Abstract
Aspergillus flavus is a diverse assemblage of strains that include aflatoxin-producing and non-toxigenic strains with cosmopolitan distribution. The most promising strategy currently being used to reduce preharvest contamination of crops with aflatoxin is to introduce non-aflatoxin (biocontrol) A. flavus into the crop environment. Whether or not introduction of biocontrol strains into agricultural fields is enough to reduce aflatoxin contamination to levels required for acceptance of the contaminated food as fit for consumption is still unknown. There is no question that biocontrol strains are able to reduce the size of the populations of aflatoxin-producing strains but the available data suggests that at most only a four- to five-fold reduction in aflatoxin contamination is achieved. There are many challenges facing this strategy that are both short term and long term. First, the population biology of A. flavus is not well understood due in part to A. flavus’s diversity, its ability to form heterokaryotic reproductive forms, and its unknown ability to survive for prolonged periods after application. Second, biocontrol strains must be selected that are suitable for the environment, the type of crop, and the soil into which they will be introduced. Third, there is a need to guard against inadvertent introduction of A. flavus strains that could impose an additional burden on food safety and food quality, and fourth, with global warming and resultant changes in the soil nutrients and concomitant microbiome populations, the biocontrol strategy must be sufficiently flexible to adapt to such changes. Understanding genetic variation within strains of A. flavus is important for developing a robust biocontrol strategy and it is unlikely that a “one size fits all” strategy will work for preharvest aflatoxin reduction.
Keywords: aflatoxin, Aspergillus flavus, biocontrol, food safety, recombination, maize, cottonseed, population diversity
DEVELOPMENT OF THE BIOCONTROL STRATEGY
Fungal growth on agricultural commodities, with or without mycotoxin production, does not occur in pure culture. Early studies found that aflatoxin production by Aspergillus flavus is reduced when it is cultivated with certain other fungi and bacteria (Ashworth et al., 1965; Weckbach and Marth, 1977; Wicklow et al., 1980, 1988; Horn and Wicklow, 1983; Ehrlich et al., 1985). The soil microbiome, mainly fungi and bacteria, affects the ability of the fungi to produce secondary metabolites (Ashworth et al., 1965). It has long been known that some plants are never contaminated with aflatoxin even though aflatoxin-producing species are present in the soil (Wogan, 1966). However, when tissues of these resistant plants are sterilized, A. flavus has no trouble producing aflatoxin on the tissue (Ehrlich and Ciegler, 1985). The ability of a fungus to compete for a host depends on many factors including pH, soil type, nitrogen and carbon availability, and water and mineral content (Eugenio et al., 1970). In 1975 an outbreak of A. flavus contamination of field corn in Iowa led to an unexpectedly low level of aflatoxin contamination of the crop (Ehrlich et al., 1985). Wicklow and others noticed that co-culture of A. flavus with A. niger caused a marked reduction in the formation of aflatoxin beyond a simple displacement of one fungus by the other (Wicklow et al., 1980). We found that co-cultivation of A. flavus with P. oxalicum had a similar effect on aflatoxin production but, in this case, not only were aflatoxin amounts reduced to a level not accountable by simple displacement, but a metabolite of P. oxalicum, secalonic acid, was also reduced (Ehrlich et al., 1985). We speculated that the co-cultivation of the two organisms caused a competition for ATP that is needed for one or more of the oxidation steps in secondary metabolite formation. The order of inoculation was also important for determining which of the two competing fungi was successful in reducing mycotoxin production. Inoculation with the P. oxalicum first greatly inhibited A. flavus production of aflatoxins even when eventual growth was similar. We also showed that A. flavus mutants reduced in their abilities to produce aflatoxins also showed similar competitor ability to that of P. oxalicum (Ehrlich, 1987). Recent work (see below) has offered a different interpretation of the mechanism behind these competition results (Sweany et al., 2011).
This early work supported the concept that competition with A. flavus isolates incapable of aflatoxin production could remediate aflatoxin contamination. This mode of biocontrol is currently the most widely used biocontrol method for reducing aflatoxin contamination of cereal crops in maize and cottonseed where aflatoxin contamination is a persistent problem for human and animal health (Wu and Khlangwiset, 2010a). Cotty and co-workers in the 1990s found that one particular non-aflatoxigenic A. flavus isolate (AF36) isolated from Arizona cottonseed, is an especially good competitor for reduction of aflatoxin content of cottonseed (Cotty and Bhatnagar, 1994). They determined that isolates from this VCG group were generally good as competitors against aflatoxin-producing isolates (Chang et al., 2012). We subsequently found that this isolate was unable to produce aflatoxin because of a point mutation in the polyketide synthase gene that is necessary for aflatoxin biosynthesis (Ehrlich and Cotty, 2004).
Cotty and co-workers developed a method to apply the non-aflatoxigenic strain to cotton-growing fields to prevent aflatoxin production by the wild-type aflatoxigenic populations present in the growing regions (Cotty, 2006; Cotty and Mellon, 2006). The method involves spreading non-aflatoxigenic A. flavus spores onto the field at particular times prior to harvest (Jaime-Garcia and Cotty, 2007). They assumed that addition of the non-aflatoxin producing strain would then allow it to out-compete the wild-type populations for access to the cottonseed and thereby displace the wild-type fungus. Therefore, based on this concept they called this strategy a “displacement” strategy for biocontrol of aflatoxin contamination (Jaime-Garcia and Cotty, 2009). Assuming this is true than the resulting treated field should never have to be treated again because, then, only the non-aflatoxigenic population of fungi would be present in the field. This method to prevent aflatoxin contamination is now in widespread use in Arizona for cotton fields and in other places in the southeast U.S for treatment of maize-growing areas. In other countries where aflatoxin contamination of maize is an endemic problem such as Kenya and parts of China, other strains have been discovered that are being used for aflatoxin remediation. In some cases the competing fungi are used as “cocktails” that include application of multiple strains of non-aflatoxigenic A. flavus (Wu et al., 2013).
