Abstract
CD8 T-cell neurological complications are a new HIV-driven condition caused by an unusually intense inflammatory reaction with influx of CD8 lymphocytes in the nervous system. Encephalitis and neuropathies have been described. We report the first case of spinal cord involvement. A 52-year-old African woman with HIV infection not profoundly immunosuppressed, and with a low plasmatic viral replication, without antiretroviral therapy, presented with transverse myelitis. Spinal MRI revealed inflammatory intraspinal gadolinium-enhanced lesions. Exhaustive workup was negative and brain biopsy revealed a significant inflammatory reaction with abundant CD8 T cells. Intravenous pulse methylprednisolone treatment led to rapid, disease-free recovery. CD8 T cells transverse myelitis in patients with HIV infection receiving antiretroviral therapy is a clinical entity that should be added to the list of HIV complications.
Background
Neurological CD8 T-lymphocyte complications are HIV-driven inflammatory conditions affecting the nerves1 and the brain.2–5 Spinal cord involvement in HIV infection is usually observed in cases of severe immunosuppression, the most common causes being the vacuolar myelopathy that is frequently concomitant with HIV-associated dementia, opportunistic infections and tumours.6 Since the introduction of combined antiretroviral therapies (cART), the incidence of spinal cord diseases has dramatically decreased.6 Our description of the first case of CD8 T cell transverse myelitis enhances the spectrum of this new neurological complication in patients with usually satisfactory indices of HIV control.3 5 As it can be healed with rapid glucocorticoid treatment and optimal control of HIV replication, this clinical condition should be rapidly diagnosed.3
Case presentation
A 52-year-old African woman was diagnosed with primary HIV infection in 2000. After an initial treatment with stavudine, didanosine and nelfinavir leading to the control of the infection, these were discontinued in 2002. From 2002 to 2007, she had rather good HIV control indices (CD4 counts of 500–600/μL and plasmatic HIV viral load (plVL) ≤1000 copies/mL), without cART. In June 2007, she was admitted for subacute paraparesis with a T12 sensory level and bilateral leg weakness responsible for walking difficulties and falls. Neurological symptoms had appeared 2 weeks earlier with burning bilateral plantar pain that gradually increased, climbing up the legs to the groin in a few days. A month before admission she had diarrhoea due to Shigella spp, which was rapidly cured by sulfamethoxazole-trimethoprime. At the onset of the neurological symptoms, the CD4 count dropped to 409/μL, the CD8 count was 1807/μL and the plVL raised to 7500 copies/mL.
Investigations
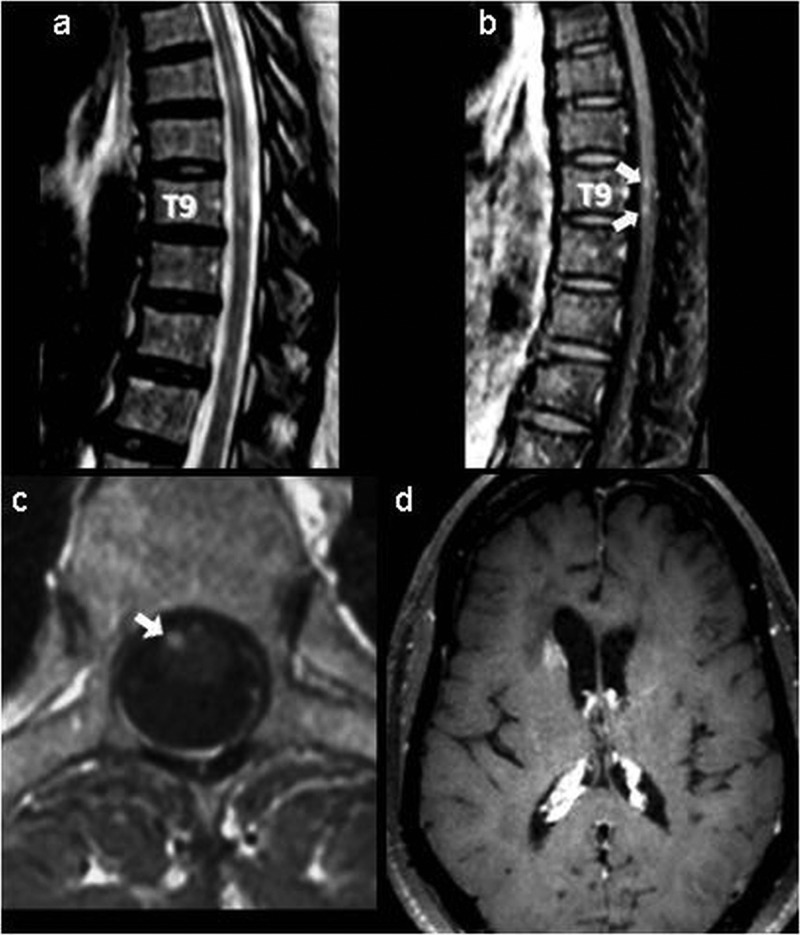
Spinal MRI showed multiple intraspinal lesions on T2-weighted images (figure 1A), some displaying gadolinium enhancement (figure 1B,C). The lumbar puncture showed normal opening pressure, normal glucose assays, mildly elevated protein (62 mg/dL), a lymphocyte count of 200/mm3, with 82% normal CD8 T cells and no abnormal cells. The cerebrospinal fluid (CSF) VL was 72 copies/mL and the concomitant plVL was 832 copies/mL. Brain MRI revealed patchy gadolinium enhancement (figure 1D). A thorough workup being unremarkable, including CSF stains and culture for virus, fungus and bacteria, and PCR for cytomegalovirus, varicella zoster virus, herpes simplex virus-1 and 2, Epstein-Barr virus and ACE, antinuclear antibodies against extractable nuclear antigens, and antidouble-standed DNA, the patient underwent a brain biopsy that revealed typical CD8 encephalitis (CD8-E) with abundant normal CD8 lymphocytes as well as numerous CD4 lymphocytes as comprehensively described elsewhere.4 Analysis for T-cell receptor-γ gene rearrangement was negative.
