Abstract
Ethnopharmacological relevance
Heartsease (Viola tricolor L.), a member of the Violaceae family, has a long history as a medicinal plant and has been documented in the Pharmacopoeia of Europe. Due to its anti-inflammatory properties it is regarded as a traditional remedy against skin diseases, for example for the treatment of scabs, itching, ulcers, eczema or psoriasis, and it is also used in the treatment of inflammation of the lungs and chest such as bronchitis or asthma. Because T-cells play an important role in the pathological process of inflammatory diseases we investigated the effect of an aqueous Viola extract on lymphocyte functions and explored the ‘active’ principle of the extract using bioactivity-guided fractionation.
Material and Methods
An aqueous Viola extract was prepared by C18 solid-phase extraction. Effects on proliferation of activated lymphocytes (using the cell membrane permeable fluorescein dye CFSE), apoptosis and necrosis (using annexin V and propidium iodide staining), interleukin-2 (IL-2) receptor expression (using fluorochrome-conjugated antibodies) and IL-2 cytokine secretion (using an ELISA-based bead array system) were measured by flow cytometry. Influence on lymphocyte polyfunctionality was characterized by Viola extract-induced production of IFN-γ and TNF-α, as well as its influence on lymphocyte degranulation activity. Fractionation and phytochemical analysis of the extract were performed by RP-HPLC and mass spectrometry.
Results
The aqueous Viola extract inhibited proliferation of activated lymphocytes by reducing IL-2 cytokine secretion without affecting IL-2 receptor expression. Similarly, effector functions were affected as indicated by the reduction of IFN-γ and TNF-α production; degranulation capacity of activated lymphocytes remained unaffected. Bioassay-guided fractionation and phytochemical analysis of the extract led to identification of circular plant peptides, so called cyclotides, as bioactive components.
Conclusion
An aqueous Viola extract contains bioactive cyclotides, which inhibit proliferation of activated lymphocytes in an IL-2 dependent manner. The findings provide a rationale for use of herbal Viola preparations in the therapy of disorders related to an overactive immune system. However, further studies to evaluate its clinical potency and potential risks have to be performed.
Keywords: Violaceae, Viola tricolor L., Immunosuppression, Cyclotides, Psoriasis, Anthroposophical medicine, Phytotherapy
1. Introduction
The management of patients with inflammatory disorders, such as atopic dermatitis or psoriasis remains a challenging aspect of clinical practice. Besides genetic and environmental factors, imbalance of the adaptive immune system is thought to play a role in their pathogenesis resulting in infiltration and accumulation of inflammatory cells, mainly T-lymphocytes, in the affected tissue (Cai et al., 2012). T-lymphocytes initiate a cell-mediated immune-inflammation process in situ and maintain activation of dendritic cells and macrophages by transforming them into tissue destructive effector cells (Cai et al., 2012). Immunosuppression, the targeted reduction of the activation or efficacy of the immune system, is an option for the treatment of these conditions. Established pharmaceuticals to treat such inflammatory diseases are (i) locally applied corticosteroids or calcineurin inhibitors, like cyclosporine A, a cyclic non-ribosomal undecapeptide of fungal origin which down regulate the immune system or (ii) systemic immunosuppressant’s used for severe conditions. Since inflammatory immune disorders are characterized by an increased proliferation of T-lymphocytes, most immunosuppressive drugs aim to block cell cycle progression of these cells (Macian, 2005). Besides the registered drugs as first-line therapy, which may have many and sometimes severe side effects (De Mattos et al., 2000), there are numerous traditional and alternative herbal treatments with promising but yet not proven efficacy (Reuter et al., 2010).
Heartsease (Viola tricolor L.) is a traditional medicinal plant and member of the Violaceae family. It has been described and used for centuries in Europe for the therapy of inflammatory lung diseases and for the treatment of inflammatory skin disorders, such as atopic dermatitis (Hoppe, 1951; Hager, 1999) or psoriasis (Amenta et al., 2000). Its traditional use as herbal remedy is documented in several handbooks of phytotherapy (Madaus, 1938; Czygan and Wichtl, 2002), as well as in complementary medine, especially in Anthroposophical Medicine (Pelikan, 1978) and is furthermore registered in the German commission E Monograph (phytotherapy and herbal substances) of the German Federal Institute for Drugs and Medical Devices (Bundesanzeiger (BAnz) 1986), as well as described in the Pharmacopoeia of Europe (European Pharmacopoeia (EP) (2011)). Viola tricolor is well-known to contain flavonoids (Vukics et al., 2008a, 2008b), polysaccharides, phenylcarbonic acids, salicylic acid derivatives, catechins and cumarins (Czygan and Wichtl, 2002). In addition, the family Violaceae and in particular Viola tricolor have been appreciated as rich source of naturally-occurring macrocyclic peptides, so called cyclotides (Schopke et al., 1993; Goransson et al., 2004). Cyclotides are ribosomally-synthesized plant compounds (Gruber et al., 2007) that display the unique structural topology of a head-to-tail cyclized backbone combined with three conserved disulfide bonds arranged in a knotted configuration, which confers them with remarkable stability (Colgrave and Craik, 2004; Colgrave et al., 2005; Clark et al., 2006). Cyclotides were recently reported to act as immunosuppressive peptides which inhibited the proliferation of T-lymphocytes (Grundemann et al., 2012).
Since lymphocytes play an important role in the pathological process of inflammatory diseases, our aim was to investigate the influence of an aqueous extract prepared from Viola tricolor herbs on the cell division and function of activated human lymphocytes in vitro. Using a bioactivity-guided fractionation approach and detailed cell-based investigations the ‘active’ principle of a complex plant crude extract was purified and analytically characterized by reversed-phase chromatography and mass spectrometry. The significance of the identified active compounds for the observed effect was highlighted in this study revealing a potential new source of immunosuppressive natural compounds.
2. Materials and methods
2.1. Ethics statement
All experiments conducted on human material were approved by the Ethics committee of the University of Freiburg (235/11; 22.06.11).
