Abstract
Chylous ascites (chyloperitoneum) is a rare clinical condition, characterized by an accumulation of lymph fluid in the peritoneal cavity. Most commonly it is associated with abdominal malignancy (usually lymphoma). We present an unusual case of a woman who developed a persistent pseudocyst and recurrent chylous ascites following acute necrotizing pancreatitis.
Background
We report a case of pancreatitis that was complicated with chylous ascites which was resistant to conservative measures, including dietary modification. More aggressive therapy in the form of fasting the patient with total parenteral nutrition (TPN) for 6 weeks allowed resolution.1
Case presentation
A previously well 53-year-old woman presented with a 10 day history of worsening abdominal pain. Examination revealed diffuse abdominal tenderness with normal vital signs. Her Ranson's criteria were two on admission (white blood cell count 26×109 leukocytes/L; lactate dehydrogenase 749 iu/L). Serum amylase was approximately 2509 IU/L and C reactive protein was 488.3 mg/L, which was equivalent to two on the Glasgow criteria and was diagnosed as acute mild pancreatitis. At 48 h, Ranson's criteria were four (base excess –4.2 mEq/L; >6 L fluid sequestered; urea rise 18.2 mmol/L; calcium 1.52 mmol/L) which corresponds to Glasgow criteria being four, and she was in acute severe pancreatitis and developed acute renal failure and respiratory distress.
Investigations
CT scan demonstrated a diffusely edematous pancreas, with an inflammatory phlegmon (figure 1) on ultrasound, and magnetic resonance cholangiopancreatography showed no evidence of gallstones, common bile duct, or pancreatic duct abnormality. Subsequent imaging demonstrated an evolving acute fluid collection, and only the uncinate process and a portion of the tail remained perfused, which corresponds to a Baltazar score of C at the early stage and D at the later stage (figure 2A, B).
Figure 1.
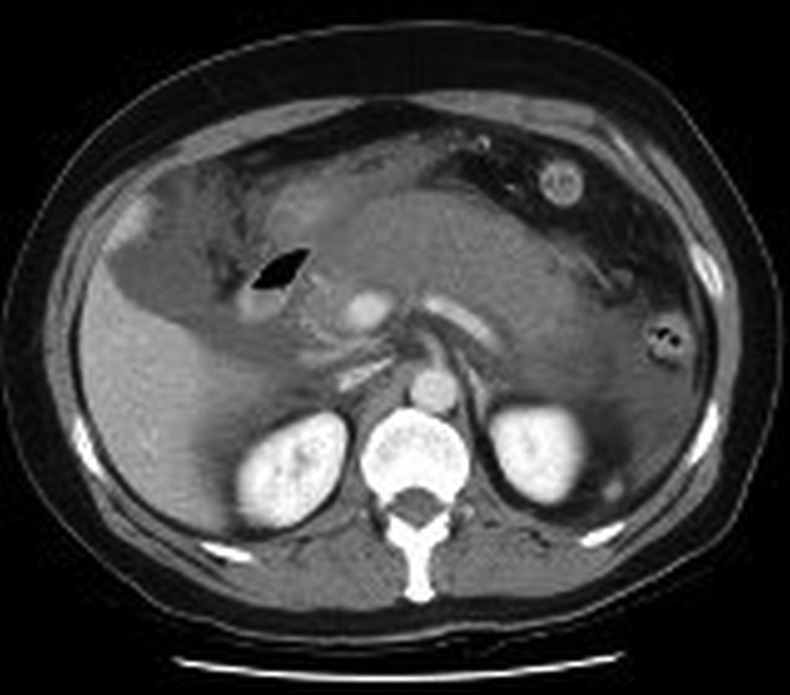
Axial CT image demonstrating diffusely an edematous poorly perfused pancreas with associated ascites.
Figure 2.