There is a growing awareness that A. flavus also produces an indole tetramic acid mycotoxin, cyclopiazonic acid (CPA), under the same conditions that it produces aflatoxin (Chang and Ehrlich, 2011). CPA is a specific inhibitor of sarcoplasmic and endoplasmic reticulum calcium-dependent ATPase, an enzyme necessary for proper muscle contraction and relaxation. Therefore, non-aflatoxigenic competitor isolates incapable of production of this metabolite are being used instead of AF36 for reduction of aflatoxin levels in maize (Abbas et al., 2011). In a subsequent section we will discuss the possibility that other mycotoxins are produced by both the non-aflatoxigenic and aflatoxigenic populations that could contribute to mycotoxin contamination and cause toxic effects in humans and animals upon consumption.
Although the biocontrol strategy for aflatoxin remediation is increasingly being adopted world wide, there are several potential pitfalls that should be addressed These include the need to better understand the natural diversity of A. flavus populations in agricultural soil, the effects of climate change on both this diversity and on plant susceptibility, the ability of the introduced biocontrol strain to outcross with existing aflatoxin-producing A. flavus, the adaptation of certain A. flavus isolates for predominant growth on the plant rather than in the soil, the difficulty in timing the application or controlling the stability of the inoculum, how the introduction of the biocontrol strain affects the soil microenvironment, the potential damage to the plant from the introduced strain, and the need to better understand the entire A. flavus toxin burden that may result from A. flavus contamination beyond that of aflatoxin. In addition the cost of the biocontrol method and the potential need to continue reapplication seasonally must also be considered in weighing the benefits of biocontrol A. flavus as a means of reducing food and feed contamination with aflatoxin.
EFFICACY OF BIOCONTROL BY NON-AFLATOXIGENIC ISOLATES OF A. flavus
More than 100 countries have enforced or proposed regulations for levels of aflatoxin in feeds and foods (Wu et al., 2013). Because these levels are so low, the regulations place a strong burden on grain intended for export. Some of these requirements are listed in Table 1. Normally maize contains only low levels of aflatoxin and usually meets these requirements (Yu et al., 2008), but in years with severe outbreaks of A. flavus, contamination levels can exceed 100–200 ppb (Johansson et al., 2006). In cottonseed grown in Arizona levels of aflatoxin frequently exceed the levels permitted for commerce and remediation by either diluting the contaminated meal with less contaminated grain or by chemical treatment to destroy aflatoxins is often necessary. Table 2 presents a summary of results from several laboratories showing aflatoxin concentrations after treatments with several different biocontrol A. flavus and of several different crops. In some studies the reported reduction in aflatoxin content in treated versus untreated fields is as much as 20-fold (Dorner et al., 2003; Dorner, 2009, 2010; Abbas et al., 2011; Zanon et al., 2013). These data are for experimental laboratory studies where treatments were presumably done under optimized and highly controlled conditions. Generally a 5- to 20-fold reduction in aflatoxin levels would be sufficient for allowing the crop to meet the standards for consumption, but if the starting levels are particularly high, even a 20-fold reduction may not be enough. Recently, in several maize-growing regions in Kenya there have been reports of aflatoxin poisoning in humans (Probst et al., 2012; Wagacha et al., 2013; Yard et al., 2013). In these cases, ingestion was of maize that was contaminated after harvest by improper storage. It is not clear that a pre-harvest biocontrol strategy would be able to prevent such exposure and there may be simpler and more cost-effective methods to prevent ingestion of post-harvest contaminated maize.
Table 1.
Allowable levels of aflatoxins in foods and feeds.
| Country | Limit in PPB |
|---|---|
| France | 0.1–10 |
| Netherlands | 0.02–5 |
| Germany | 5 |
| Japan | 10 |
| Austria | 0.2–1 |
| United Kingdom | 10 |
| India | 30 |
| Malaysia | 35 |
| Mexico | 20 |
| United States | 20 |
Table 2.
Efficacy of biocontrol treatments.
| Crop | Non-AF agent | Range of AF reduction % (treated control) | Reference |
|---|---|---|---|
| Maize | K49 | 83–98 | Abbas etal. (2012) |
| Afla-guard | 9–75 | Dorner (2009) | |
| Afla-guard | 85–88 | Dorner (2010) | |
| Peanut | Afla-guard | 89–96 | Dorner etal. (2003) |
| AFCHG2 | 75 | Zanon etal. (2013) | |
| Cotton | AF36 | 20–88 | Cotty and Bhatnagar (1994) |
LIFE CYCLE AND DIVERSITY OF A. flavus
Aspergillus flavus is the most common species associated with aflatoxin contamination of agricultural crops (Cotty et al., 1994; Cotty, 1997). A. flavus is found in temperate and tropical regions in soil and, in agricultural areas, most commonly, on maize, cotton, tree, and ground nuts (Samuel et al., 2013) and less frequently on rice (Chen et al., 2013). A. flavus populations are highly diverse and their stability in the soil and on the plant is not well understood. An atoxigenic relative of A. flavus, A. oryzae, is widely used in soybean and rice fermentation (Chang and Ehrlich, 2010). It is now increasingly clear that A. oryzae is not a separate species but actually is only one of many examples of atoxigenic variants of A. flavus (Geiser et al., 2000; Chang et al., 2006). Other aflatoxin-producing fungi have been implicated in contamination of agricultural commodities. A. parasiticus has been associated with contaminations of peanuts in the United States (Horn, 2005), Argentina (Vaamonde et al., 2003), and West Africa (Ismail, 2001), but generally, the predominant contaminating organism is A. flavus (Cotty et al., 1994). A. flavus appears to be more invasive and out-competes A. parasiticus when both species are together in the soil. A. nomius is more rarely found in the soil, and usually is not associated with agricultural contamination episodes (Cotty et al., 1994; Bhatnagar et al., 2001; Cardwell and Cotty, 2002). Mis-identification of the contaminating organism, in some cases is possible. For example, in Thailand, some aflatoxin B-and G-producing organisms, found to be common in the soil resemble A. flavus, but have been conclusively identified as a new clade of A. nomius (Ehrlich et al., 2007a).
