Abstract
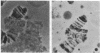
An x-ray-dependent mutator on chromosome 3 of Drosophila melanogaster is described that specifically increases the recovery of deletions for chromosomal tip regions. Such deficiencies can be induced on any chromosome. More centromere proximal mutations, as assayed by the sex-linked recessive lethal test, are not increased over the wild-type control. As far as can be determined by genetic, cytological, and molecular assays, the deletions extend to the very end of the chromosome involved. In addition, the frequency of these deletions is directly proportional to x-ray dose, suggesting that they are one-break rearrangements. It is proposed that the mutator is blocked in a major pathway for the repair of DNA double-strand breaks, and that a minor repair pathway is responsible for the addition of new telomeres under these conditions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedbrook J. R., Jones J., O'Dell M., Thompson R. D., Flavell R. B. A molecular description of telometic heterochromatin in secale species. Cell. 1980 Feb;19(2):545–560. doi: 10.1016/0092-8674(80)90529-2. [DOI] [PubMed] [Google Scholar]
- Boswell R. E., Klobutcher L. A., Prescott D. M. Inverted terminal repeats are added to genes during macronuclear development in Oxytricha nova. Proc Natl Acad Sci U S A. 1982 May;79(10):3255–3259. doi: 10.1073/pnas.79.10.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. S., Tye B. K. Organization of DNA sequences and replication origins at yeast telomeres. Cell. 1983 Jun;33(2):563–573. doi: 10.1016/0092-8674(83)90437-3. [DOI] [PubMed] [Google Scholar]
- Dawson D., Herrick G. Telomeric properties of C4A4-homologous sequences in micronuclear DNA of Oxytricha fallax. Cell. 1984 Jan;36(1):171–177. doi: 10.1016/0092-8674(84)90086-2. [DOI] [PubMed] [Google Scholar]
- De Lange T., Borst P. Genomic environment of the expression-linked extra copies of genes for surface antigens of Trypanosoma brucei resembles the end of a chromosome. Nature. 1982 Sep 30;299(5882):451–453. doi: 10.1038/299451a0. [DOI] [PubMed] [Google Scholar]
- Green M. M. Genetic instability in Drosophila melanogaster: De novo induction of putative insertion mutations. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3490–3493. doi: 10.1073/pnas.74.8.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. M., Lefevre G., Jr The cytogenetics of mutator gene-induced X-linked lethals in Drosophila melanogaster. Mutat Res. 1972 Sep;16(7):59–64. doi: 10.1016/0027-5107(72)90064-4. [DOI] [PubMed] [Google Scholar]
- King B. O., Yao M. C. Tandemly repeated hexanucleotide at Tetrahymena rDNA free end is generated from a single copy during development. Cell. 1982 Nov;31(1):177–182. doi: 10.1016/0092-8674(82)90417-2. [DOI] [PubMed] [Google Scholar]
- Lefevre G. The distribution of randomly recovered X-ray-induced sex-linked genetic effects in Drosophila melanogaster. Genetics. 1981 Nov-Dec;99(3-4):461–480. doi: 10.1093/genetics/99.3-4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J. K., Snyder L. A. The mutagenic effects of two monofunctional alkylating chemicals on mature spermatozoa of drosophila. Mutat Res. 1968 Jul-Aug;6(1):129–137. doi: 10.1016/0027-5107(68)90109-7. [DOI] [PubMed] [Google Scholar]
- Lindsley D. L., Sandler L., Baker B. S., Carpenter A. T., Denell R. E., Hall J. C., Jacobs P. A., Miklos G. L., Davis B. K., Gethmann R. C. Segmental aneuploidy and the genetic gross structure of the Drosophila genome. Genetics. 1972 May;71(1):157–184. doi: 10.1093/genetics/71.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock B. The Behavior in Successive Nuclear Divisions of a Chromosome Broken at Meiosis. Proc Natl Acad Sci U S A. 1939 Aug;25(8):405–416. doi: 10.1073/pnas.25.8.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock B. The Fusion of Broken Ends of Chromosomes Following Nuclear Fusion. Proc Natl Acad Sci U S A. 1942 Nov;28(11):458–463. doi: 10.1073/pnas.28.11.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock B. The Stability of Broken Ends of Chromosomes in Zea Mays. Genetics. 1941 Mar;26(2):234–282. doi: 10.1093/genetics/26.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Szostak J. W. Construction of artificial chromosomes in yeast. Nature. 1983 Sep 15;305(5931):189–193. doi: 10.1038/305189a0. [DOI] [PubMed] [Google Scholar]
- Novitski E., Grace D., Strommen C., Puro J. Terminal chromosome attachments. Am J Hum Genet. 1981 Jan;33(1):55–60. [PMC free article] [PubMed] [Google Scholar]
- Novitski E., Grace D., Strommen C. The entire compound autosomes of Drosophila melanogaster. Genetics. 1981 Jun;98(2):257–273. doi: 10.1093/genetics/98.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts P. A. A cytogenetic analysis of x-ray induced "visible" mutations at the yellow locus of Drosophila melanogaster. Mutat Res. 1974 Feb;22(2):139–144. doi: 10.1016/0027-5107(74)90094-3. [DOI] [PubMed] [Google Scholar]
- Roberts P. A. In support of the telomere concept. Genetics. 1975 May;80(1):135–142. doi: 10.1093/genetics/80.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M. Isolation of a telomeric DNA sequence from Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1041–1046. doi: 10.1101/sqb.1978.042.01.104. [DOI] [PubMed] [Google Scholar]
- Strobel E., Dunsmuir P., Rubin G. M. Polymorphisms in the chromosomal locations of elements of the 412, copia and 297 dispersed repeated gene families in Drosophila. Cell. 1979 Jun;17(2):429–439. doi: 10.1016/0092-8674(79)90169-7. [DOI] [PubMed] [Google Scholar]
- Sutton E. Terminal Deficiencies in the X Chromosome of Drosophila Melanogaster. Genetics. 1940 Nov;25(6):628–635. doi: 10.1093/genetics/25.6.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Blackburn E. H. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982 May;29(1):245–255. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- Szostak J. W. Replication and resolution of telomeres in yeast. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1187–1194. doi: 10.1101/sqb.1983.047.01.134. [DOI] [PubMed] [Google Scholar]
- Yao M. C., Yao C. H. Repeated hexanucleotide C-C-C-C-A-A is present near free ends of macronuclear DNA of Tetrahymena. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7436–7439. doi: 10.1073/pnas.78.12.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B. S., Pession A., Traverse K. L., French C., Pardue M. L. Telomere regions in Drosophila share complex DNA sequences with pericentric heterochromatin. Cell. 1983 Aug;34(1):85–94. doi: 10.1016/0092-8674(83)90138-1. [DOI] [PubMed] [Google Scholar]
- Yudkin J. Sugar and disease. Nature. 1972 Sep 22;239(5369):197–199. doi: 10.1038/239197a0. [DOI] [PubMed] [Google Scholar]