Abstract
Tuberculosis is the most common chronic central nervous system infection in developing countries like India. Non-osseous spinal cord involvement is a rare manifestation of tuberculosis. The use of MRI, as an imaging modality of choice, has revolutionised the imaging of tuberculomas with reasonable certainty and thereby avoiding unnecessary surgical intervention. We report an unusual presentation of intradural extramedullary and intracranial tuberculomas with communicating syringomyelia complicated with tubercular meningitis.
Background
Non-osseous spinal cord involvement is a rare manifestation of tuberculosis (TB). There has been a frequent mention of intramedullary tuberculomas in the literature;1 2 but tubercular meningitis (TBM), although relatively common, is rare in association with syringomyelia and further presence of intradural extramedullary (IDEM) tuberculoma. The proposed mechanism of syringomyelia formation is inflammatory arachnoiditis and ischaemic myelomalacia secondary to inflammatory occlusion of the spinal cord vessels. Focal scarring causes a block in the cerebrospinal fluid (CSF) circulation, thus forcing the CSF into the central canal of the spinal cord via Virchow-Robin spaces.3 Syrinx formation is thought to be secondary to either an obliterative endarteritis causing ischaemic injury and softening or postinflammatory scarring which leads to spinal block4 5 thereby producing an increase in relative pressure inside the spinal cord. Repetitive formation of pressure gradient at the end of CSF pulse from central canal leads to leakage of CSF and interstitial oedema, which further increases the size of syrinx. Pathologically, syringomyelia can be divided into communicating and non-communicating types.6 In communicating type there is a connection of central canal of spinal cord and the fourth ventricle, which was evident in MRI of our patient.
Case presentation
A 23-year-old man presented with cervical pain followed by asymmetric, progressive weakness involving initially both upper limbs and then lower limbs and tightness around chest for the past 4 years. Weakness was followed by wasting of both upper limbs distally involving both the hands. Furthermore, this was followed by progressive difficulty in clearing both feet from the ground with frequent tripping. Prior to this he had a history of low-grade fever with chronic headache and vomiting for which he underwent a lumbar puncture, which revealed increased CSF opening pressure of 300 mm H2O, elevated protein content and cellular response with lymphocytic pleocytosis. He was prescribed antitubercular treatment, but was non-compliant and discontinued the medication after 4 months. On examination, the patient was fully conscious and oriented, cranial nerves and bilateral fundus examination was normal as well. The patient had asymmetrical (right>left) wasting of thenar and hypothenar muscles of both the hands and bilateral foot drop. Tone was increased except in distal lower limbs. The patient was ambulatory with distal predominant weakness in both upper and lower limbs. Deep tendon reflexes except of supinator and ankle bilaterally were exaggerated and plantars were extensor. The patient had dissociative sensory loss below C5 level, wherein there was impairment of pain and temperature with sparing of posterior column sensations. No cerebellar or meningeal signs were present and spinal examination was normal.
Investigations
Routine haematology and biochemistry was normal apart from raised erythrocyte sedimentation rate (50 mm). Chest X-ray, vasculitis profile, HIV serology, ECG and CSF analysis were normal as well. Brain MRI (figure 1A–D) showed gross communicating hydrocephalus with ring enhancing lesion on the right side of basal cistern. Cervicodorsal spine MRI T1-weighted sagittal (figure 2A) sections showed cervicomedullary syrinx with communication with fourth ventricle; and postcontrast sagittal and axial (figure 2B–E) sections showed ring enhancing granulomas at C2–3 and D5–6 vertebral levels. Dorsolumbar spine MRI (figure 3A–C) T2-weighted sagittal and axial sections showed syringomyelia extending until the conus with ring enhancing lesion opposite to D7–10 vertebra suggestive of tuberculomas (figure 3A–C). In the meantime, his reports on CSF for TB PCR were positive and CSF culture by BACTEC 460 TB method was positive for Mycobacterium tuberculosis as well.