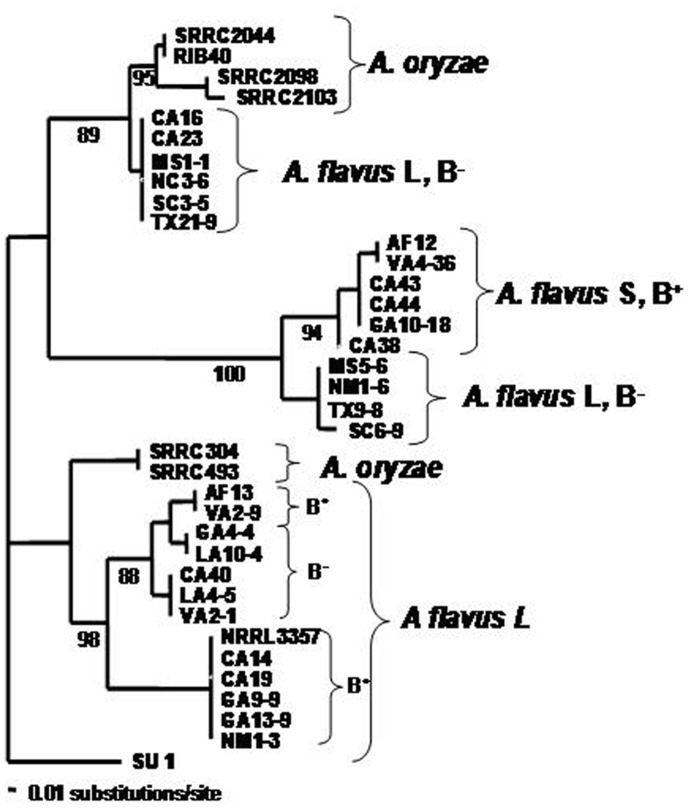
A. flavus is a diverse assemblage of strains which include toxin-producing and non-toxigenic strains, sclerotial type variants, strains with variability in response to light, strains residing in multiple vegetative compatibility groups (VCGs), and strains with variable ability to colonize living plant tissue. A cladogram showing A. flavus diversity is shown in Figure 1. As a predominantly saprophytic fungus, A. flavus resides in the soil, but as an opportunist it is readily able to colonize most environments whenever there is a rich source of carbon and nitrogen. A. flavus’s diversity, therefore, appears to be an evolutionary response to its cosmopolitan distribution. Its main mode of replication is by asexual sporulation but under some conditions, A. flavus forms sclerotia, hardened masses of desiccated and melanized mycelia that are able to survive adverse environmental and nutritional conditions.
FIGURE 1.
Cladogram illustrating the diversity of A. flavus isolates selected from several different cotton and maize-growing areas in the United States. RIB40 is the Japanese A. oryzae strain used for soy and rice fermentations.
Aspergillus flavus soil populations contain isolates from two morphologically distinct sclerotial size variants, termed the L-strain [also called A. flavus Group IB (Geiser et al., 2000)] for isolates with average sclerotial size >400 μm and the S-strain (Group IA) for isolates with sclerotial size less that 400 μm (Cotty, 1997). Both S-and L-strains of A. flavus are found globally in maize-growing regions of the world. On typical laboratory growth media, when grown in the dark, S-strain isolates produce higher levels of aflatoxins, more abundant sclerotia, and fewer conidia. Atoxigenic S-strain isolates are very rarely found in natural environments (Orum et al., 1997). A. flavus lacks the ability to produce G-aflatoxins due to a gap in the cluster that includes a required cytochrome P450-encoding gene, cypA. The size of the deletion that causes loss of a portion of cypA is 1.5 kb for S-strain isolates and 0.8 kb for L-strain isolates. Differences in sclerotial morphology correlate with the differences between the S- and L-strain A. flavus in the size of the deletion in the norB-cypA gene (Ehrlich et al., 2004). Soil populations of A. flavus are typically composed of isolates from hundreds of different VCGs. Although frequent genetic exchange among these groups has not been observed, historical recombination in populations probably has occurred. Because the 0.8 kbdeletion in S-strain isolates is identical to the deletion in those A. oryzae isolates that possess most of the aflatoxin cluster, such isolates may have descended from a common ancestor that had the S-strain-type norB-cypA gene deletion (Chang et al., 2005). On average 30% of the A. flavus soil isolates in Arizona were identified as belonging to the S-strain (Cotty, 1997; Orum et al., 1997). Because S-strain isolates consistently produce more aflatoxin than L-strain isolates and aflatoxin production in this strain is not as strongly affected by nitrogen source, the concentration of S-strain isolates in the soil appears to be better correlated with major outbreaks of aflatoxin contamination in cotton-growing areas in Arizona and Texas (Orum et al., 1997; Jaime-Garcia and Cotty, 2006). Furthermore, up to 40% of the L-strain soil isolates of A. flavus found in Arizona and other regions of the United States (Horn and Dorner, 1999) were incapable of producing aflatoxins while S-strain isolates rarely were atoxigenic (Cotty et al., 1994). Interestingly, Two of the biocontrol strains used in the United States, AF36 and K49, have the S-strain type norB-cypA deletion which may correlate with their competitor abilities (Chang et al., 2012).
POPULATION DYNAMICS OF A. flavus IN AGRICULTURAL ENVIRONMENTS
Aspergillus flavus was considered to be incapable of forming a sexual state and therefore was expected to maintain an entirely asexual life-style. Populations are divided into VCGs. Vegetative compatibility was believed to be a strong barrier to genetic exchange and in A. flavus was thought to be controlled by as many as 12 genetic loci (Bayman and Cotty, 1991; Ehrlich et al., 2007b). We found that A. flavus isolates from different VCGs formed genetically distinct groups suggesting that recombination is at most an infrequent event (Ehrlich et al., 2007b). Genetic isolation has been suggested by an additional study that found no evidence of gene flow between VCGs, including VCGs of opposite mating-type. Their results suggest that the VCGs diverged before domestication of agricultural hosts (> 10,000 year before the present; Grubisha and Cotty, 2009).
