Summary
Epithelial to Mesenchymal Transition (EMT) is important for many developmental events and has been linked to tumor dissemination and therapeutic resistance. Salt and colleagues identify how EMT impacts how proliferation signals flow to PI3 kinase in non-small cell lung cancer.
Originally identified as a key developmental process, Epithelial to Mesenchymal Transition (EMT) has since been implicated in many aspects of cancer biology. EMT is a dynamic process with cells transitioning in either direction between the epithelial and mesenchymal differentiation states. Heterogeneity within a tumor is often observed with respect to the broad range of phenotypes along the EMT spectrum, as well as a range of differentiation states. As mesenchymal cells are more motile and invasive, the mesenchymal phenotype has been linked to increased invasion/metastasis in multiple types of cancer. In addition, EMT has been associated with the acquisition of cancer stem cell characteristics, and ultimately, poor patient prognosis (1). A growing body of evidence indicates that epithelial cells are more likely to initially respond to therapy, and an EMT is often observed in cancers that acquire resistance to treatment (2). Therefore it is critical to uncover the mechanistic details underlying the induction of EMT and the resulting phenotypic changes. Thus far, the TGFβ signaling pathway has been identified as a primary driver, and a group of transcription factors, including Twist, Snail, Slug and ZEB1, shown to be essential to invoke the broad changes in gene expression associated with the mesenchymal cell type (1). Less clear are the modifications to the signaling networks that regulate proliferation and survival occurring as a result of an EMT and that can lead to targeted therapeutic resistance. The differential ability of epithelial and mesenchymal cancer cells harboring the same driver oncogene to survive in the presence of a targeted therapy serves as evidence to suggest that re-wiring of these pathways is occurring under selective pressure in cancer cells, but the overall scope and specific details of these changes are not yet well understood.
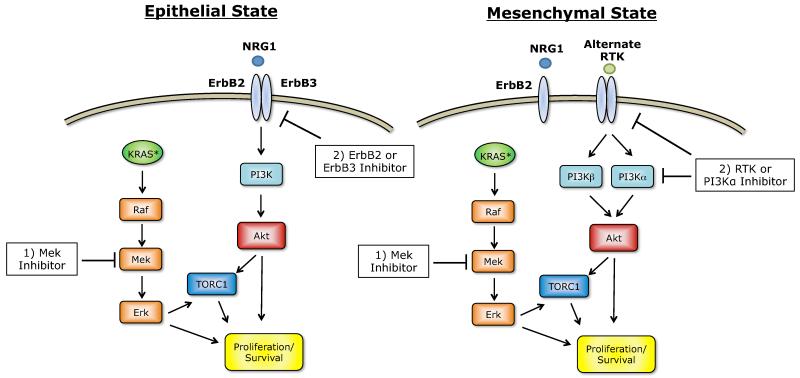
The study by Salt and colleagues in this issue of Cancer Discovery (3), utilized Twist and Snail inducible expression models of EMT to shed light on the changes in PI3K/Akt pathway signaling that occur following an EMT in KRAS-mutant non-small cell lung cancers (NSCLCs). Using this model, the authors showed that transition towards the mesenchymal state renders the cells more dependent on serum. This was explained by an NRG1/ErbB2 autocrine loop present in the epithelial state but lost in the mesenchymal state. Comparison of key proliferative signals indicated that the PI3K/Akt pathway, but not the ERK1/2 (MAPK1/2) pathway, was less active in the mesenchymal cells. Further, the reduction in Akt activity was a result of the loss of its upstream activator, ErbB3, and restoration of ErbB3/Akt signaling could rescue the diminished proliferation of the mesenchymal cells in low serum. In the epithelial state, inhibition of any of the components of the NRG1/ErbB2/Akt pathway was sufficient to impair proliferation. Moreover, in tumor samples analyzed as part of The Cancer Genome Atlas (TCGA) mesenchymal lung cancers had decreased levels of ErbB3 and are enriched for higher expression and/or amplification of the PIK3CA gene.
The authors also explored potential mechanisms by which mesenchymal cells can reactivate Akt signaling to promote proliferation. Exogenous addition of several different growth factors or overexpression of PI3Kα was able to restore mesenchymal cell proliferation in low serum conditions. Related to this, two recent studies have shown how specific growth factors can provide compensatory signaling and render cells resistant to targeted therapeutics (4, 5). During lineage differentiation, the transition towards mesenchymal state alters the panel of growth factors that can control cellular proliferation and consequently, provides a means of therapeutic resistance. Importantly, in the study by Salt et al. mesenchymal cell proliferation driven by growth factor treatment can be reversed by the addition of a PI3K inhibitor (3). Taken together, these data are consistent with a re-wiring of PI3K pathway signaling in mesenchymal cells such that it is no longer under the control of ErbB3, yet still remains essential to the proliferation of these cells. A number of previous studies have implicated PI3K re-activation in resistance to therapies including during EMT. For example, in a subset of breast cancers, TGFβ promotes activation of PI3K by upregulating ErbB ligands rather than the receptors themselves in order to increase ErbB3 phosphorylation (6). Clearly, the specific mechanisms underlying EMT-induced therapeutic resistance will be context specific, which poses a major challenge when developing therapeutic strategies. In addition, recent reports revealing that Akt can phosphorylate TWIST1 and lead to further activation of the pro-EMT pathways further underscores the complexity of the networks regulating proliferation and differentiation and the crosstalk that can occur between them (7).