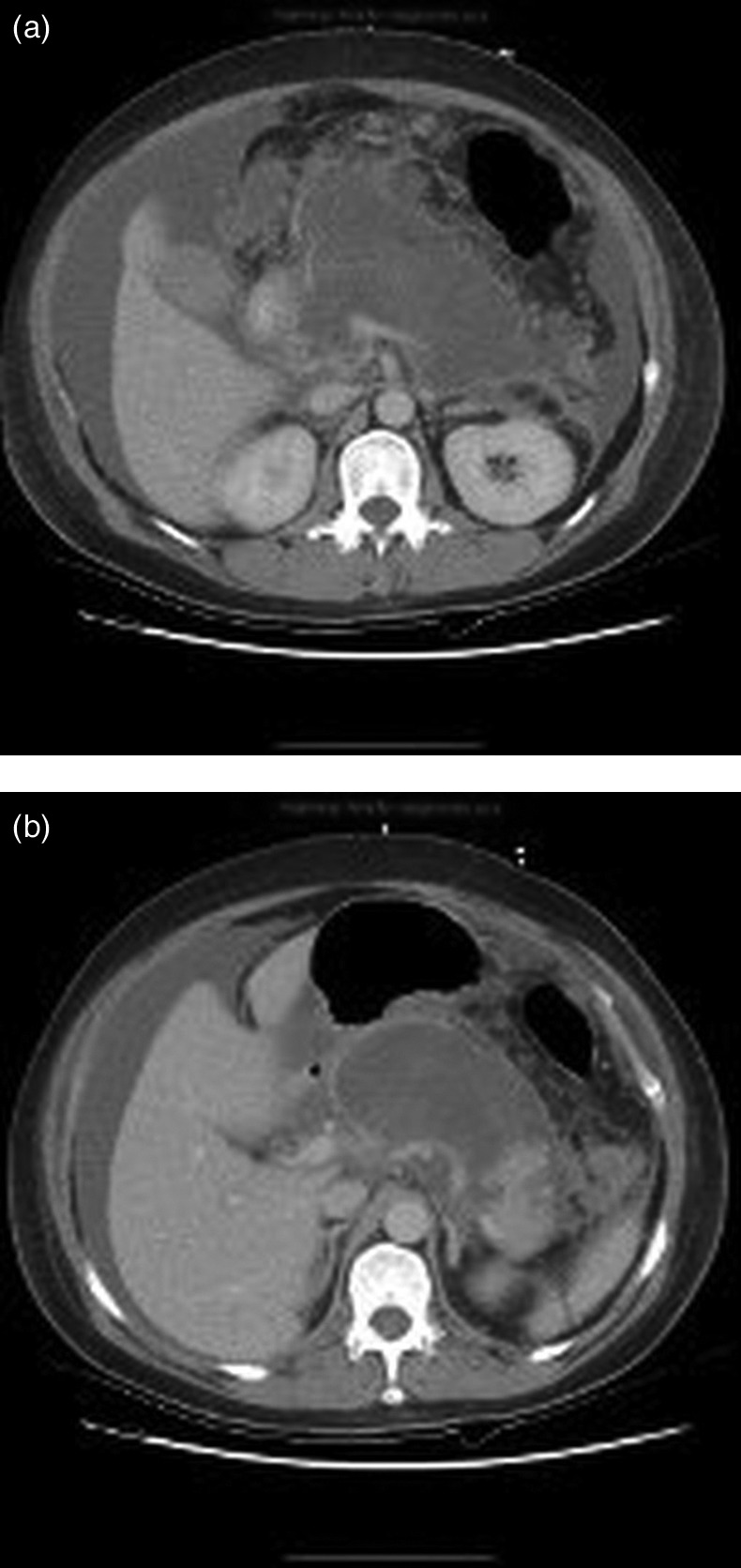
(A) Axial CT demonstrating necrosis of the pancreas with associated collection of only a portion of the uncinate. (B) The distal tail remained perfused.
She was managed in the intensive care unit with full support from medical, surgical, dietician, physiotherapist, and other supportive therapies. She improved and was discharged home on day 41. There was no history of alcohol excess, and no definite cause for the pancreatitis was found. She did have a past medical history of hyperlipidemia and was receiving atorvastatin, but her lipid profile and parathyroid hormone were normal.2
A follow-up ultrasound demonstrated an 11×7.5 cm pseudocyst. While awaiting endoscopic ultrasound she presented (at 18 weeks) with marked ascites. Drainage of ascites was undertaken which resolved her acute symptoms. The paracentesis demonstrated chylous ascites. The color of the ascites was milky, with an elevated triglyceride level of 3538 mg/dL, and normal glucose and amylase levels. Total protein was 3.9 g/dL and a high leukocyte count, and all cultures were negative. The patient's biochemical profile was as follows: sodium 135 mmol/L, potassium 4 mmol/L, urea 4 mmol/L, creatinine 63 µmol/L, albumin 27 g/L, and amylase 84 U/L. Culture was negative, including for mycobacterium. No malignant cells were seen. Endoscopic ultrasound demonstrated a grossly abnormal pancreas with extensive parenchymal calcification, the visualized pancreatic duct appeared normal, and no stone in the pancreatic duct and thoracic duct appeared patent. Specialist opinion recommended total paracentesis by percutaneous aspiration of cyst. Chylous fluid (5 L) was drained under CT guidance (figure 3). This was carried out under CT guidance rather than gastrocystostomy as per the patient's preference, followed by dietary modification.