Recently A. flavus and many other presumed asexual fungi have been found to be capable of sexual reproduction, when grown in the dark under nutrient deprived conditions. A. flavus, as a heterothallic fungus, has two mating type loci, Mat1-1 and Mat1-2, maintained separately in homokaryotic isolates (Ramirez-Prado et al., 2008). Early evidence from genetic analysis suggested that A. flavus populations are able to undergo recombination (Geiser et al., 1998). Recent studies found that A. flavus in different VCG are able to outcross, and that VCG is not a strong barrier to sexual recombination (Olarte et al., 2012a). In fact such outcrossing among VCGs leads to new VCGs, and thereby, increased diversity (Olarte et al., 2012b). Most of these recombination studies have been done under laboratory conditions. Recombination can occur within conidia or sclerotia when they harbor multiple nuclei of different mating type. Fusion of nuclei containing different fluorescent markers revealed that, while conidial populations are predominantly homokaryotic, a small percentage can become heterokaryotic and, thereby, capable of recombination. The frequency of mating-type genes in the population was found to be correlated with recombination in the aflatoxin gene cluster.
Recombination has been detected between aflatoxigenic and non-aflatoxigenic A. flavus with some of the offspring regaining the ability to produce aflatoxins (Olarte et al., 2012a; Horn et al., 2013). Clearly, such recombination is a source of diversity within A. flavus. Because of this ability to recombine, it is critical to assess the frequency of such events in agricultural environments where atoxigenic biocontrol A. flavus have been introduced. A recent study found that, under an agricultural environment, a small percentage of the sclerotia that can form on contaminated maize can be heterokaryotic if the seed is contaminated with isolates of both mating types. Upon contact with non-sterile soil, these sclerotia can develop into ascocarps, the sexual reproductive developmental forms (Horn et al., 2013). A separate study found that soil populations in agricultural environments that were not treated with biocontrol A. flavus had approximately equal populations of fungi of both mating types. The population of fungi obtained from the plant (maize) was skewed to overrepresent isolates with Mat1-2 loci fungi (Sweany et al., 2011). These recent studies show that both asexual/sexual reproduction and ecological factors influence recombination.
It has been shown that the populations of A. flavus in an agricultural environment contain abundant amounts of non-aflatoxigenic A. flavus (Horn and Dorner, 1999). This suggests that loss of aflatoxin-producing ability in A. flavus could be a consequence of adaptation to a carbon-rich environment that makes the aflatoxin cluster less genetically stable. The ability to produce aflatoxins (and other mycotoxins) may give the fungi a long-term advantage over a non-aflatoxigenic biocontrol strain for survival in the soil, but in agricultural environments this adaptive pressure may be partially lost. Larger effective population sizes tend to increase mean population mutation and recombination rates (Hartl and Clark, 1997), further driving the evolution of new VCGs, some of which have lost aflatoxin-producing ability due to mutations within the biosynthetic cluster or due to large chromosomal deletions resulting in losses of entire telomeric regions (Chang et al., 2006). Since the aflatoxin and CPA clusters reside near the telomere of chromosome 3 in A. flavus, such mutations result in a high frequency of loss of aflatoxin and CPA-producing ability. The detection of linkage disequilibrium blocks in partial clusters indicates that recombination has played a large a role in cluster disassembly, and multilocus coalescent analyses of cluster and non-cluster regions indicate lineage-specific gene loss in A. flavus (Moore et al., 2007).
The long-term fate of the non-aflatoxigenic biocontrol strain in the agricultural environment has not yet been fully addressed. A preliminary report found that in cotton fields treated with biocontrol A. flavus the introduced biocontrol isolate while the highly toxigenic strain S increased to reach an equilibrium in which the population of the biocontrol strain was about 10% that of the aflatoxin-producing isolate after 4 years. After only 1 year, the soil of treated fields had A. flavus populations with greater than 50% of the biocontrol isolate. This result suggests that long-term longevity of the biocontrol A. flavus could be an important consideration in establishing treatment protocols (Jaime-Garcia and Cotty, 2013). Survival of sclerotia in soil has been studied (Wicklow et al., 1993). After 36 months exposure to an agricultural soil, 68–100% of the sclerotia survived, with the main loss being due to nematode fungivory (Wicklow et al., 1993; McCormick, 2013). While soil sclerotia are largely stable in soil, the conidial inoculum is less stable and is subject to losses due to ingestion and degradation by soil bacteria and that formulations not using wheat or barley as a carrier are desirable (Accinelli et al., 2009).
OTHER SECONDARY METABOLITE GENE CLUSTERS IN A. flavus
Aspergillus flavus is able to produce toxic secondary metabolites in addition to aflatoxins. This suggests that caution is needed in considering what isolates should be used as non-aflatoxigenic biocontrol agents (Rank et al., 2012). Among these secondary metabolites are the indole-diterpenes, aflatrem, paxillenes, paspalicines, and aflavinines, as well as is the ergot-like alkaloids CPA and pseurotin (Figure 2). While none of these metabolites is currently regulated as a food or feed contaminant, toxicity studies indicate that they could have neurotoxic and nephrotoxic effects on animals. Other metabolites are also frequent metabolites of A. flavus, including the Substance P neurotransmitter antagonist, ditryptophenaline. We have recently determined that some of these metabolites are produced in greater quantities in S strain A. flavus than in L strain and are produced by some of the non-aflatoxigenic competitor strains. The reported toxic effects on humans of ingestion of A. flavus-contaminated maize was growth retardation, immune suppression, and liver damage, the latter being manifested the most in people with hepatitis C infection (Probst et al., 2007; Probst et al., 2010; Wu and Khlangwiset, 2010b). These toxic effects have usually been ascribed to ingestion of aflatoxins. We suggest that simultaneous ingestion of other toxic A. flavus metabolites may contribute to these observed toxicities in people who have eaten aflatoxin-contaminated maize. The S morphotype A. flavus, the Aspergillus strain is most associated with the recent outbreaks of toxicity to humans in Kenya and Nigeria and may be far more toxic than the L strain (Donner et al., 2009; Mehl and Cotty, 2010; Probst et al., 2010). A. flavus is also able to produce metabolites that are usually not considered to be particularly toxic but could affect animal health. These include metabolites such as orcellinic acid, aspergillic acid and kojic acid (Varga et al., 2012) as well as iron-chelating siderophores similar to ferricrocin (Wallner et al., 2009). What effect these additional metabolites might have on animal and plant health is unknown.
FIGURE 2.
Some known non-aflatoxin metabolites from A. flavus.