Figure 1.
Spinal MRI: (A) sagittal T2-weighted, (B) postgadolinium T1-weighted images and (C) axial postgadolinium T1-weighted images. Arrows indicate multiple intramedullary T2 high-signal intensity lesions, some of them at the T9 level, displaying T1 enhancement. (D) Brain MRI postgadolinium T1-weighted image. The arrowhead indicates patchy enhancement of the head of the right caudate nucleus.
Treatment
Intravenous methylprednisolone (1 g daily for 5 days) was started as soon as the diagnosis was confirmed by biopsy, 24 days after the onset of first neurological features, with a tapering administration of prednisone by mouth (1 mg/kg/day) for 2 months. cART was started 3 weeks after the initiation of corticosteroid therapy, with zidovudine, lamivudine and lopinavir/ritonavir.
Outcome and follow-up
The patient improved in less than 1 week with corticosteroid therapy, and was able to walk within a month. Six months later, she was totally asymptomatic and neurological examination was normal. Spinal MRI revealed the persistence of high-signal lesions in T2-weighted sequences without gadolinium enhancement. In January 2013, while the patient was being treated with abacavir, lamivudine and raltegravir, the neurological examination was normal. MRI still showed some high-signal lesions in T2-weighted sequences without any contrast enhancement, and the CD4 count was 1190/μL, the CD8 count 1186/μL and the plVL <20 copies/mL.
Discussion
Acute transverse myelitis (ATM) is an inflammatory parainfectious complication affecting the spinal cord, preceded in 30–60% of cases by a systemic infectious process or vaccination.6 7 It is also observed in the context of various autoimmune disorders.7 Only a few cases of ATM have been related to primary HIV infection.8 Symptoms typically develop over hours to days and worsen over days to weeks.9 Exhaustive workup in our case, as recommended in such conditions,7 9 ruled out infections other than HIV, connective tissue diseases, multiple sclerosis or neoplasia. ATM can have devastating neurological consequences with up to two-thirds of patients having a moderate-to-severe degree of residual disability.9 Acute management of these patients is dictated by which aetiology is suspected and rapid initiation of that treatment portends a more favourable outcome.9
CD8-E is an immune-mediated syndrome triggered by a novel HIV presentation, favoured by the interruption of cART, virological escape or a concomitant minor infection.3 The cART-induced immune reconstitution inflammatory syndrome was thought to be the main trigger in the early descriptions of the disorder,2 but more than 40% of CD8-E cases are preceded by a minor infection that mediates the resurgence of transient HIV replication, as in our case.3 The hallmark of CD8 T-cell neurological complications is the intense angiocentric infiltration by CD8 cytotoxic T lymphocytes (CTL), which perturbs the delicate intraparenchymal balance of the CD8/CD4 T cells at the origin of the immunopathological reaction.4 5
CD8 CTL are increasingly recognised as potential drivers of neuronal damage in various inflammatory central nervous system (CNS) disorders.10 While they are essential for viral clearance in virus-induced encephalitis, they may also cause severe tissue-destructive immunopathology. Thus, Rasmussen's encephalitis is considered to be a prime example of CD8 T-cell-mediated encephalitis.10 Theiler's murine encephalomyelitis viral model established the important concept that viral persistence in the CNS can generate an autoimmune response, and highlighted the important role of the CD8 T-cell immune response in determining the balance between viral elimination and persistence.11 Even in patients with stabilised HIV infection, ongoing immunoactivation in the CNS due to low levels of viral replication seems to persist.12 An inflammatory response, once established, may give rise to an automaintained state of cellular activation. Autoreactivity of CD8 CTL may lead to CD8 myelopathy through a self-amplifying loop of CD8 T-cell-mediated CNS tissue destruction.4 6
Once infection has been ruled out, acute management of ATM is aimed at stopping the inflammatory process that is occurring in the spinal cord.9 The standard recommendation is the administration of high-dose intravenous methylprednisolone, started as early as possible.9 The acute administration of high-dose corticosteroids for CD8-E is also recommended.3 In our personal experience aggressive treatment early in the course of such CD8-mediated neurological complications was found to be crucial for a favourable response.3 13 All patients who died or had partial recovery with sequalae had delayed or no corticosteroid therapy.5 Since such cases may have been misdiagnosed in the past, we should take into account the present potential for neurological recovery offered by the proper management of these disorders. Prospective studies are currently under way to design the best therapeutic strategy.
