Google Scholar]
- Cox S.W., Ebersole L.E., Carpenter G.H., Proctor G.B. (2007) Effects of autonomic agonists and immunomodulatory cytokines on polymeric immunoglobulin receptor expression by cultured rat and human salivary and colonic cell lines. Archives of Oral Biology 52, 411-416 [DOI] [PubMed] [Google Scholar]
- Diamond G., Beckloff N., ryan L.K. (2008) Host defense peptides in the oral cavity and the lung: similarities and differences. Journal of Dental Research 87, 915-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Fronso S., Nakamura F.Y., Bortoli L., Robazza C., Bertollo M. (2013) Stress/recovery balance in basketball amateur players: Differences by gender and preparation phases. International Journal of Sports Physiology and Performance, in press [DOI] [PubMed] [Google Scholar]
- Fahlman M.M., Engels H.J. (2005) Mucosal IgA and URTI in American college football players: a year longitudinal study. Medicine andScience in Sports and Exercise 37, 374-380 [DOI] [PubMed] [Google Scholar]
- Foster C. (1998) Monitoring training in athletes with reference to overtraining syndrome. Medicine and Science in Sports and Exercise 30, 1164-1168 [DOI] [PubMed] [Google Scholar]
- Foster C., Florhaug J.A., Franklin J., Gottschall L., Hrovatin L.A., Parker S., Doleshal P., Dodge C. (2001) A new approach to monitoring exercise training. Journal of Strength and Conditioning Research 15, 109-115 [PubMed] [Google Scholar]
- Gleeson M., Bishop N., Oliveira M., McCauley T., Tauler P. (2011) Sex differences in immune variables and respiratory infection incidence in an athletic population. Exercise Immunology Review 17, 122-135 [PubMed] [Google Scholar]
- Gleeson M., Bishop N., Oliveira M., Tauler P. (2013) Influence of training load on upper respiratory tract infection incidence and antigen-stimulated cytokine production. Scandivian Journal of Medicine and Science in Sports 23, 451-457 [DOI] [PubMed] [Google Scholar]
- Gleeson M., McDonald W.A., Pyne D.B., Clancy R.L., Cripps A.W., Francis J.L, Fricker P.A. (2000) Immune status and respiratory illness for elite swimmers during a 12-week training cycle. International Journal of Sports Medicine 21, 302-307 [DOI] [PubMed] [Google Scholar]
- Gleeson M., McDonald W.A., Pyne D.B., Cripps A.W., Francis J.L., Fricker P.A., Clancy R.L. (1999) Salivary IgA levels and infection risk in elite swimmers. Medicine and Science in Sports and Exercise 31, 67-73 [DOI] [PubMed] [Google Scholar]
- Impellizzeri F.M., Rampinini E., Coutts A.J., Sassi A., Marcora S.M. (2004) Use of RPE-based training load in soccer. Medicine and Science in Sports and Exercise 36, 1042-1047 [DOI] [PubMed] [Google Scholar]
- Kellmann M., Altenburg D., Lormes W., Steinacker JM. (2001) Assessing stress and recovery during preparation for the world championship in rowing. The Sport Psycologyst 15, 151-167 [Google Scholar]
- Koch A.J., Wherry A.D., Petersen M.C., Johnson J.C., Stuart M.K., Sexton W.L. (2007) Salivary immunoglobulin A response to a collegiate rugby game. Journal of Strength and Conditioning Research 21, 86-90 [DOI] [PubMed] [Google Scholar]
- Leicht C.A., Bishop N.C., Paulson T.A., Griggs K.E., Goosey-Tolfrey V.L. (2012) Salivary immunoglobulin A and upper respiratory symptoms during five months of training in elite tetraplegic athletes. International Journal of Sports Physiology and Performance 7, 210-217 [DOI] [PubMed] [Google Scholar]
- Lucia A., Hoyos J., Santalla A., Earnest C., Chicharro J.L. (2003) Tour de France versus vuelta a Espana: which is harder? Medicine and Science in Sports and Exercise 35, 872-878 [DOI] [PubMed] [Google Scholar]