GLOBAL WARMING AND BIOCONTROL
Global warming has increased daily high temperatures in the mid west and northern maize-growing regions of the United States and Canada. The resulting temperatures are predicted to eventually resemble those in the southern United States where aflatoxin contamination of maize is a frequent problem. Aflatoxin contamination of mid west maize has not been recognized as a problem as yet. Besides temperature shifts, global warming can cause climate changes which result in more unpredictable weather problems for agricultural areas. Aflatoxin contamination events are more prevalent during times of high heat and drought, which may stress the host plant thereby facilitating A. flavus infection (Schmidt-Heydt et al., 2009; Roze et al., 2012; Mohale et al., 2013; Reverberi et al., 2013). Fungal stress has been correlated with increased expression of genes involved in both secondary metabolism production and sexual recombination as discussed above. Agricultural areas experiencing drought often suffer aflatoxin contamination outbreaks, and unpredictable changes in climate that result in drought may occur with increased frequency. Currently, incidences of aflatoxin contamination of crops are limited to tropical and sub-tropical areas (between latitudes 40°N and 40°S) around the world (Samuel et al., 2013). Because the average global surface temperature has increased by 0.8°C since 1901, with most of that increase occurring in the last 30 years, it is possible that by the end of the 21st century the favorable climate for aflatoxin contamination may encompass more of the maize-growing regions of the U.S. and outbreaks will become more frequent in occurrence.
Another potential consequence of climate change is that the biocontrol strain could be an inadvertent cause of increased damage to the plant, especially if growing conditions are less favorable for cultivation. Concomitantly, changes in the soil environment and its microbiome due to temperature elevation, could also subject the crop to increased damage. Understanding genetic variation within strains of A. flavus is important for developing a robust biocontrol strategy and it is unlikely that a “one size fits all” strategy will work.
CONCLUSION
The ultimate goal for using non-aflatoxigenic A. flavus as a biocontrol agent should be the long-term protection of crops against aflatoxin contamination. Current strategies utilize a program of annual re-application of biocontrol strains, and the fate of the biocontrol strains after one growing season is still unknown. Even a low rate of recombination for aflatoxigenic fungi could be significant for future food safety. There exist other challenges to the biocontrol strategy for remediation of aflatoxin contamination. The inherent diversity of A. flavus populations makes a biocontrol strategy more difficult because A. flavus populations differ in their abilities to produce aflatoxins and other toxic secondary metabolites. Some of these other secondary metabolites could be important for assessing the full toxic burden when grains contaminated with A. flavus are ingested. Climate change could increase stress on the plant and the fungus and environmental stress could increase plant susceptibility to the fungus and is a known inducer of secondary metabolite production. Stress could also affect the ability of the fungus to outcross with native populations of A. flavus. Also, in use of the biocontrol A. flavus care must be taken to prevent undue crop damage or damage to the soil microflora that might result.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I thank my co-workers who contributed to the increased understanding of the population biology of Aspergillus flavus, in particular, Peter Cotty, Perng-Kuang Chang, Jeffrey Cary, Deepak Bhatnagar, and Geromy Moore. I also thank Ken Damann (LSU Baton Rouge) and Ignazio Carbone North Carolina State University) for their contributions to understanding the fundamentals of population diversity of A. flavus.
REFERENCES
- Abbas H. K., Weaver M. A., Horn B. W., Carbone I., Monacell J. T., Shier W. T. (2011). Selection of Aspergillus flavus isolates for biological control of aflatoxins in corn. Toxin Rev. 30 59–70 10.3109/15569543.2011.591539 [DOI] [Google Scholar]
- Abbas H. K., Zablotowicz R. M., Horn B. W., Phillips N. A., Johnson B. J., Jin X., et al. (2012). Comparison of major biocontrol strains of non-aflatoxigenic Aspergillus flavus for the reduction of aflatoxins and cyclopiazonic acid in maize. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 28 198–208 10.1080/19440049.2010.544680 [DOI] [PubMed] [Google Scholar]
- Accinelli C., Sacca M. L., Abbas H. K., Zablotowicz R. M., Wilkinson J. R. (2009). Use of a granular bioplastic formulation for carrying conidia of a non-aflatoxigenic strain of Aspergillus flavus. Bioresour. Technol. 100 3997–4004 10.1016/j.biortech.2009.03.010 [DOI] [PubMed] [Google Scholar]
- Ashworth L. J., Schroeder H. W., Langley B. C. (1965). Aflatoxins: environmental factors governing occurrence in Spanish peanuts. Science 148 1228–1229 10.1126/science.148.3674.1228 [DOI] [PubMed] [Google Scholar]
- Bayman P., Cotty P. J. (1991). Vegetative compatibility and genetic diversity in the Aspergillus flavus population of a single field. Can. J. Bot. 69 1707–1711 10.1139/b91-216 [DOI] [Google Scholar]
- Bhatnagar D., Cotty P. J., Cleveland T. E. (2001). “Genetic and biological control of aflatoxigenic fungi,” in Microbial Food Contamination eds. Wilson C. L., Droby S. (Boca Raton: CRC Press; ) 208–240 [Google Scholar]