2.2. Plant material and extraction
Pulverized aerial parts of Viola tricolor L. were purchased from Kottas Pharma GmbH (Vienna, Austria) (Herba Violae tricoloris plv.). Plant material was extracted overnight in dichloromethane:methanol (1:1, v/v). The cyclotide containing fraction was separated from the bulk of hydrophilic compounds by addition of 0.5 volumes of water and liquid/liquid phase separation. The upper methanol/water phase was concentrated on a roto-evaporator and lyophilized. The dried extract was dissolved in solvent A (100% H2O with 0.1% trifluoroacetic acid) and in-batch pre-purified with C18 solid-phase extraction (ZEOprep 60 Å, C18 irregular 40–63 μm; ZEOCHEM, Uetikon, Switzerland). The C18 cartridges were washed with 20% solvent B (90% acetonitrile in H2O with 0.08% trifluoroacetic acid) and eluted with 80% solvent B. The resulting eluate was lyophilized and reconstituted in phosphate-buffered saline (PBS) at 10 mg/mL for biological assays what is referred to as aqueous Viola extract.
2.3. Preparation and cultivation of human peripheral lymphocytes
Human peripheral lymphocytes were isolated from the blood of healthy adult donors obtained from the Blood Transfusion Centre (University Medical Center, Freiburg, Germany). Venous blood was centrifuged on a LymphoPrep™ gradient (density: 1.077 g/cm3, 20 min, 500g, 20 °C; Progen, Heidelberg, Germany). Cells were washed twice with medium and cell viability as well as concentration was determined using the trypan blue exclusion test. Cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (PAA, Pasching, Austria), 2 mM l-glutamine, 100 U/mL penicillin and 100 U/mL streptomycin (all from Life Technologies, Paisley, UK). The cells were cultured at 37 °C in a humidified incubator with a 5% CO2/95% air atmosphere.
2.4. Activation and treatment of lymphocytes
Lymphocytes were either stimulated with anti-human CD3 (clone OKT3) and anti-human CD28 (clone 28.2) mAbs (each 100 ng/mL; both from eBioscience, Frankfurt, Germany) or PHA-L (10 μg/mL; Roche Diagnostics, Basel, Switzerland) as indicated in the presence of medium, cyclosporine A (5 μg/mL) respectively; Sandimmun® 50 mg/mL, Novartis Pharma, (Basel, Switzerland), camptothecin (CPT; 30 μg/mL): Tocris, (Bristol, UK) and 0.5% Triton-X 100 or various concentrations of the Viola extract. After cultivation, the cells were assessed in biological tests as described.
2.5. Determination of cell proliferation and cell division
For cell proliferation and cell division tracking analysis, cells were harvested and washed twice in cold PBS and resuspended in PBS at a concentration of 5 × 106 cells/mL. Cells were incubated for 10 min at 37 °C with 5 μM 5(6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Sigma-Aldrich, St. Louis, MO). The staining reaction was quenched by washing twice with complete medium and the cell division progress was analyzed using flow cytometry and the affiliated number of total events for each experiment was ≥5000.
2.6. Determination of lymphocyte apoptosis and necrosis using annexin V and propidium iodide staining
The effects of apoptosis were determined by independent experiments using the Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (eBioscience, Frankfurt, Germany) according to the manufacturer’s instructions. After annexin V staining, propidium iodide (PI; eBioscience) was added and the cells were incubated for an additional 10 min in the dark, followed by flow cytometric analysis using a BD FACSCalibur flow cytometer and BD CellQuest Pro Software to quantify the levels of apoptosis and necrosis. The number of total events measured by FACS was ≥5000 for each experiment.
2.7. IL-2 receptor expression analysis
Cultured cells were washed with PBS and stained with R-phycoerythrin (PE)-labeled anti-human CD25 mAbs (IL-2 receptor) and allophycocyanin (APC)-labeled anti-human CD8 mAbs for 15 min at 4 °C (all antibodies were purchased from eBioscience). Afterwards, the cells were washed twice, resuspended with PBS and transferred into FACS vials. The expression of the surface markers CD25 and CD8 were measured by flow cytometry. The number of total events affiliated by FACS for each experiment was ≥5000.
2.8. Determination of cytokines and degranulation analysis
Cells were treated with medium, cyclosporine A (5 μg/mL) or the aqueous extract. (100 μg/mL) for 24 h and were restimulated with PMA (50 ng/mL) and ionomycin (500 ng/mL) for an additional 6 h. Supernatants were harvested by centrifugation and were stored at −20 °C until further analysis. The amount of cytokines was measured and quantified using the FlowCytomix™ technique according to manufacturer’s instructions (eBioscience). The number of total events affiliated by FACS for each experiment was ≥500. For the CD107a degranulation assay, cells were restimulated with PMA (50 ng/mL) and ionomycin (500 ng/mL) and 5 μL (~0.25 μg) PE-conjugated anti-CD107a mAb (eBioscience) were added to each well containing 100 μL of the cell suspension. After incubation at 37 °C for 1 h, 2 μL of 1/10 diluted GolgiStop solution (Becton Dickinson) was added per well and the plates were incubated for further 2.5 h. Samples were analyzed by flow cytometry and the affiliated number of total events for each experiment was ≥5000.
2.9. Fractionation of the aqueous Viola tricolor extract
The lyophilized eluate from C18-batch purification was reconstituted in solvent A and analyzed by MALDI-TOF mass spectrometry (MS) and analytical RP-HPLC. Fractionation was carried out with linear gradients from 20% to 80% solvent B using preparative (Phenomenex Jupiter, 10 μm, 300 Å, 250 × 21.2 mm; 8 mL/min), semi-preparative (Kromasil C18, 5 μm, 100 Å, 250 × 10 mm; 3 mL/min) and analytical (Kromasil C18, 5 μm, 100 Å, 250 × 4.6 mm; 1 mL/min) RP-C18 HPLC columns on an Ultimate 3000 HPLC controlled by the Chromeleon 6.80 software application (Dionex, Amsterdam, The Netherlands). Eluting compounds were monitored with UV-absorbance at wavelengths of 214, 254 and 280 nm, automatically collected and lyophilized. All fractions were analyzed by analytical HPLC and MALDI-TOF MS or LC–MS, and stored at −20 °C for further analysis.