- Manzi V., Castagna C., Padua E., Lombardo M., D’Ottavio S., Massaro M., Volterrani M., Iellano F. (2009) Dose-response relationship os autonomic nervous system responses to individualized training impulse in marathon runners. Americam Journal of Physiology Heart and Circulatory Physiology 296, 1733-1740 [DOI] [PubMed] [Google Scholar]
- Milanez V.F., Pedro R.E., Moreira A., Boullosa D.A., Salle-Neto F., Nakamura F.Y. (2011) The role of aerobic fitness on session-rating of perceived exertion in futsal players. International Journal of Sports Physiology and performance 6, 358-366 [DOI] [PubMed] [Google Scholar]
- Moreira A., Arsati F., de Oliveira Lima-Arsati Y.B., de Freitas C.G., de Araujo V.C. (2011a) Salivary immunoglobulin A responses in professional top-level futsal players. Journal of Strength and Conditioning Research 25, 1932-1936 [DOI] [PubMed] [Google Scholar]
- Moreira A., Arsati F., Lima-Arsati Y.B.O., Simões A.C., de Araújo V.C. (2011b) Monitoring stress tolerance and occurrences of upper respiratory illness in basketball players by means of psychometric tools and salivary biomarkers. Stress and Health 27, 166-172 [Google Scholar]
- Moreira A., Delgado L., Moreira P., Haahtela T. (2009) Does exercise increase the risk of upper respiratory tract infections? Britsh Medical Bulletin 90, 111-131 [DOI] [PubMed] [Google Scholar]
- Neville V., Gleeson M., Folland J.P. (2008) Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes. Medicine and Science in Sports and Exercise 40, 1228-1236 [DOI] [PubMed] [Google Scholar]
- Nicholls A.R., Backhouse S.H., Polman R.C., McKenna J. (2009) Stressors and affective states among professional rugby union players. Scandinavian Journal of Medicine and Science in Sports 19, 121-128 [DOI] [PubMed] [Google Scholar]
- Nieman D.C. (1997) Exercise immunology: practical applications. International Journal of Sports Medicine 1, S91-100 [DOI] [PubMed] [Google Scholar]
- Novas A., Rowbottom D., Jenkins D. (2002) Total daily energy expenditure and incidence of upper respiratory tract infection symptoms in young females. International Journal of Sports Medicine 23, 465-470 [DOI] [PubMed] [Google Scholar]
- Novas A.M., Rowbottom D.G., Jenkins D.G. (2003) Tennis, incidence of URTI and salivary IgA. International Journal of Sports Medicine 24, 223-229 [DOI] [PubMed] [Google Scholar]
- Papacosta E., Gleeson M., Nassis G.P. (2013) Salivary hormones, IgA and performance during intense training and tapering in judo athletes. Journal of Strength and Conditioning Research, 27, 2569-2580 [DOI] [PubMed] [Google Scholar]
- Rushall B.S. (1990) A tool for measuring stress tolerance in elite athletes. Journal of Applied Sports Psychology 2, 51-66 [Google Scholar]
- Spence L., Brown W.J., Pyne D.B., Nissen M.D., Sloots T.P., McCormack J.G., Locke A.S., Fricker P.A. (2007) Incidence, etiology, and symptomatology of upper respiratory illness in elite athletes. Medicine and Science in Sports and Exercise 39, 577-586 [DOI] [PubMed] [Google Scholar]
- Tsai M.L., Chou K.M., Chang C.K., Fang S.H. (2011) Changes of mucosal immunity and antioxidation activity in elite male Taiwanese taekwondo athletes associated with intensive training and rapid weight loss. Britsh Journal of Sports Medicine 45, 729-734 [DOI] [PubMed] [Google Scholar]
- Vardiman J.P., Riggs C.E., Galloway D.L., Waxman M.B., Touchberry C.D., Gallagher P.M. (2011) Salivary IgA is not a reliable indicator of upper respiratory infection in collegiate female soccer athletes. Journal of Strength and Conditioning Research 25, 1937-1942 [DOI] [PubMed] [Google Scholar]
- Walsh N.P., Gleeson M., Shephard R.J., Woods J.A., Bishop N.C., Fleshner M., Green C., Pedersen B.K., Hoffman-Goetz L., Rogers C.J., Northoff H., Abbasi A., Simon P. (2011) Position statement. Part one: Immune function and exercise. Exercise Immunology Review 17, 6-63 [PubMed] [Google Scholar]