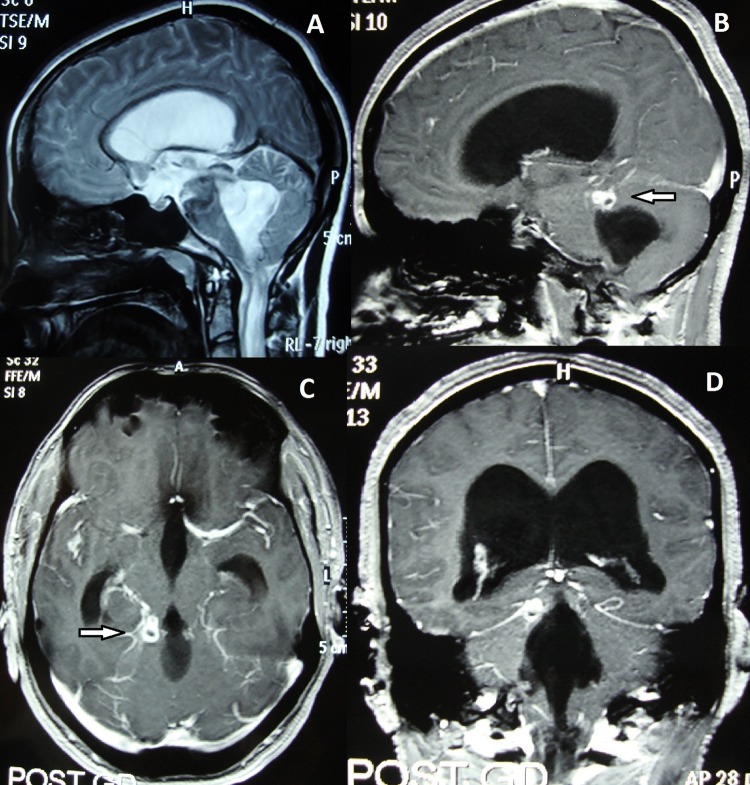
Figure 1.
(A) Brain MRI T2-weighted sagittal sections showing dilation of lateral and fourth ventricles with communication with cervical syrinx. (B–D) Brain MRI postcontrast sagittal, axial and coronal images showing dilation of lateral and fourth ventricles with ring enhancing conglomerate granulomas at right basal cisterns (white arrows).
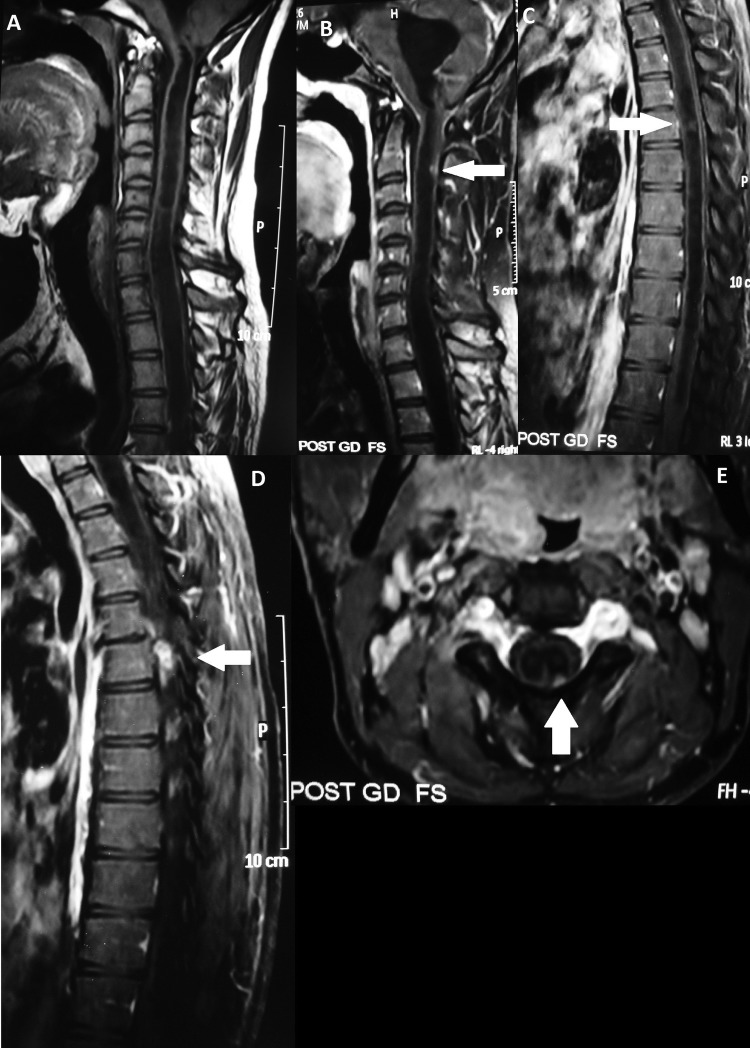
Figure 2.
(A) Cervical spine MRI T1-weighted sagittal images showing syringomyelia with communication with fourth ventricle. (B–D)Cervical spine MRI T1-weighted postcontrast images showing communicating syrinx cavity from cervicomedullary junction until lower dorsal cord with multiple ring enhancing granulomas at the C2–3 and D5–6 vertebral levels (white arrows). (E) Cervical spine MRI T1-weighted postcontrast axial section showing intradural extramedullary ring enhancing granuloma (white arrows).