2.10. Nano LC–MS and MALDI-TOF/TOF analysis
Collected fractions were analyzed by nano LC–MS on a Dionex Ultimate 3000 nano HPLC system. For LC analysis, samples of fractions (1–20 μL) were injected, pre-concentrated using Dionex PepMap™ C18 cartridges (300 μm × 5 mm, 5 μm, 100 Å) and separated by nano-RP-HPLC prior to online MS analysis using a Dionex Acclaim PepMap™ C18 column (150 mm × 75 μm, 3 μm, 100 Å; 300 nL/min). The mobile phase consisted of solvent A (0.1% aqueous triflouracetic acid), solvent C (0.5% aqueous formic acid) and solvent D (80% acetonitrile, 20% H2O with 0.4% formic acid). Peptides were chromatographed using linear gradients of 4–44% D in 80 min and 44–90% in 20 min, followed by 5-min hold at 90% D and equilibration to the starting conditions for 20-min. The separation system was directly coupled to a hybrid quadrupole/linear ion trap mass spectrometry 4000 QTRAP system (AB Sciex, Framingham, MA) running with the Analyst 1.5.1 software package. The 4000 QTRAP equipped with a nano-spray source was operated in positive ionization mode. Analysis and identification by mass, isotope distribution and charge state was performed in Enhanced Multiple Scan mode of the linear ion trap using 1000 Da/s scan speed for a scan range of 750–2000 m/z and a total cycle time of 8 s. MS Reconstruct Tool was applied for spectra deconvolution in the mass range of 2500–4000 Da and a S/N threshold to obtain reliable peak identification. Additionally, all spectra were manually processed to eliminate false automatic assignments.
For MALDI-MS, Viola tricolor extract and fractions were analyzed on a MALDI-TOF/TOF 4800 analyzer (AB Sciex, Framingham, MA) operated in reflector positive mode acquiring 3000 total shots per spectrum with a fixed laser intensity set at 4000. Experiments were carried out using α-cyano hydroxyl cinnamic acid (saturated in 50% (v/v) acetonitrile) for sample preparation. Desalted samples (0.5 μL) were mixed with 3 μL of matrix and 0.5 μL of the mixture was spotted on the target plate. Spectra were acquired and processed using the 4800 mass analyzer and Data Explorer Software.
2.11. Data- and statistical analysis
For statistical analysis, data were processed with Microsoft Excel and SPSS software. Values are presented as mean ± SD for the indicated number of independent experiments. Statistical significance was determined by one-way ANOVA followed by Dunnett’s post hoc pairwise comparisons. P values <0.05 are considered as statistically significant.
3. Results
3.1. Effects of Viola tricolor on lymphocyte proliferation
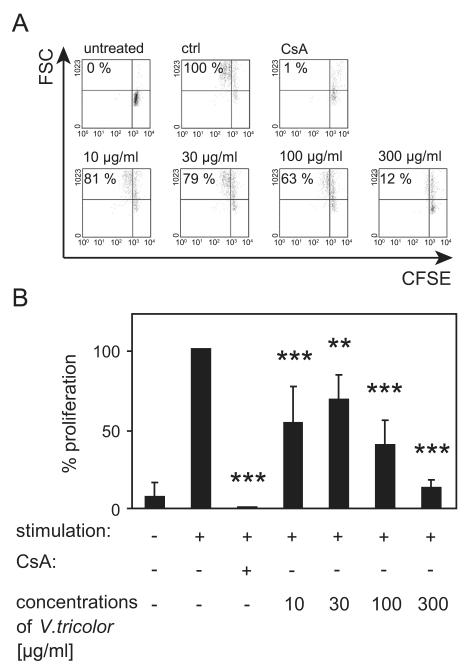
We analyzed the impact of an aqueous methanol extract prepared from Viola tricolor on cell division of activated human lymphocytes using CFSE-labeled cells (Fig. 1). CFSE is inherited by daughter cells after cell division and each dividing cell consequently loses fluorescent intensity. The dye does not influence the viability of the stained cells. Activated CFSE+ lymphocytes were analyzed by flow cytometry in the presence of control medium or various concentrations of the Viola extract (10–300 μg/mL). In the presence of cyclosporine A (0.7% ± 0.5) the proliferation was strongly decreased compared to stimulated cells alone (=100%). The presence of the extract exhibited an inhibitory effect (10 μg/mL: 53% ± 23; 30 μg/mL: 68% ± 15; 100 μg/mL: 40% ± 16; and 300 μg/mL: 13% ± 5) on lymphocyte proliferation.
Fig. 1. Influence of Viola tricolor on proliferation of activated lymphocytes.
CFSE-labeled lymphocytes (2 × 105 cells) were cultured in the presence of medium, cyclosporine A (CsA, 5 μg/mL) or different concentrations of the Viola extract (10–300 μg/mL) and activated with PHA-L (10 μg/mL) for 3 days. The proliferation of lymphocytes was analyzed using flow cytometry. The individual values (%) were listed in representative dot plots and living lymphocytes were gated as shown in (A). Data of four independent experiments are summarized in (B) shown as mean ± SD. The values are normalized (=100%) to untreated stimulated cells (ctrl). The asterisks represent significant differences of Viola extract-treated cells in comparison to control cells (**P<0.01 and ***P<0.001).
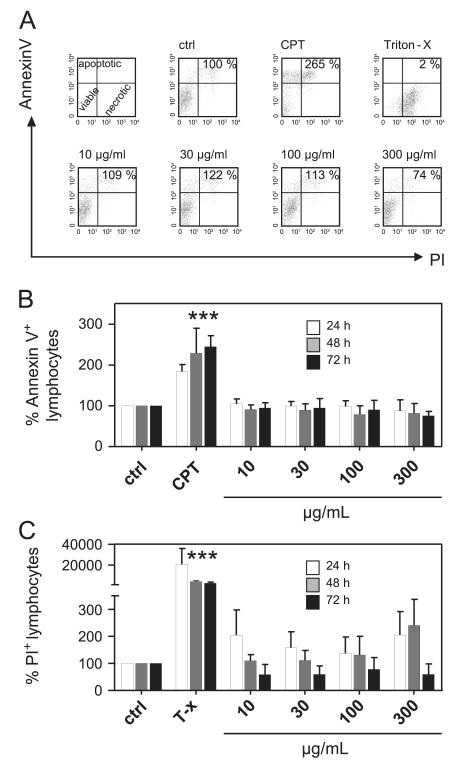
To exclude possible cytotoxic effects as cause of the observed anti-proliferative effects of the Viola extract, we quantified apoptotic and necrotic effects of the extract over time (Fig. 2). Annexin V and propidium iodide staining allowed the discrimination and parallel analysis of viable (annexin−/PI−), apoptotic (annexin+/PI+) and necrotic (annexin−/PI+) cells. As shown in Fig. 2, the extract has no significant influence on the induction of apoptosis. Necrosis was slightly increased at higher concentrations (Fig. 2C). The positive controls for apoptosis (24 h: 183% ± 18; 48 h: 228% ± 62; and 72 h: 245% ± 28) and necrosis (24 h: 20718% ± 15232; 48 h: 4236% ± 652; and 72 h: 2434% ± 1163), campthotecin (30 μg/mL) and detergent (Triton-X 100; 0.5%), respectively, significantly increased the fractions of these cells in general compared to control levels (=100%). These data indicate that the Viola extract was not cytotoxic to lymphocytes.