Salt and colleagues’ findings have several potential implications on the treatment of KRAS mutant lung cancers. Previous studies have shown that inhibition of both the PI3K and MEK pathways is required for maximal efficacy in this context (8). The toxicity of this combination in the clinic may potentially limit its implementation. Alternative strategies could target the upstream driver of PI3K signaling, which differs between normal tissues and may limit toxicity, increasing the therapeutic window. Recently, it has been demonstrated that different RTKs can promote PI3K signaling in KRAS mutant cells in a context specific manner (9). This variability suggests that the most effective inhibitor to combine with MEK inhibitor may need to be identified on a patient-by-patient basis. Here, proteomics and other approaches might be necessary to identify key RTKs to inhibit along with MEK in each tumor. However, additional in vitro studies are required to determine if the magnitude of RTK activity correlates directly to the control of PI3K/Akt in this setting.
As noted above, data from the TCGA reveal that PIK3CA expression is upregulated specifically in mesenchymal cells. This could mean that PI3Kα is the major functional PI3K isoform in this context and that PI3Kα specific inhibitors could be effective at slowing mesenchymal cell growth. These isoform selective drugs are entering the clinic and their increased specificity will likely lead to fewer side effects, raising the interesting possibility that a MEK/PI3Kα inhibitor combination will be less toxic, allowing for a higher dose regimen that could be efficacious in some KRAS mutant cancers.
Re-wiring of signaling is a recurrent theme in therapeutic resistance: To circumvent inhibition by tyrosine kinase inhibitors (TKIs), cancer cells frequently utilize a second RTK to generate a bypass track in order to reroute key signaling pathways around the inhibited driver oncogene (10). In a subset of EGFR mutant cancers, resistance to EGFR TKI is conferred by amplification of the MET receptor gene which then maintains activation of the PI3K pathway (11). In vitro studies on these cancers revealed that in both the pre-resistant and post-resistant cells, PI3K signaling is being regulated through ErbB3. In the case of mesenchymal transition, alterations to signaling pathways might provide an additional means of survival to cells no longer relying solely on the driver oncogene and could potentially serve as a target for therapeutic intervention. For instance, mesenchymal NSCLCs resistant to EGFR TKI have been shown to downregulate EGFR and increase expression of PDGFR, FGFR, and AXL (10).
The plasticity of cancer cells allows them to grow and survive despite frequent changes to their environment. The process of EMT is an example of a dramatic shift in cell state that gives cancer cells the ability to migrate to and colonize a new environment as well as survive therapeutic insult. More generally, even less dramatic changes in the differentiation state could shift how different extracellular cues regulate proliferation and survival. Indeed, relatively subtle, non-genetic changes might strongly influence the efficacy of targeted therapeutics. For example, highly differentiated NSCLCs are more susceptible to EGFR inhibitors consistent with a strong control of epithelial differentiation and maintenance by EGFR signals. Thus, selective pressure and fluidity along differentiation axes can yield cancer cells that have changed their portfolio of environmental cues dependencies, including growth factors, cytokines and adhesion molecules. Interestingly, oncogenes such as Ras can cooperate with EMT inducing signals implying that some oncogenic drivers might favor EMT as a mechanism of therapeutic resistance (12). Re-wiring of cell proliferation and survival pathways such as described by Salt et al. provides a specific molecular explanation for these adaptive phenotypes. Future studies should provide additional insights into how different genomic contexts shaped by driving oncogenes can re-wire upon differentiation and become susceptible to alternate therapeutic strategies. Ideally, these escape routes will be mapped out in enough detail that they can be suppressed at the onset of treatment, rather than upon the emergence of therapeutic resistance and can be leveraged to counter intrinsic resistance and improve initial response. Although much remains to be understood about EMT and its impact on prognosis and therapeutics, studies such as the one in this issue have begun to define specific mechanisms underlying the impact of EMT on therapeutic response and provide a road towards better anti-cancer therapies.
Figure 1.
EMT status dictates treatment strategy in KRAS mutant NSCLCs. In epithelial cancers, the PI3K pathway can be blocked by inhibiting ErbB2 or ErbB3, while in mesenchymal cells, downregulation of ErbB3 and upregulation of other RTKs and PI3K suggest that these kinases should be targeted following an EMT.
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest.
References
- 1.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 2.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–51. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salt MB, Bandyopadhyay S, McCormick F. Epithelial to mesenchymal transition rewires the molecular path to PI3-Kinase-dependent proliferation. Cancer Discov. 2013 doi: 10.1158/2159-8290.CD-13-0520. [DOI] [PubMed] [Google Scholar]
- 4.Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–9. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–4. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang SE, Xiang B, Guix M, Olivares MG, Parker J, Chung CH, et al. Transforming growth factor beta engages TACE and ErbB3 to activate phosphatidylinositol-3 kinase/Akt in ErbB2-overexpressing breast cancer and desensitizes cells to trastuzumab. Molecular and cellular biology. 2008;28:5605–20. doi: 10.1128/MCB.00787-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue G, Restuccia DF, Lan Q, Hynx D, Dirnhofer S, Hess D, et al. Akt/PKBmediated phosphorylation of Twist1 promotes tumor metastasis via mediating cross-talk between PI3K/Akt and TGF-beta signaling axes. Cancer Discov. 2012;2:248–59. doi: 10.1158/2159-8290.CD-11-0270. [DOI] [PubMed] [Google Scholar]
- 8.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–6. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebi H, Corcoran RB, Singh A, Chen Z, Song Y, Lifshits E, et al. Receptor tyrosine kinases exert dominant control over PI3K signaling in human KRAS mutant colorectal cancers. J Clin Invest. 2011;121:4311–21. doi: 10.1172/JCI57909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niederst MJ, Engelman JA. Bypass mechanisms of resistance to receptor tyrosine kinase inhibition in lung cancer. Sci Signal. 2013;6:re6. doi: 10.1126/scisignal.2004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 12.Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]