Figure 3.
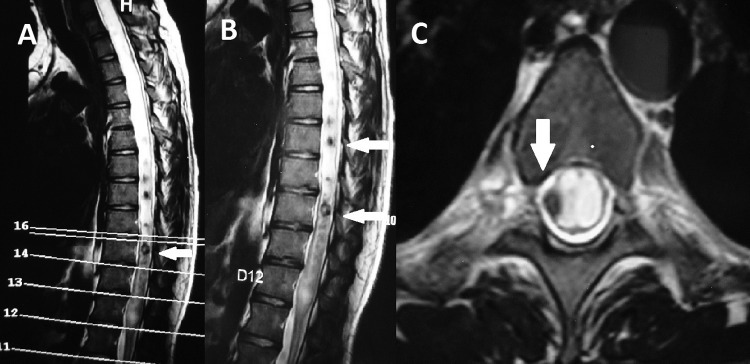
(A and B) Dorsolumbar spine MRI T2-wieghted sagittal images showing syringomyelia extending until D12 vertebral level with multiple inflammatory granulomas with hypointense core from D7 to D10 vertebrae (white arrows). (C) Dorsal spine MRI T2-weighted axial image showing extensive dilation of central canal and intradural extramedullary granuloma with hypointense core (white arrows).
Differential diagnosis
Neurocysticercosis
Meningioma
Lipoma
Treatment
The patient was then put on a five drug daily antitubercular regimen including isoniazid (5 mg/kg), rifampin (10 mg/kg), ethambutol (15–20 mg/kg), pyrazinamide (15–30 mg/kg) and streptomycin (15 mg/kg) with adjunctive corticosteroids. Initially, intravenous dexamethasone was given at a dose of 0.4 mg/kg/day which was subsequently reduced by 0.1 mg/kg on weekly basis. After the fourth week oral dexamethasone was prescribed which was subsequently reduced by 1 mg each week over 3 weeks. Five-drug therapy was continued for 2 months initially and then switched over to continuation phase comprising isoniazid and rifampin for a total of 12 months.
Outcome and follow-up
The patient was unwilling for any neurosurgical intervention for syrinx; however, he did well on follow-up visits with reduction in frequency and intensity of headaches. The patient had no improvement in his residual weakness but remained ambulatory.
Discussion
TB is an important disease with increasing incidence especially in the developing countries. IDEM tuberculoma presents with subacute-to-chronic cord compression. Most of the cases7 8 develop during or following the treatment. Cascino and Dibble9 postulated that a tubercle forms in the cord, ruptures into the subarachnoid space and causes chemical meningitis. The patient may respond to the treatment, but during recovery a tuberculoma forms at the site of the original lesion and later produces cord compression. Similarly, our patient also had a history of meningitis which was treated with antitubercular drug treatment, and after 4 months developed multiple tuberculomas in neuraxis. TBM is a well-known extrapulmonary complication, but syringomyelia has been a rare complication with a few sporadic reports.3 10 Also, IDEM tuberculomas constitutes only 1% of spinal tuberculomas.5 Even in developing countries where a variety of complications to TB are encountered, IDEM tuberculomas are still a rare entity.11–13 To the best of our knowledge, IDEM tuberculoma and concurrent syringomyelia after treatment for TBM have been reported so far in only two case reports. Hui et al4 reported a young immunocompetent woman who developed TBM with hydrocephalus. Their patient was treated with antitubercular drugs and ventriculoperitoneal shunting was carried out. Subsequently, she developed paraparesis with spine MRI revealing spinal arachnoiditis, extramedullary granulomas and extensive syringomyelia. Gul et al14 also reported similar findings in a young man developing progressive paraparesis within 3 months of antitubercular therapy for TBM. Our patient was diagnosed as TBM but was non-compliant to antitubercular drugs and subsequently developed progressive quadriparesis with communicating syringomyelia and IDEM tuberculomas.
Muthukumar and Sureshkumar15 reported a young woman developing paraparesis after receiving antitubercular therapy for TBM, where MRI revealed extensive syrinx from C2 to conus medullaris with IDEM tuberculoma. In our patient's hydrocephalus, intracranial tuberculoma were seen along with extensive syrinx and IDEM tuberculomas.
Thus, there is a possibility that our patient during initial treatment for TBM with antitubercular regimen developed intracranial and IDEM tuberculomas which might have resulted in obliterative endarteritis and postinflammatory scarring producing a relative increase in CSF pressures within the cord resulting in an extensive syrinx.