Fig. 2. Influence of Viola tricolor on lymphocyte apoptosis and necrosis.
Lymphocytes (2 × 105 cells) were activated with PHA-L (10 μg/ml) alone or in the presence of camptothecin (CPT; 30 μg/mL), Triton-X 100 (0.5%) or different concentrations of the Viola extract (10–300 μg/mL) for 24 (white bars), 48 (gray bars) and 72 h (black bars). The amount of apoptotic (annexin V+/PI− and annexin V+/PI−) cells and necrotic cells (annexin V−/PI+) were assessed by FACS analysis using annexin V and propidium iodide staining. Representative dot plots and proliferation values (%) of total lymphocytes are demonstrated in (A). Results from total lymphocyte analysis of apoptotic (B) and necrotic (C) cells are summarized and presented as mean ± SD of three to four independent experiments. The values are normalized (=100%) to untreated stimulated cells (ctrl). The asterisks represent significant differences of Viola extract-treated cells in comparison to control cells (***P<0.001).
3.2. Effects of Viola tricolor on IL-2 biology of activated lymphocytes
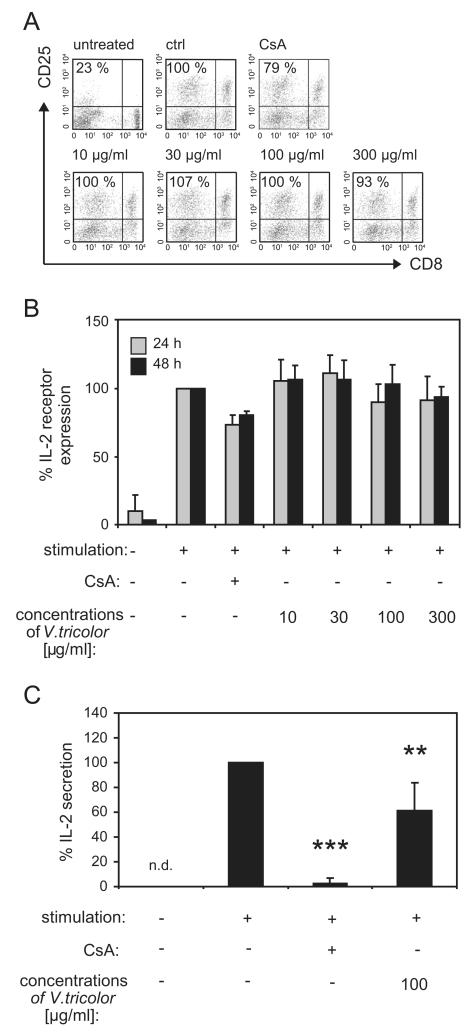
T-cell proliferation is initiated by activation and expression of growth factor interleukin 2 (IL-2), which promotes interaction with the receptor CD25 that is up-regulated on the surface of activated T-cells (Malek, 2008). Therefore, the influence of the Viola extract on expression of the IL-2 receptor CD25 was analyzed on lymphocytes using flow cytometry (Fig. 3). The data indicate that 24 and 48 h after activation the percentage of CD25+ lymphocytes (Fig. 3A and B) is strongly induced in activated lymphocytes compared to unstimulated cells. The percentage of IL-2 receptor positive lymphocytes during the presence of cyclosporine A was slightly reduced at both days (24 h: 73% ± 7 and 48 h: 81% ± 3) after stimulation. The expression of IL-2 receptor was not influenced by the Viola extract at any time. Besides IL-2 receptor expression, T-cell proliferation is regulated by endogenous release of IL-2. The control compound cyclosporine A inhibits the production of IL-2 (Shevach, 1985) and because IL-2 is a pivotal lymphokine during an immune response, inhibition of its production may explain the immunosuppressive effects of the Viola extract. Therefore the capacity of the Viola extract to influence the direct release of IL-2 from lymphocytes (Fig. 3C) was determined. Cells were treated with different concentrations of the extract or cyclosporine A and the cells were activated using mitogen stimulation for 24 h followed by re-stimulation with PMA and ionomycin. The IL-2 release of lymphocytes was reduced by the treatment with the Viola extract (61% ± 23) as well as for the positive control cyclosporine A (2% ± 4) compared to control cells (=100%). This indicated that the anti-proliferative effect of the Viola extract might be mediated through inhibition of IL-2 secretion.
Fig. 3. Influence of Viola tricolor on IL-2 biology of activated lymphocyte.
Untreated lymphocytes (2 × 105 cells), mitogen-activated (PHA-L; 10 μg/mL) cells alone or in the presence of cyclosporine A (CsA, 5 μg/mL) or various concentrations of the Viola extract were cultured for 24 or 48 h. The presence of the IL-2 receptor alpha chain CD25 on CD8+ and CD8− T-lymphocytes was analyzed with anti-human mAbs and by flow cytometry. Representative dot plots (A) indicate the data from proliferating lymphocytes and values (%) from living lymphocytes were gated and presented. Data (mean ± SD) from two to three independent experiments were summarized in (B). For IL-2 secretion analysis cells getting a re-stimulation impuls with PMA (50 ng/mL) and ionomycin (500 ng/mL) after 24 h of culture for 6 h before analysis of the secreted proteins, expressed by amounts of IL-2. The amount of IL-2 was measured in the supernatant of cultured lymphocytes using an ELISA-based cytokine detection method and flow cytometry. Limit of quantification for the IL-2 ELISA was 16.4 pg/mL. The values are normalized (=100%) to untreated stimulated cells (ctrl). Results are summarized in graphs and data are presented as mean7SD from four independent experiments (D). The asterisks represent significant differences of Viola extract-treated cells in comparison to control cells (**P<0.01 and ***P<0.001).