Learning points.
CD8 T-cell neurological complications are HIV-driven, immune-mediated conditions, characterised by a massive perivascular infiltration of polyclonal CD8 lymphocytes.
Once another infection than HIV has been ruled out, CD8 myelitis manifests as an acute inflammatory myelitis with sensory alteration, weakness and a clear sensory level. Lumbar puncture discloses lymphocytic meningitis with a majority of CD8 T cells (>65%). Spinal MRI demonstrates tiny multiple intramedullary lesions displaying patchy gadolinium enhancement.
The overall prognosis of such complications is poor if diagnosis is delayed. CD8 myelitis can be healed with rapid glucocorticoid therapy and optimal control of HIV replication. CD8 transverse myelitis should be considered when managing controlled patients with HIV infection admitted for an acute myelitis.
Footnotes
Contributors: All the authors have seen, discussed, contributed significantly and agreed with the contents of the manuscript. AM, F-XL and JS drafted/revised the manuscript for content, including medical writing for content. PC made critical revision of the manuscript for important intellectual content.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Moulignier A, Authier FJ, Baudrimont M, et al. Peripheral neuropathy in human immunodeficiency virus-infected patients with the diffuse infiltrative lymphocytosis syndrome. Ann Neurol 1997;41:438–45 [DOI] [PubMed] [Google Scholar]
- 2.Miller RF, Isaacson PG, Hall-Craggs M, et al. Cerebral CD8+ lymphocytosis in HIV-1 infected patients with immune restoration induced by HAART. Acta Neuropathol 2004;108:17–23 [DOI] [PubMed] [Google Scholar]
- 3.Lescure F-X, Moulignier A, Savatovsky J, et al. CD8 encephalitis in HIV-infected patients receiving cART: a treatable entity. Clin Infect Dis 2013;57:101–8 [DOI] [PubMed] [Google Scholar]
- 4.Gray F, Lescure F-X, Adle-Biassette H, et al. Encephalitis with infiltration by CD8+ lymphocytes in HIV patients receiving combined antiretroviral treatment. Brain Pathol 2013;23:525–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucas S, Wong KT, Mahadeva U. Fatal CD8+ T cell encephalitis: an observational autopsy study of an emerging complication in HIV-1-infected African patients on effective long-term ART. 18th Conference on Retroviruses and Opportunistic Infections; Boston, 2011:398 [Google Scholar]
- 6.Cho TA, Vaitkevicius H. Infectious myelopathies. Continuum (Minneap Minn) 2012;18:1351–73 [DOI] [PubMed] [Google Scholar]
- 7.Transverse Myelitis Consortium Working Group Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 2002;59:499–505 [DOI] [PubMed] [Google Scholar]
- 8.Hamada Y, Watanabe K, Aoki T, et al. Primary HIV infection with acute transverse myelitis. Intern Med 2011;50:1615–17 [DOI] [PubMed] [Google Scholar]
- 9.West TW, Hess C, Cree BA. Acute transverse myelitis: demyelinating, inflammatory, and infectious myelopathies. Semin Neurol 2012;32:97–113 [DOI] [PubMed] [Google Scholar]
- 10.Melzer N, Meuth SG, Wiendl H. CD8+ T cells and neuronal damage: direct and collateral mechanisms of cytotoxicity and impaired electrical excitability. FASEB J 2009;23:3659–73 [DOI] [PubMed] [Google Scholar]
- 11.Fazakerley JK, Walker R. Virus demyelination. J Neurovirol 2003;9:148–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gisolf EH, van Praag RM, Jurrians S, et al. Increasing cerebrospinal fluid chemokine concentrations despite undetectable cerebrospinal fluid HIV RNA in HIV-1-infected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr 2000;25:426–33 [DOI] [PubMed] [Google Scholar]
- 13.Venkataramana A, Pardo CA, McArthur JC, et al. Immune reconstitution inflammatory syndrome in the CNS of HIV-infected patients. Neurology 2006;67:383–8 [DOI] [PubMed] [Google Scholar]