- Cardwell K., Cotty P. J. (2002). Distribution of Aspergillus Section Flavi among field soils from the four agroecological zone of the Republic of Benin, West Africa. Plant Dis. 86 434–439 10.1094/PDIS.2002.86.4.434 [DOI] [PubMed] [Google Scholar]
- Chang P. K., Abbas H. K., Weaver M. A., Ehrlich K. C., Scharfenstein L. L., Cotty P. J. (2012). Identification of genetic defects in the atoxigenic biocontrol strain Aspergillus flavus K49 reveals the presence of a competitive recombinant group in field populations. Int. J. Food Microbiol. 154 192–196 10.1016/j.ijfoodmicro.2012.01.005 [DOI] [PubMed] [Google Scholar]
- Chang P. K., Ehrlich K. C. (2010). What does genetic diversity of Aspergillus flavus tell us about Aspergillus oryzae? Int. J. Food Microbiol. 138 189–199 10.1016/j.ijfoodmicro.2010.01.033 [DOI] [PubMed] [Google Scholar]
- Chang P.-K., Ehrlich K. C. (2011). Cyclopiazonic acid biosynthesis by Aspergillus flavus. Toxin Rev. 30 79–89 10.3109/15569543.2011.576795 [DOI] [Google Scholar]
- Chang P.-K., Ehrlich K. C, Hua S.-S. H. (2006). Cladal relatedness among Aspergillus oryzae isolates and Aspergillus flavus S and L morphotypes. Int. J. Food Microbiol. 108 172–177 10.1016/j.ijfoodmicro.2005.11.008 [DOI] [PubMed] [Google Scholar]
- Chang P. K., Horn B. W., Dorner J. W. (2005). Sequence breakpoints in the aflatoxin biosynthesis gene cluster and flanking regions in nonaflatoxigenic Aspergillus flavus isolates. Fungal Genet. Biol. 42 914–923 10.1016/j.fgb.2005.07.004 [DOI] [PubMed] [Google Scholar]
- Chen J. G., Egner P. A., Ng D., Jacobson L. P., Munoz A., Zhu Y. R., et al. (2013). Reduced aflatoxin exposure presages decline in liver cancer mortality in an endemic region of China. Cancer Prevent. Res. 6 1038–1045 10.1158/1940-6207.CAPR-13-0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotty P. J. (1997). Aflatoxin-producing potential of communities of Aspergillus section flavi from cotton producing areas in the United States. Mycol. Res. 101 698–704 10.1017/S0953756296003139 [DOI] [Google Scholar]
- Cotty P. J. (2006). “Biocompetitive exclusion of toxigenic fungi," in The Mycotoxin Factbook, Food and Feed Topics eds Barug D., Bhatnagar D., Van Egmond H. P., Van der Kamp J. W., Van Osenbruggen W. A., Visconti A. (Wageningen: Wageningen Academic Publishers; ) 179–197 [Google Scholar]
- Cotty P. J., Bayman D. S., Egel D. S., Elias K. S. (1994). “Agriculture, aflatoxins and Aspergillus,” in The Genus Aspergillus ed. Powell K. (New York: Plenum Press; ) 1–27 [Google Scholar]
- Cotty P. J., Bhatnagar D. (1994). Variability among atoxigenic Aspergillus flavus strains in ability to prevent aflatoxin contamination and production of aflatoxin biosynthetic pathway enzymes. Appl. Environ. Microbiol. 60 2248–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotty P. J., Mellon J. E. (2006). Ecology of aflatoxin producing fungi and biocontrol of aflatoxin contamination. Mycotoxin Res. 22 110–117 10.1007/BF02956774 [DOI] [PubMed] [Google Scholar]
- Donner M., Atehnkeng J., Sikora R. A., Bandyopadhyay R., Cotty P. J. (2009). Distribution of Aspergillus section flavi in soils of maize fields in three agroecological zones of Nigeria. Soil Biol. Biochem. 41 37–44 10.1016/j.soilbio.2008.09.013 [DOI] [Google Scholar]
- Dorner J. W. (2009). Biological control of aflatoxin contamination in corn using a nontoxigenic strain of Aspergillus flavus. J. Food Prot. 72 801–804 [DOI] [PubMed] [Google Scholar]
- Dorner J. W. (2010). Efficacy of a biopesticide for control of aflatoxins in Corn. J. Food Prot. 73 495–499 [DOI] [PubMed] [Google Scholar]
- Dorner J. W., Cole R. J., Connick W. J., Daigle D. J., McGuire M. R., Shasha B. S. (2003). Evaluation of biological control formulations to reduce aflatoxin contamination in peanuts. Biol. Control 26 318–324 10.1016/s1049-9644(02)00139-131 [DOI] [Google Scholar]
- Ehrlich K. (1987). Effect on aflatoxin production of competition between wild-type and mutant strains of Aspergillus parasiticus. Mycopathologia 97 93–96 10.1007/BF00436844 [DOI] [PubMed] [Google Scholar]
- Ehrlich K., Ciegler A. (1985). Effect of phytate on aflatoxin formation by Aspergillus parasiticus grown on different grains. Mycopathologia 92 3–6 10.1007/BF00442651 [DOI] [PubMed] [Google Scholar]
- Ehrlich K., Ciegler A., Klich M. A., Lee L. (1985). Fungal competition and mycotoxin production on corn. Experientia 41 691–693 10.1007/BF02007725 [DOI] [Google Scholar]
- Ehrlich K. C., Chang P.-K., Yu J., Cotty P. J. (2004). Aflatoxin biosynthesis cluster gene cypA is required for G aflatoxin formation. Appl. Environ. Microbiol. 70 6518–6524 10.1128/AEM.70.11.6518-6524.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich K. C., Cotty P. J. (2004). An isolate of Aspergillus flavus used to reduce aflatoxin contamination in cottonseed has a defective polyketide synthase. Appl. Microbiol. Biotechnol. 65 473–478 10.1007/s00253-004-1670-y [DOI] [PubMed] [Google Scholar]
- Ehrlich K. C., Kobbeman K., Montalbano B. G., Cotty P. J. (2007a). Aflatoxin-producing Aspergillus species from Thailand. Int. J. Food Microbiol. 114 153–159 10.1016/j.ijfoodmicro.2006.08.007 [DOI] [PubMed] [Google Scholar]
- Ehrlich K. C., Montalbano B. G., Cotty P. J. (2007b). Analysis of single nucleotide polymorphisms in three genes shows evidence for genetic isolation of certain Aspergillus flavus vegetative compatibility groups. FEMS Microbiol. Lett. 268 231–236 10.1111/j.1574-6968.2006.00588.x [DOI] [PubMed] [Google Scholar]
- Eugenio C., Christensen C., Mirocha C. (1970). Factors affecting production of the mycotoxin F-2 by Fusarium roseum. Phytopathology 60 1055–1057 10.1094/Phyto-60-1055 [DOI] [PubMed] [Google Scholar]