3.3. Effects of Viola tricolor on effector function of activated lymphocytes
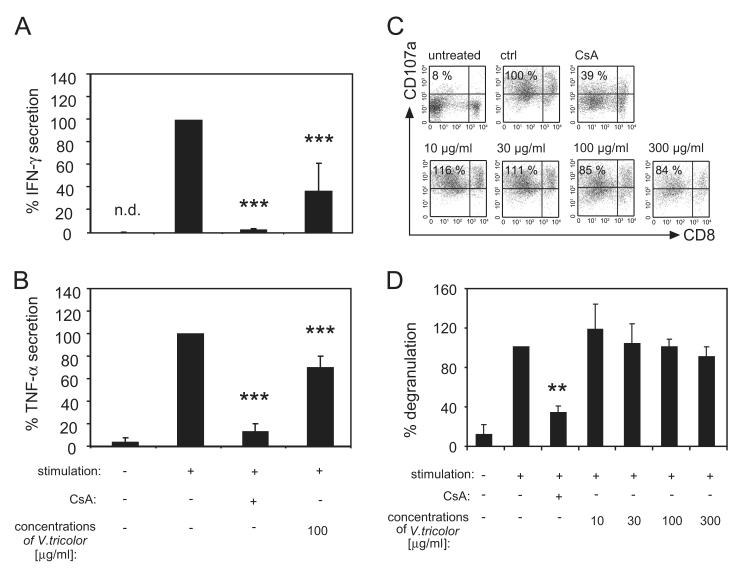
Consequently it is of interest to determine whether Viola extract solely inhibits proliferation or also affects the effector function of T-lymphocytes; the latter would directly relate to changes in IFN-γ and TNF-α. Therefore, the production of both mediators was analyzed following treatment of activated T-lymphocytes with the Viola extract or cyclosporine A (Fig. 4A and B). The results demonstrate that the release of both effector mediators was diminished by cyclosporine A (IFN-γ: 2% ± 2 and TNF-α: 13% ± 7) compared to control cells (=100%) and also in the presence of the Viola extract (IFN-γ: 37% ± 25 and TNF-α: 70% ± 10). Furthermore, it is of interest to determine the influence of the Viola extract on the degranulation activity of T-lymphocytes as a further marker of functionality (Fig. 4C and D). Activation of T-lymphocytes leads to release of cytolytic granules that contain lysosomal-associated membrane protein 1 (CD107; LAMP-1). The granule vesicle membranes fuse with the membranes of activated T-lymphocytes and therefore LAMP-1 can be used as a marker protein for the cytotoxic activity of these cells. This process is reflected by Fig. 4C and D, where the frequency of CD107a+ lymphocytes in non-stimulated controls is low (11% ± 7) and increased to high levels (100%) after activation. The degranulation capacity was reduced by cyclosporine A (34% ± 8), but not by the presence of different concentrations of the Viola extract. This suggests that treatment with the Viola extract diminished effector functions, as indicated by the reduced IFN-γ and TNF-α production, but did not affect the level of degranulation activity.
Fig. 4. Influence of Viola tricolor on IFN-γ and TNF-α production and on degranulation capacity of lymphocytes.
Purified lymphocytes (2 × 105 cells) were incubated for 36 h with cyclosporine A (CsA, 5 μg/mL) or the Viola extract (100 μg/mL), were stimulated with PHA-L (10 μg/mL) and getting a re-stimulation impulse with PMA (50 ng/mL) and ionomycin (500 ng/mL) before analysis of IFN-γ (A) and TNF-α (B) in the supernatant. Activated cells incubated with medium alone were used as controls. Limit of quantification for IFN-γ and TNF-α ELISA was 1.6 and 3.2 pg/mL, respectively. The degranulation capacity was detected by using the LAMP-1 (CD107a) assay and anti-human CD8 mAb staining. Supernatants and cells were analyzed by flow cytometry. Representative dot plots indicate the data (%) from gated living lymphocytes (C). Results are summarized in graphs and data are presented as mean ± SD from three to four independent experiments (D). n.d.: Not detected in the assay. The asterisks represent significant differences of Viola extract-treated cells in comparison to control cells (**P<0.01 and ***P<0.001).
3.4. Bioassay-guided fractionation and phytochemical analysis of the immunosuppressive Viola tricolor extract
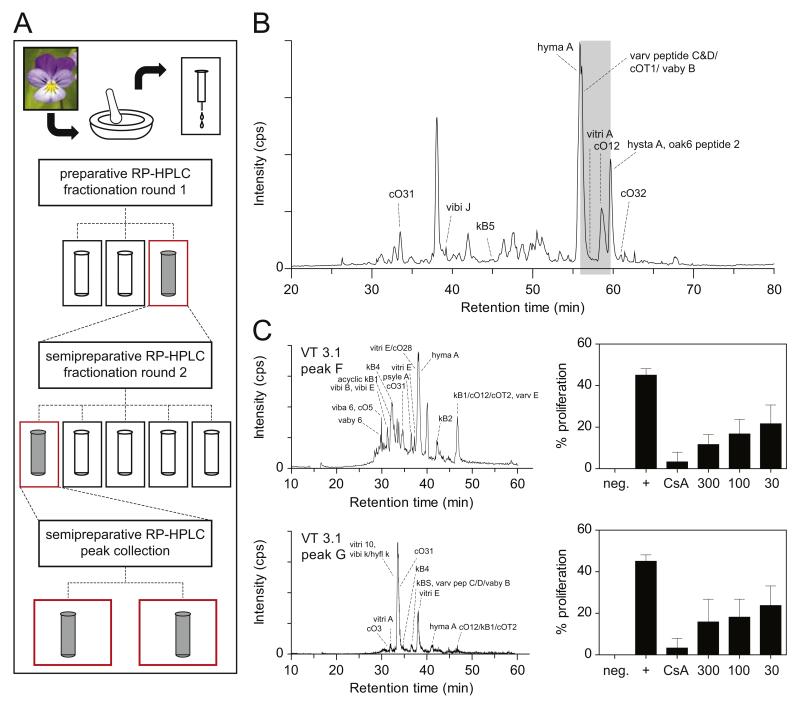
Viola tricolor is known to contain a variety of cyclotides (Svangard et al., 2004). Accordingly the extract characterized by LC–MS analysis (Fig. 5), analytical HPLC and MALDI-TOF MS (Fig. S1) confirmed the presence of cyclotides. Following a multistep bioassay-guided fractionation approach (Figs. 5A, S1 and S2) several subfractions of the initial Viola extract were generated using RP-HPLC. In line with the observed activity of the Viola extract (Fig. 1), two semipure cyclotide fractions, i.e. VT 3.1F and VT 3.1G displayed significant inhibition of lymphocyte proliferation (Figs. 5 and S2). These fractions contained thirteen and five known cyclotides (Table 1), respectively, including vibi B, vitri E, kalata B1 and hyma A (fraction VT3.1 F) and vitri 10, vitri E and cycloviolacin 31 (fraction VT3.1 G), as well as novel cyclotides.
Fig. 5. LC–MS analysis of Viola tricolor extract and subfractions.