Ideal treatment of syringomyelia has always been a region of debate. Various surgical procedures like syringostomy or syringe-peritoneal shunting have been tried with variable results. The success of procedures has always been dubious because of the presence of arachnoiditis at different spinal levels in TBM. We thus preferred conservative management in our patient with antitubercular drugs and corticosteroids.
We conclude that for central nervous system TB, neuroimaging of entire neuraxis is warranted to look for the presence of spinal tuberculomas and syrinx, and timely treatment can possibly avoid complications with a significant morbidity.
Learning points.
Central nervous system tuberculosis is common in developing countries, but intradural extramedullary and intracranial tuberculomas with extensive syringomyelia is an extremely rare co-occurrence.
Neuroimaging of entire neuraxis is warranted to look for spinal tuberculomas and syrinx in tubercular patients with meningitis.
MRI is the neuroimaging modality of choice which can detect tuberculomas with reasonable certainty.
Obliterative endarteritis and postinflammatory scarring due to tuberculoma results in relative rise in cerebrospinal fluid pressures within the cord thereby resulting in syrinx formation.
Footnotes
Contributors: On behalf of her coauthors BS takes sole responsibility for the content of this manuscript and all the coauthors were equally involved in developing the content of the manuscript and literature.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ramdurg SR, Gupta DK, Suri A, et al. Spinal intramedullary tuberculosis: a series of 15 cases. Clin Neurol Neurosurg 2009;111:115–18 [DOI] [PubMed] [Google Scholar]
- 2.Sharma MC, Arora R, Deol PS, et al. Intramedullary tuberculoma of the spinal cord: a series of 10 cases. Clin Neurol Neurosurg 2002;104:279–84 [DOI] [PubMed] [Google Scholar]
- 3.Kaynar MY, Koçer N, Gençosmanoğlu BE, et al. Syringomyelia—as a late complication of tuberculous meningitis. Acta Neurochir 2000;142:935–9 [DOI] [PubMed] [Google Scholar]
- 4.Hui AC, Chan YL, Kay R. Syrinx and tuberculoma formation in tuberculous arachnoiditis. Can J Neurol Sci 2001;28:148–9 [DOI] [PubMed] [Google Scholar]
- 5.Caplan LR, Norohna AB, Amico LL. Syringomyelia and arachnoiditis. J Neurol Neurosurg Psychiatry 1990;53:106–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoodley MA, Jones NR, Yang L, et al. Mechanisms underlying the formation and enlargement of non communicating syringomyelia: experimental studies. Neurosurg Focus 2000;8:E2. [DOI] [PubMed] [Google Scholar]
- 7.Cruickshank GS, Johnston RA. Intradural, extramedullary spinal cord compression from tuberculous granuloma. Br J Neurosurg 1996;10:93–5 [DOI] [PubMed] [Google Scholar]
- 8.Skendros P, Kamaria F, Kontopoulos V, et al. Intradural, extramedullary tuberculoma of the spinal cord as a complication of tuberculous meningitis. Infection 2003;31:115–17 [DOI] [PubMed] [Google Scholar]
- 9.Cascino J, Dibble JB. Tuberculoma of spinal cord. J Am Med Assoc 1956;162:461–2 [DOI] [PubMed] [Google Scholar]
- 10.Tiamkao S, Tanapaisal C, Kanpittaya J, et al. Syringomyelia as a complication of tuberculous meningitis. J Med Assoc Thai 2001;84:125–9 [PubMed] [Google Scholar]
- 11.Mathuriya SN, Khosla VK, Banerjee AK. Intradural extramedullary tuberculous spinal granulomas. Clin Neurol Neurosurg 1988;90:155–8 [DOI] [PubMed] [Google Scholar]
- 12.Natarajan M. Intraspinal granulomas. Neurol India 1974;22:163–8 [PubMed] [Google Scholar]
- 13.Makkar G, Srivastava A, Aggarwal AK. Intradural extramedullary spinal tuberculoma—an uncommon entity. Indian J Radiol Imaging 2003;13:103–4 [Google Scholar]
- 14.Gul S, Celebi G, Kalayci M, et al. Syringomyelia and intradural extramedullary tuberculoma of the spinal cord as a late complication of tuberculous meningitis. Turk Neurosurg 2010;20:561–5 [DOI] [PubMed] [Google Scholar]
- 15.Muthukumar N, Sureshkumar V. Concurrent syringomyelia and intradural extramedullary tuberculoma as late complications of tuberculous meningitis. J Clin Neurosci 2007;14:1225–30 [DOI] [PubMed] [Google Scholar]