- Geiser D. M., Dorner J. W., Horn B. W., Taylor J. W. (2000). The phylogenetics of mycotoxin and sclerotium production in Aspergillus flavus and Aspergillus oryzae. Fungal Genet. Biol. 31 169–179 10.1006/fgbi.2000.1215 [DOI] [PubMed] [Google Scholar]
- Geiser D. M., Pitt J. I., Taylor J. W. (1998). Cryptic speciation and recombination in the aflatoxin-producing fungus, Aspergillus flavus. Proc. Natl. Acad. Sci. U.S.A. 95 388–393 10.1073/pnas.95.1.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubisha L. C., Cotty P. J. (2009). Twenty-four microsatellite markers for the aflatoxin-producing fungus Aspergillus flavus. Mol. Ecol. Resour. 9 264–267 10.1111/j.1755-0998.2008.02378.x [DOI] [PubMed] [Google Scholar]
- Hartl D., Clark A. (1997). Principles of Population Genetics. Sunderland, MA: Sinauer Associates [Google Scholar]
- Horn B., Sorensen R., Lamb M., Sobolev V., Olarte R., Worthington C., et al. (2013). Sexual reproduction in Aspergillus flavus sclerotia naturally produced in corn. Phytopathology 10.1094/PHYTO-05-13-0129-R [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Horn B. W. (2005). Colonization of wounded peanut seeds by soil fungi: selectivity for species from Aspergillus section flavi. Mycologia 97 202–217 10.3852/mycologia.97.1.202 [DOI] [PubMed] [Google Scholar]
- Horn B. W., Dorner J. W. (1999). Regional differences in production of aflatoxin B1 and cyclopiazonic acid by soil isolates of Aspergillus flavus along a transect within the United States. Appl. Environ. Microbiol. 65 1444–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn B. W., Wicklow D. T. (1983). Factors influencing the inhibition of aflatoxin production in corn by Aspergillus niger. Can. J. Microbiol. 29 1087–1091 10.1139/m83-167 [DOI] [PubMed] [Google Scholar]
- Ismail M. A. (2001). Deterioration and spoilage of peanuts and desiccated coconuts from two sub-Saharan tropical East African countries due to the associated mycobiota and their degradative enzymes. Mycopathologia 150 67–84 10.1023/A:1010863507652 [DOI] [PubMed] [Google Scholar]
- Jaime-Garcia R., Cotty P. J. (2006). Spatial distribution of Aspergillus flavus and its toxigenic strains on commercial cottonseed from south Texas and its relationship to aflatoxin contamination. Plant Pathol. 55 358–366 10.1111/j.1365-3059.2006.01358.x [DOI] [Google Scholar]
- Jaime-Garcia R., Cotty P. J. (2007). Influences of application timing on sporulation of the biocontrol product Aspergillus flavus AF36 in cotton fields of Arizona. Phytopathology 97 S168–S168 [Google Scholar]
- Jaime-Garcia R., Cotty P. J. (2009). Effect of application time on displacement of aflatoxin producers by the atoxigenic strain Aspergillus flavus AF36. Phytopathology 99 S58–S58 [Google Scholar]
- Jaime R., Cotty P. J. (2013). Temporal dynamic of Aspergillus flavus community structure in soils of fields treated with the atoxigenic biocontrol A. flavus AF36 in Arizona. Phytopathology 103 66 [Google Scholar]
- Johansson A. S., Whitaker T. B., Hagler W. M., Jr., Bowman D. T., Slate A. B., Payne G., et al. (2006). Predicting aflatoxin and fumonisin in shelled corn lots using poor-quality grade components. J. AOAC Int. 89 433–440 [PubMed] [Google Scholar]
- McCormick S. P. (2013). Microbial detoxification of mycotoxins. J. Chem. Ecol. 39 907–918 10.1007/s10886-013-0321-0 [DOI] [PubMed] [Google Scholar]
- Mehl H. L., Cotty P. J. (2010). Variation in competitive ability among isolates of Aspergillus flavus from different vegetative compatibility groups during maize infection. Phytopathology 100 150–159 10.1094/phyto-100-2-0150 [DOI] [PubMed] [Google Scholar]
- Mohale S., Medina A., Magan N. (2013). Effect of environmental factors on in vitro and in situ interactions between atoxigenic and toxigenic Aspergillus flavus strains and control of aflatoxin contamination of maize. Biocontrol Sci. Technol. 23 776–793 10.1080/09583157.2013.794895 [DOI] [Google Scholar]
- Moore G. G., Ramirez-Prado J. H., Horn B. W., Carbone I. (2007). Evidence of extensive recombination in the aflatoxin gene cluster of Aspergillus flavus. Phytopathology 97 S79–S79 [Google Scholar]
- Olarte R. A., Horn B. W., Dorner J. W., Monacell J. T., Singh R., Stone E. A., et al. (2012a). Effect of sexual recombination on population diversity in aflatoxin production by Aspergillus flavus. Phytopathology 103 8 [DOI] [PubMed] [Google Scholar]
- Olarte R. A., Horn B. W., Dorner J. W., Monacell J. T., Singh R., Stone E. A., et al. (2012b). Effect of sexual recombination on population diversity in aflatoxin production by Aspergillus flavus and evidence for cryptic heterokaryosis. Mol. Ecol. 21 1453–1476 10.1111/j.1365-294X.2011.05398.x [DOI] [PubMed] [Google Scholar]
- Orum T. V., Bigelow D. M., Nelson M. R., Howell D. R., Cotty P. J. (1997). Spatial and temporal patterns of Aspergillus flavus strain composition and propagule density in Yuma County, arizona, soils. Plant Dis. 81 911–916 10.1094/PDIS.1997.81.8.911 [DOI] [PubMed] [Google Scholar]
- Probst C., Callicott K. A., Cotty P. J. (2012). Deadly strains of Kenyan Aspergillus are distinct from other aflatoxin producers. Eur. J. Plant Pathol. 132 419–429 10.1007/s10658-011-9887-y [DOI] [Google Scholar]
- Probst C., Njapau H., Cotty P. J. (2007). Outbreak of an acute aflatoxicosis in Kenya in 2004: identification of the causal agent. Appl. Environ. Microbiol. 73 2762–2764 10.1128/aem.02370-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst C., Schulthess F., Cotty P. J. (2010). Impact of Aspergillus section flavi community structure on the development of lethal levels of aflatoxins in Kenyan maize (Zea mays). J. Appl. Microbiol. 108 600–610 10.1111/j.1365-2672.2009.04458.x [DOI] [PubMed] [Google Scholar]