(A) Flowchart illustrating the bioactivity-guided fractionation of the Viola extract. Three initial subfractions were generated from the extract applying preparative HPLC, and the most active one was chosen for further fractionation yielding five subfractions by semi-preparative HPLC. Subsequently, the most abundant peaks of the most active subfractions were purified using semi-preparative HPLC in isocratic elution mode to yield active fractions VT3.1F and VT3.1 G. (B, C) Base peak chromatograms from LC–MS analysis of the Viola extract (B, left panel) and the subfractions VT 3.1F and G obtained from manual peak collection are presented (C, left panel). The subfractions showed an inhibition of proliferation as measured by flow cytometry (B, C; right panels). Cyclotides were identified by LC–MS reconstruct and database search as described in Section 2. A corresponding list of cyclotides identified in the active fractions is given in Table 1. Phytochemical and anti-proliferative data of all tested extracts and fractions are provided as Supplementary Material (Supplementary Figs. S1 and S2).
Table 1. Cyclotide identification in active subfractions VT 3.1F and VT 3.1G.
| Cyclotidea | Sequenceb | MW (mono.)c |
Evidenced | Theoretical MW (mono.) (Da)e |
ΔMW [ppm]f |
Cyclotide classg |
|---|---|---|---|---|---|---|
| VT 3.1F | ||||||
| [R28A]kalata B1 | GLPVCGETCVGGTCNTPG---CTCSWPV-CTAN | 2805.73 | I | 2805.08 | 231 | M |
| cO12 (varv E) | GLPICGETCVGGTCNTPG---CSCSWPV-CTRN | 2890.35 | IC | 2890.14 | 73 | B |
| kalata B1 (kB1) | GLPVCGETCVGGTCNTPG---CTCSWPV-CTRN | M | ||||
| kalata B4 | GLPVCGETCVGGTCNTPG---CTCSWPV-CTRD | 2892.05 | I | 2891.12 | 324 | M |
| hyma A partial | ----CGETCLFIPCIFSVVG-CSCSSKV-CYRN | 2894.72 | I | 2893.22 | 519 | B |
| acyclic kalata B1 | GLPVCGETCVGGTCNTPG---CTCSWPV-CTRN | 2908.66 | I | 2908.15 | 176 | |
| vitri E | GLPVCGETCVGGTCNTPG---CSCSWPV-CFRN | 2922.61 | I | 2922.14 | 161 | M |
| vibi B | GXPVCGETCFGGTCNTPG---CTCSYPI-CTRN | 2928.65 | IC | 2929.14 | 167 | M |
| [P20D,V21K]kB1 | GLPVCGETCVGGTCNTPG---CTCSWDK-CTRN | 2936.63 | I | 2937.14 | 174 | M |
| kalata B2 | GLPVCGETCFGGTCNTPG---CSCTWPI-CTRD | 2953.20 | I | 2953.14 | 20 | M |
| vaby D | GLPVCGETCFGGTCNTPG---CTCDPWPVCTRN | 3064.94 | I | 3063.19 | 573 | M |
| kalata B3 | GLPTCGETCFGGTCNTPG---CTCDPWPICTRD | 3080.87 | I | 3080.17 | 229 | M |
| vibiE | -GIPCAESCVWIPCTVTALIGCGCSNKV-CYN- | 3080.17 | 229 | B | ||
| mram11 | GHPTCGETCLLGTCYTPG---CTCKRPV-CYKN | 3079.02 | 603 | B | ||
| cO29 | -GIPCGESCVWIPCISGAIG—CSCKSKV-CYKN | 3080.29 | 190 | B | ||
| caripe 4h | -LICSSTCLRIPCLSPR—CTCRHHI---CYLN | 3080.35 | 171 | M | ||
| viba 6 | -GIPCGESCVLIPCISSVIG-CSCKSKV-CYRN | 3093.38 | I | 3093.4 | 5 | B |
| cO5 | -GTPCGESCVWIPCISSAVG-CSCKNKV-CYKN | 3111.83 | I | 3111.32 | 164 | B |
| globa A | -GIPCGESCVFIPCITAAIG-CSCKTKV-CYRN | 3111.39 | 141 | B | ||
| VT 3.1G | ||||||
| kalata B4 | GLPVCGETCVGGTCNTPG---CTCSWPVCTRD | 2892.74 | IC | 2891.12 | 561 | M |
| vitri E | GLPVCGETCVGGTCNTPG---CSCSWPVCFRN | 2922.55 | IC | 2922.14 | 141 | M |
| viba 10 | -GIPCAESCVYLPCVTIVIG-CSCKDKVCY-N | 3056.62 | I | 3055.34 | 418 | B |
| Hyfl k | -GTPCGESCVYIPCFTAVVG-CTCKDKVCYLN | 3148.60 | 3148.33 | 85 | B | |
| vibi k | -GIPCGESCVWIPCLTSAVG-CPCKSKVCYRN | 3148.39 | 66 | B | ||
| vitri A | -GIPCGESCVWIPCITSAIG-CSCKSKVCYRN | 3153.86 | I | 3152.38 | 469 | B |
| cO3 | -GIPCGESCVWIPCLTSAIG-CSCKSKVCYRN | 3152.38 | 469 | B | ||
| cO7 | -SIPCGESCVWIPCTITALAGCKCKSKVCYN- | 3152.41 | 459 | B | ||
| cO20 | -GIPCGESCVWIPCLTSAIG-CSCKSKVCYRD | 3153.36 | 158 | B |
Identification by comparison of determined mass using MS Reconstruct tool of Analyst 1.5.1 software of a LC-MS EMS Scan (1000 Da/s, scan sum = 2) with CyBase database using a mass tolerance of ± 1 Da.
Sequences from http://www.cybase.org.
Observed molecular weight.
Score providing evidence for the quality of the deconvoluted mass of a hit (≤ 1 Da) and criteria for identification: I=isotope distribution and C=charge state of all observed multi charged ions.
Theoretical masses calculated from amino acid sequences via free available mass calculator ProtParam tool from http://www.expasy.org and addition of exact mass of 24.063 Da to cyclization and folding.
Peptide mass tolerance: (theoretical mass-detected mass)-theoretical mass × 106 [ppm].
M=Möbius and B=bracelet.
Sequence from transcriptome-mining approach reported in (Koehbach et al., 2013).