Figure 3.
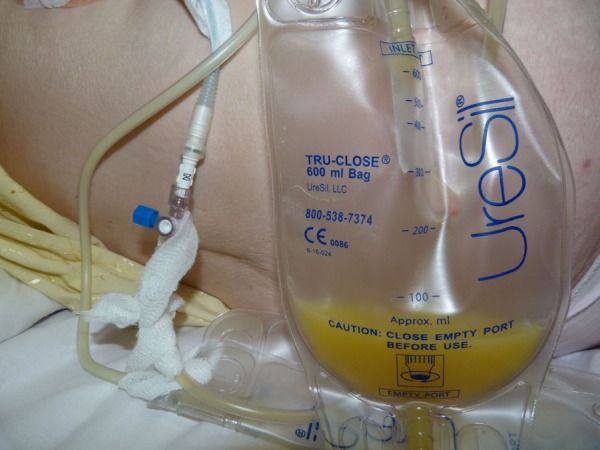
Chylous ascites draining.
Treatment
Initially the patient was commenced on a medium chain triglyceride (MCT) rich diet, but her adherence to this was, by her own account, less than optimal. Over the following 7 months, the patient presented on nine occasions to our service with gross ascites (and on one occasion to a hospital in France, where she was holidaying). The interval between presentations varied between 14 and 36 days (average ∼21 days), and on each occasion between 5 and 7 L of chylous fluid were aspirated; once the ascites were aspirated, the patient was anxious for discharge.
Outcome and follow-up
She was subsequently commenced on TPN with MCTs, and a somatostatin analog (lanreotide) for 6 weeks. This reduced her chylous ascites to such an extent that she has not required any further paracentesis.
Discussion
The word ascites is derived from the Greek word askos meaning bag or bladder.3 In developed countries, the commonest causes of chylous ascites are intra-abdominal malignancy (lymphomas) and cirrhosis. In developing countries, the commonest etiology is infection with tuberculosis and filariasis. Other etiologies are congenital, traumatic (including postoperative), and inflammatory causes, such as acute or chronic pancreatitis,4 5 or following radiotherapy. More rare causes which have been described include constrictive pericarditis (increases lymph production by increasing hepatic venous flow), retroperitoneal fibrosis,6 sarcoidosis,7 and Whipple's disease.8
In children, causes include congenital lymphatic abnormalities such as primary lymphatic hypoplasia, but obstructive or idiopathic lesions caused by malrotation, intussusception, lymphangioma, and incarcerated hernia can occur. Battered child syndrome must be excluded in children with chylous ascites as it is the underlying cause in up to 10% of pediatric cases.9
Three main pathophysiological mechanisms have been postulated for chylous ascites10:
obstruction of the flow of lymph fluid caused by external pressure (eg, a mass) leading to leakage from dilated subserosal lymphatic channels into the peritoneal cavity;
lymph exuding through the walls of dilated retroperitoneal vessels without valves, which leak fluid through a fistula into the peritoneal cavity, as in congenital lymphangectasia; and
traumatic obstruction of the thoracic duct causing direct leakage of lymph through a lymphoperitoneal fistula.
The treatment options are many and varied, and although the underlying cause should be addressed whenever possible, a conservative approach may be successful. If this is unsuccessful, patients are often prescribed a diet with high levels of proteins and low levels of lipids, and for these lipids to be exclusively in the form of MCTs to reduce peritoneal lymph flow.