- Ramirez-Prado J. H., Moore G. G., Horn B. W., Carbone I. (2008). Characterization and population analysis of the mating-type genes in Aspergillus flavus and Aspergillus parasiticus. Fungal Genet. Biol. 45 1292–1299 10.1016/j.fgb.2008.06.007 [DOI] [PubMed] [Google Scholar]
- Rank C., Klejnstrup M. L., Petersen L. L., Kildgaard S., Frisvad J. C., Gotfredsen C. H., et al. (2012). Comparative Chemistry of Aspergillus oryzae (RIB40) and A. flavus (NRRL 3357). Metabolites 2 39–56 10.3390/metabo2010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverberi M., Punelli M., Smith C. A., Zjalic S., Scarpari M., Scala V., et al. (2013). How peroxisomes affect aflatoxin biosynthesis in Aspergillus flavus. PLoS ONE 7:e48097 10.1371/journal.pone.0048097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roze L. V., Chanda A., Wee J., Awad D., Linz J. E. (2012). Stress-related transcription factor AtfB integrates secondary metabolism with oxidative stress response in aspergilli. J. Biol. Chem. 286 35137–35148 10.1074/jbc.M111.253468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel S. M., Aiko V., Panda P., Mehta A. (2013). Aflatoxin B-1 occurrence, biosynthesis and its degradation. J. Pure Appl. Microbiol. 7965–971 [Google Scholar]
- Schmidt-Heydt M., Abdel-Hadi A., Magan N., Geisen R. (2009). Complex regulation of the aflatoxin biosynthesis gene cluster of Aspergillus flavus in relation to various combinations of water activity and temperature. Int. J. Food Microbiol. 135 231–237 10.1016/j.ijfoodmicro.2009.07.026 [DOI] [PubMed] [Google Scholar]
- Sweany R. R., Damann K. E., Jr., Kaller M. D. (2011). Comparison of soil and corn kernel Aspergillus flavus populations: evidence for niche specialization. Phytopathology 101 952–959 10.1094/PHYTO-09-10-0243 [DOI] [PubMed] [Google Scholar]
- Vaamonde G., Patriarca A., Fernandez Pinto V., Comerio R., Degrossi C. (2003). Variability of aflatoxin and cyclopiazonic acid production by Aspergillus section flavi from different substrates in Argentina. Int. J. Food Microbiol. 88 79–84 10.1016/S0168-1605(03)00101-6 [DOI] [PubMed] [Google Scholar]
- Varga J., Frisvad J. C., Samson R. A. (2012). Two new aflatoxin producing species, and an overview of Aspergillus section flavi. Stud. Mycol. 69 57–80 10.3114/sim.2011.69.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagacha J. M., Mutegi C., Karanja L., Kimani J., Christie M. E. (2013). Fungal species isolated from peanuts in major Kenyan markets: emphasis on Aspergillus section flavi. Crop Protect. 52 1–9 10.1016/j.cropro.2013.05.004 [DOI] [Google Scholar]
- Wallner A., Blatzer M., Schrettl M., Sarg B., Lindner H., Haas H. (2009). Ferricrocin, a siderophore involved in intra- and transcellular iron distribution in Aspergillus fumigatus. Appl. Environ. Microbiol. 75 4194–4196 10.1128/AEM.00479-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckbach L. S., Marth E. H. (1977). Aflatoxin production by Aspergillus parasiticus in a competitive environment. Mycopathologia 62 39–45 10.1007/BF00491994 [DOI] [PubMed] [Google Scholar]
- Wicklow D. T., Hesseltine C. W., Shotwell O. L., Adams G. L. (1980). Interference competition and aflatoxin levels in corn. Phytopathology 70 761–764 10.1094/Phyto-70-761 [DOI] [Google Scholar]
- Wicklow D. T., Horn B. W., Shotwell O. L., Hesseltine C. W., Caldwell R. W. (1988). Fungal interference with Aspergillus flavus infection and aflatoxin contamination of maize grown in a controlled environment. Phytopathology 78 68–74 10.1094/Phyto-78-68 [DOI] [Google Scholar]
- Wicklow D. T., Wilson D. M., Nelsen T. C. (1993). Survival of Aspergillus flavus sclerotia and conidia buried in soil in Illinois and Georgia. Phytopathology 83 1141–1147 10.1094/Phyto-83-1141 [DOI] [Google Scholar]
- Wogan G. (1966). “Mycotoxin contamination of foodstuffs,” in Advances in Chemistry, World Protein Resources (Washington, DC: American Chemical Society; ) 195–215 [Google Scholar]
- Wu F., Khlangwiset P. (2010a). Evaluating the technical feasibility of aflatoxin risk reduction strategies in Africa. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 27 658–676 10.1080/19440041003639582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Khlangwiset P. (2010b). Health economic impacts and cost-effectiveness of aflatoxin-reduction strategies in Africa: case studies in biocontrol and post-harvest interventions. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 27 496–509 10.1080/19440040903437865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Stacy S. L., Kensler T. W. (2013). Global risk assessment of aflatoxins in maize and peanuts: are regulatory standards adequately protective? Toxicol. Sci. 135 251–259 10.1093/toxsci/kft132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yard E. E., Daniel J. H., Lewis L. S., Rybak M. E., Paliakov E. M., Kim A. A., et al. (2013). Human aflatoxin exposure in Kenya, 2007: a cross-sectional study. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 30 1322–1331 10.1080/19440049.2013.789558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Payne G. A., Campbell B. C., Guo B. Z., Cleveland T. E., Robens J. F., et al. (2008). “Mycotoxin production and prevention of aflatoxin contamination in food and feed,” in The ASPERGILLI: Genomics, Medical Aspects, Biotechnology, and Research Methods eds Osmani S., Goldman G. (Boca Raton, FL: CRC Press; ) 457–472 [Google Scholar]
- Zanon A., Chiotta M., Giaj-Merlera G., Barros G. (2013). Evaluation of potential biocontrol agent for aflatoxin in Argentinean peanuts. Int. J. Food Microbiol. 162 220–225 10.1016/j.ijfoodmicro.2013.01.017 [DOI] [PubMed] [Google Scholar]