4. Discussion
Viola tricolor is a well-known and documented medicinal plant and its traditional use as an herbal remedy against inflammatory immune diseases is widespread all over Europe. Interested to understand the molecular basis for its use in phytomedicine we characterized the influence of an aqueous Viola extract on the proliferation of human lymphocytes using cell-based assays in comparison to cyclosporine A, which is the benchmark of immunosuppressive pharmaceuticals, to define its immunotherapeutic activity. The plant extract exhibited a dose-dependent inhibition of the cell division of activated T-lymphocytes. This effect was not of cytotoxic origin since the Viola extract induced neither apoptosis nor necrosis over time (Figs. 1 and 2). T-lymphocyte proliferation is initiated by ligation of the T-cell receptor to external stimuli that initiate secretion of the autocrine growth factor IL-2 that in turn promotes interaction with its surface receptor that is up-regulated on activated T-cells. Therefore, we analyzed the influence of the Viola extract on IL-2 receptor expression and IL-2 cytokine secretion. Treatment with the Viola extract inhibited the secretion of IL-2, but had no significant effect on the expression of the IL-2 surface receptor, whereas cyclosporine A affected the cytokine production and receptor expression as expected for its documented immunosuppressive properties (Shevach, 1985). Influence of the Viola extract on polyfunctionality of lymphocytes was characterized by diminished effector functions, as indicated by the reduced IFN-γ and TNF-α production, but the level of degranulation activity remained unaffected (Figs. 3 and 4). Previously, our laboratories demonstrated immunosuppressive activity of a peptide-containing Oldenlandia affinis DC. (Rubiaceae) plant extract towards activated human lymphocytes (Grundemann et al., 2012). The cyclotide kalata B1 was determined as one of the activity-bearing compounds of Oldenlandia affinis. Knowing that Viola tricolor is a rich source of cyclotides, the observed immunosuppressive effects may be due to cyclotides. Therefore, the Viola extract was analyzed by LC–MS, analytical HPLC and MALDI-TOF, which confirmed the presence of cyclotides as active anti-proliferative ingredients of the Viola extract (Fig. 5, Table 1).
Purified kalata B1, a typical cyclotide isolated from Oldenlandia affinis, and synthetic kalata B1 analogs suppressed T-cell polyfunctionality and arrested the proliferation of immune-competent cells through inhibiting IL-2 biology at more than one site (Grundemann et al., 2013). Furthermore cyclotides induced a reduction of the IL-2 cell surface marker expression and IL-2 cytokine secretion and gene expression (Grundemann et al., 2013). In contrast, the Viola extract has no influence on the expression of the IL-2 receptor, which confirms that the extract comprises a multicomponent cyclotide mixture probably exhibiting multiple effects on IL-2 biology. Another direction for this multi-range activity is the fact that the Viola extract has no influence on the degranulation activity. Cyclotides have a broad range of immunological properties, which are, in some instances even contrary (Grundemann et al., 2012) and therefore might explain diverse effects of different cyclotide containing extracts. In consideration to Viola tricolor’s immunosuppressive ingredients, attention should be paid for potential side effects of Viola extracts on individuals with a diminished immune status.
It is of great interest to discuss the potential and future use of Viola extracts as phytotherapy for inflammatory disorders, especially for that of the skin. Aqueous Viola tricolor extracts are traditionally applied topically for mild forms of inflammatory skin diseases and these indications are in line with the German commission E Monograph (phytotherapy and herbal substances) of the German Federal Institute for Drugs and Medical Devices (Bundesanzeiger (BAnz) 1986) or theEuropean Pharmacopoeia (EP) (2011). There are in general only few investigations available concerning the bioactivity of Viola tricolor, specifying on its (i) haemolytic (Schopke et al., 1993), (ii) diuretic (Hager, 1999), (iii) anti-cancer (Goun et al., 2002; Svangard et al., 2004), (iv) anti-microbial (Witkowska-Banaszczak et al., 2005), (v) anti-inflammatory (Toiu et al., 2007) and (vi) anti-oxidant activity (Slomka et al., 2008; Vukics et al., 2008a, 2008b). Respective to this literature, there are no other studies existing, concerning the influence of Viola extracts on immunocompetent cell-based systems. Our specially prepared, cyclotide-containing extract may be an improvement of the traditionally used tea preparation since we demonstrated its effects on T-cell biology which plays an important role in the pathology of inflammatory skin diseases. In addition to monotherapeutical applications of Viola extract it is possible to apply combinations with well established immunosuppressive drugs because most of these chemical substances are associated with side effects if given at an effective dosage. Once dosage is reduced below a certain level, both, side effects and drug effect cease at the same time. To minimize adverse effects but maintain drug efficacy, efforts have been made to combine a reduced dosage of the drug with additional substances that have similar properties (Hackstein et al., 2007). Although effective conventional remedies are available, approximately 30% of patients in Europe use complementary therapies, to avoid side-effects (Mainardi et al., 2009). Hereby, our results give a rational base for the use of Viola extracts and open new avenues for treatment of inflammatory skin disease.
Supplementary Material
Acknowledgments
We would like to thank M. Garcia-Käufer for statistical analysis.