The logic of limiting fat intake to MCTs is that by abstaining from long chain triglycerides, the production of monoglycerides and free fatty acids (formed when these are hydrolyzed) are avoided, which are carried as chylomicrons to the intestinal lymph ducts. MCTs, on the other hand, are absorbed directly by the intestinal mucosal cell and transported to the liver as free fatty acids and glycerol via the portal vein. This is to enable any chylous fistula to heal naturally. This has been shown to give at best a partial effect; two studies looking at outcomes in a pediatric population showed a limited response.11 12
There is some debate as to whether this is best delivered by oral dietary measures or by enteric rest with TPN. TPN has the advantage of counteracting the potential for dietary insufficiency (including any increased loss of fat and protein following repeated paracenteses), with the added advantage of associated fasting, which further reduces intestinal lymph flow.
Somatostatin is an inhibitory hormone which inhibits the release of growth hormone, thyroid stimulating hormone, and gastrointestinal hormones (such as cholecystokinin, vasoactive intestinal peptide, and gastrin), and also lowers the rate of gastric emptying as well as reducing smooth muscle contraction and blood flow within the gut. Octreotide and lanreotide are synthetic analogs of somatostatin, which decrease intestinal blood flow, thereby reducing lymph secretion through somatostatin receptors in the intestinal wall.13
Octreotide has previously been used to treat postpancreatitic chylous ascites in a report from Nova Scotia14 but there have been no reports of lanreotide being used successfully. However, the combination of both a somatostatin analog and TPN has been shown in a Chinese study to be successful, although the numbers involved were low.
Learning points.
We report a rare complication of a condition routinely seen in general surgical practice, which failed to respond to conservative measures, including dietary modification, but resolved following more aggressive therapy which necessitated fasting the patient for 6 weeks plus total parenteral nutrition and somatostatin analog.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Khan FY, Matar I. Chylous ascites secondary to hyperlipidemic pancreatitis with normal serum amylase and lipase. World J Gastroenterol 2007;13:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pepper OHP. Medical etymology. Philadelphia: Saunders, 1949 [Google Scholar]
- 3.Zhang ZY, Howard JM. Chylous ascites. A late complication of massive peripancreatic necrosis. Int J Pancreatol 1997;21:259–61 [DOI] [PubMed] [Google Scholar]
- 4.Khan FY, Matar I. Chylous ascites secondary to hyperlipidemic pancreatitis with normal serum amylase and lipase. World J Gastroenterol 2007;13:480–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilkeson GS, Allen NB. Retroperitoneal fibrosis. A true connective tissue disease. Rheum Dis Clin North Am 1996;22:23–38 [DOI] [PubMed] [Google Scholar]
- 6.Cappell MS, Friedman D, Mikhail N. Chyloperitoneum associated with chronic severe sarcoidosis. Am J Gastroenterol 1993;88:99–101 [PubMed] [Google Scholar]
- 7.Isenberg JI, Gilbert SB, Pitcher JL. Ascites with peritoneal involvement in Whipple's disease. Report of a case. Gastroenterology 1971;60:305–10 [PubMed] [Google Scholar]
- 8.Beshay VE, Beshay JE, Rosenberg AJ. Chylous ascites: a case of child abuse and an overview of a rare condition. J Pediatr Gastroenterol Nutr 2001;32:487–9 [DOI] [PubMed] [Google Scholar]
- 9.Browse NL, Wilson NM, Russo F, et al. Aetiogy and treatment of chylous ascites. Br J Surg 1992;79:1145–50 [DOI] [PubMed] [Google Scholar]
- 10.Unger SW, Chandler JG. Chylous ascites in infants and children. Surgery 1983;93:455–61 [PubMed] [Google Scholar]
- 11.Man DW, Spitz L. The management of chylous ascites in children. J Pediatr Surg 1985;20:72–5 [DOI] [PubMed] [Google Scholar]
- 12.Bhatia C, Pratap U, Slavik Z. Octreotide therapy: a new horizon in treatment of iatrogenic chyloperitoneum. Arch Dis Child 2001;85:234–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Ghamdi MY, Bedi A, Reddy SB, et al. Chylous ascites secondary to pancreatitis: management of an uncommon entity using parenteral nutrition and octreotide. Dig Dis Sci 2007 [DOI] [PubMed] [Google Scholar]
- 14.Huang Q, Jiang ZW, Jiang J, et al. Chylous ascites: treated with total parenteral nutrition and somatostatin. World J Gastroenterol 2004;10:2588–91 [DOI] [PMC free article] [PubMed] [Google Scholar]