Funding: This work was financially supported by the Software AG foundation/DAMUS-Donata (DO-P 9383) and the Austrian Science Fund (FWF, P24743-B21). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- Amenta R, Camarda L, Di Stefano V, Lentini F, Venza F. Traditional medicine as a source of new therapeutic agents against psoriasis. Fitoterapia. 2000;71(Suppl. 1):S13–S20. doi: 10.1016/s0367-326x(00)00172-6. [DOI] [PubMed] [Google Scholar]
- Bundesanzeiger (BAnz) Mar 13, 1986. (Kommission E)
- Cai Y, Fleming C, Yan J. New insights of T cells in the pathogenesis of psoriasis. Cell. Mol. Immunol. 2012;9:302–309. doi: 10.1038/cmi.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RJ, Daly NL, Craik DJ. Structural plasticity of the cyclic-cystine-knot framework: implications for biological activity and drug design. Biochem. J. 2006;394:85–93. doi: 10.1042/BJ20051691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgrave ML, Craik DJ. Thermal, chemical, and enzymatic stability of the cyclotide kalata B1: the importance of the cyclic cystine knot. Biochemistry. 2004;43:5965–5975. doi: 10.1021/bi049711q. [DOI] [PubMed] [Google Scholar]
- Colgrave ML, Jones A, Craik DJ. Peptide quantification by matrix-assisted laser desorption ionisation time-of-flight mass spectrometry: investigations of the cyclotide kalata B1 in biological fluids. J. Chromatogr. A. 2005;1091:187–193. doi: 10.1016/j.chroma.2005.07.094. [DOI] [PubMed] [Google Scholar]
- Czygan F-C, Wichtl M. Teedrogen und Phytopharmaka: ein Handbuch für die Praxis auf wissenschaftlicher Grundlage. Wiss. Verl.-Ges.; Stuttgart: 2002. [Google Scholar]
- De Mattos AM, Olyaei AJ, Bennett WM. Nephrotoxicity of immunosuppressive drugs: long-term consequences and challenges for the future. Am. J. Kidney Dis. 2000;35:333–346. doi: 10.1016/s0272-6386(00)70348-9. [DOI] [PubMed] [Google Scholar]
- European Pharmacopoeia (EP) European Directorate for the Quality of Medicines Healthcare (EDQM) : Volumes 1 and 2, Supplements 1 and 2. 7th ed. Council of Europe; Strasbourg, France: 2011. [Google Scholar]
- Goransson U, Svangard E, Claeson P, Bohlin L. Novel strategies for isolation and characterization of cyclotides: the discovery of bioactive macrocyclic plant polypeptides in the Violaceae. Curr. Protein Pept. Sci. 2004;5:317–329. doi: 10.2174/1389203043379495. [DOI] [PubMed] [Google Scholar]
- Goun EA, Petrichenko VM, Solodnikov SU, Suhinina TV, Kline MA, Cunningham G, Nguyen C, Miles H. Anticancer and antithrombin activity of Russian plants. J. Ethnopharmacol. 2002;81:337–342. doi: 10.1016/s0378-8741(02)00116-2. [DOI] [PubMed] [Google Scholar]
- Gruber CW, Cemazar M, Clark RJ, Horibe T, Renda RF, Anderson MA, Craik DJ. A novel plant protein-disulfide isomerase involved in the oxidative folding of cystine knot defense proteins. J. Biol. Chem. 2007;282:20435–20446. doi: 10.1074/jbc.M700018200. [DOI] [PubMed] [Google Scholar]
- Grundemann C, Thell K, Lengen K, Garcia-Kaeufer M, Huang Y-H, Huber R, Craik DJ, Schabbauer G, Gruber CW. Cyclotides suppress T-lymphocyte proliferation by an interleukin 2-dependent mechanism. PLoS One. 2013;8(6):e68016. doi: 10.1371/journal.pone.0068016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundemann C, Koehbach J, Huber R, Gruber CW. Do plant cyclotides have potential as immunosuppressant peptides? J. Nat. Prod. 2012;75:167–174. doi: 10.1021/np200722w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstein H, Steinschulte C, Fiedel S, Eisele A, Rathke V, Stadlbauer T, Taner T, Thomson AW, Tillmanns H, Bein G, Hölschermann H. Sanglifehrin a blocks key dendritic cell functions in vivo and promotes long-term allograft survival together with low-dose CsA. Am. J. Transplant. 2007;7:789–798. doi: 10.1111/j.1600-6143.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Hager H. Hagers Handbuch der pharmazeutischen Praxis. Springer-Verlag, Berlin; New York: 1999. [Google Scholar]
- Hoppe HA. Europaeische Drogen. Cram, de Gruyter & CO; Hamburg: 1951. [Google Scholar]
- Koehbach J, Berger A, Hellinger R, Kutchan T, Carpenter E, Rolf M, Sonibare MA, Moody JO, Wong GK, Dessein S, Greger H, Gruber CW. Cyclotide discovery in Gentianales revisited – identification and characterization of cyclic cystine knotted peptides and their phylogenetic distribution in Rubiaceae plants. Biopolymers. 2013;100:438–452. doi: 10.1002/bip.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macian F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- Madaus G. Lehrbuch der Biologischen Heilmittel. Georg Thieme Verlag; Leipzig: 1938. [Google Scholar]
- Mainardi T, Kapoor S, Bielory L. Complementary and alternative medicine: herbs, phytochemicals and vitamins and their immunologic effects. J. Allergy Clin. Immunol. 2009;123:283–294. doi: 10.1016/j.jaci.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Malek TR. The biology of interleukin-2. Annu. Rev. Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- Pelikan W. Heilpflanzenkunde III. Verlag am Goetheanum; CH-4143 Dornach: 1978. [Google Scholar]
- Reuter J, Merfort I, Schempp CM. Botanicals in dermatology: an evidence-based review. Am. J. Clin. Dermatol. 2010;11:247–267. doi: 10.2165/11533220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Schopke T, Hasan Agha MI, Kraft R, Otto A, Hiller K. Haemolytisch aktive komponenten aus Viola tricolor L. und Viola arvensis Murray. Sci. Pharm. 1993;61:145–153. [Google Scholar]
- Shevach EM. The effects of cyclosporin A on the immune system. Annu. Rev. Immunol. 1985;3:397–423. doi: 10.1146/annurev.iy.03.040185.002145. [DOI] [PubMed] [Google Scholar]
- Slomka A, Libik-Konieczny M, Kuta E, Miszalski Z. Metalliferous and nonmetalliferous populations of Viola tricolor represent similar mode of antioxidative response. J. Plant Physiol. 2008;165:1610–1619. doi: 10.1016/j.jplph.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Svangard E, Goransson U, Hocaoglu Z, Gullbo J, Larsson R, Claeson P, Bohlin L. Cytotoxic cyclotides from Viola tricolor. J. Nat. Prod. 2004;67:144–147. doi: 10.1021/np030101l. [DOI] [PubMed] [Google Scholar]
- Toiu A, Parvu AE, Oniga I, Tamas M. Evaluation of anti-inflammatory activity of alcoholic extract from Viola tricolor. Revista medico-chirurgicala a Societatii de Medici si Naturalisti din Iasi. 2007;111:525–529. [PubMed] [Google Scholar]
- Vukics V, Kery A, Bonn GK, Guttman A. Major flavonoid components of heartsease (Viola tricolor L.) and their antioxidant activities. Anal. Bioanal. Chem. 2008a;390:1917–1925. doi: 10.1007/s00216-008-1885-3. [DOI] [PubMed] [Google Scholar]
- Vukics V, Kery A, Guttman A. Analysis of polar antioxidants in Heartsease (Viola tricolor L.) and Garden pansy (Viola × wittrockiana Gams.) J. Chromatogr. Sci. 2008b;46:823–827. doi: 10.1093/chromsci/46.9.823. [DOI] [PubMed] [Google Scholar]
- Witkowska-Banaszczak E, Bylka W, Matlawska I, Goslinska O, Muszynski Z. Antimicrobial activity of Viola tricolor herb. Fitoterapia. 2005;76:458–461. doi: 10.1016/j.fitote.2005.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.