Abstract
The progressive increase in the elderly population worldwide has resulted in higher numbers of individuals affected by age-associated diseases, such as neurodegenerative and heart diseases, metabolic impairment, or cancer, with the subsequent burden for national health systems. Therapeutic interventions aimed to increase the quality of life at advanced age are visualized as important demands for the future, both at the level of individuals and society. Novel advances in telomerase function from several independent laboratories have resulted in potential new therapeutic strategies which appear as promising new venues to prevent cellular and tissue dysfunction and organismal decline, thereby increasing the so-called “health span”. Here, we analyze these recent advances.
Ageing as the cause of disease
Ageing, previously though has an unmodifiable trait, is nowadays viewed as a dynamic process. Furthermore, aging is currently seen as a causative factor for tissue dysfunction and increased risk for developing various age-associated diseases, including cancer. This highlights the importance of understanding the molecular and genetic causes of aging for the developed world, which is experiencing a dramatic increase in the elderly population [1]. In particular, a better understanding of how ageing results in tissue dysfunction and/or cancer and how we can circumvent ageing-associated decline are important questions at the present time. It has been demonstrated that ageing could be modulated and respond to several biological pathways [2]. A number of these pathways are conserved in different species, demonstrating that ageing can involve common cellular processes, which are conserved over evolution. In particular, pathways involved in genome stability, nutrient sensing, oxidative damage balance, and growth, seem to be central in ageing modulation [2,3]. In this review, we will focus on the relatively recent notion that aging is produced by accumulation of DNA damage associated to cell division. In particular, we will discuss recent advances for health improvement in mammals (in particular laboratory mice), based on prevention of the accumulation of critically short telomeres, a particularly deleterious type of DNA damage which induces a persistent DNA damage response (DDR), leading to cell death and senescence at the cellular level, and to loss of regenerative capacity of tissues at the organismal level [4-6]. This review will examine what is known on the historical role of telomerase in ageing, paying special attention to recent works which undoubtedly demonstrate that ageing can be actually reversed (and not only retarded) through telomerase re-expression.
Telomerase, DNA damage, and ageing
Tissue degeneration occurs in long-lived organisms. The accumulation of dysfunctional cells, together with a limited regenerative capacity of tissues, is thought to determine the age-related decline of body organs [7,8] and, in some situations, settle a basis for cancer progression [9]. Dysfunctional cells, usually characterized by the presence of short telomeres, are a barrier for tumor progression when presenting an intact DNA damage response which directs cells for senescence or cell death [8,10,11], although recent evidence demonstrated that transient telomere dysfunction per se could promote chromosomal instability and carcinogenesis in telomerase-proficient mice [12]. If DNA damage response barriers are bypassed (for instance through deletion of p53 [11]) short telomere cells resume and accelerate transformation phenotypes. In this scenario, re-activation of telomerase further enables full malignancy [13].
Therapies that prevent the appearance or that decrease the number of damaged cells are therefore viewed as potentially effective in slowing the ageing progression. In this regard, increased gene dose of tumor suppressor genes that eliminate damage cells from the organism through apoptosis and senescence such as p53 and p16 are known to increase life span [14,15] furthermore, clearance of senescent cells from already-adult organisms also delays aging, thus confirming the involvement of damaged cells in tissue dysfunction [16]. Similarly, prevention of metabolic damage also increases health span, as recently shown for SIRT1 and PTEN gain of function mouse models [17-19]. Interestingly, a link between telomeres and mitochondrial function has been also proposed [20,21]. In particular, aging provoked by telomere-dysfunction leads to changes in key metabolic genes that involve a functional p53 and are characterized by a repression of PGC-1α. In turn, telomerase re-activation in old wild-type mice, results in increased PGC-1α levels [21]. These metabolic changes associated to telomere dysfunction could potentially synergize with the DNA damage response triggered by short telomeres and contribute to senescence and/or apoptosis, and the eventual organismal failure associated to the aging process. Here we will focus in strategies aimed to decrease the accumulation of persistent DNA damage associated to short/dysfunctional telomeres by using telomerase reactivation strategies, which has been extensively linked to organismal aging [4,22,23].
Telomerase phenotypes
Telomerase is a multiprotein complex encompassing a reverse transcriptase catalytic subunit (Tert) and an associated RNA component (Terc) [24]. Telomerase adds DNA repeats (TTAGGG in mammals) to chromosome ends, thereby counteracting telomere shortening associated to DNA replication (the so-called end-replication problem) or to DNA degrading activities [25-28]. Animal models with mutations in telomerase or telomere-associated proteins (shelterin) have been instrumental to unveil the role of telomeres in cancer and ageing [4-6,22,23,29-33]. In particular, knockout mice for Tert or Terc with critically short telomeres are characterized by an increased incidence of age-related diseases and premature tissue degeneration which mostly, but not only, affects tissues with elevated cellular turnover such as the bone marrow or the gastrointestinal system [34]. In this regard, a role for telomerase and telomere integrity in stem cell functionality has been shown for different adult stem cell niches, including the skin and the bone marrow [29,35-38]. In particular, some adult stem cell compartments are telomerase positive and present longer telomeres than the surrounding tissues [37, 39, 40]. Further supporting a role for stem cells in tissue functionality, mice with mutations directly affecting the pools of stem cells are characterized by accelerated aging [41]. Late generation Tert or Terc knockout mice present a decrease in mean telomere length and a higher percentage of short telomeres in several organs (including the pools of stem cells), which correlate with an incapacity of tissues to regenerate and result, ultimately, in an accelerated tissue degeneration and a concomitant decrease of the lifespan [5,6,42]. These seminal studies characterizing telomerase deficient mice have placed telomeres and telomerase as key elements for organismal aging. Further supporting it, there is recent evidence demonstrating that telomere size measured early in life is a bona fide predictor of lifespan in birds [43], and telomere dynamics seems to similarly correlate with the lifespan of laboratory mice (Vera E.; Bernardes de Jesus B.; Foronda M.; Blasco MA.; Cell Reports, In Press, [2012]).
Anti-ageing role of telomerase
Telomerase constitutive expression by using mouse transgenesis in adult tissues has pinpointed a role for telomerase in tissue fitness and prevention of aging, although at the expense of an slightly increased incidence of cancer [22,44,45], Importantly, when cancelling the increased cancer incidence associated to constitutive transgenic telomerase expression by generating telomerase transgenic mice in a cancer resistant background owing to increase gene dosage of tumors suppressor genes [p16, Arf and p53], this resulted in an improved extension of lifespan of 43% when comparing to the corresponding WT mice [22].
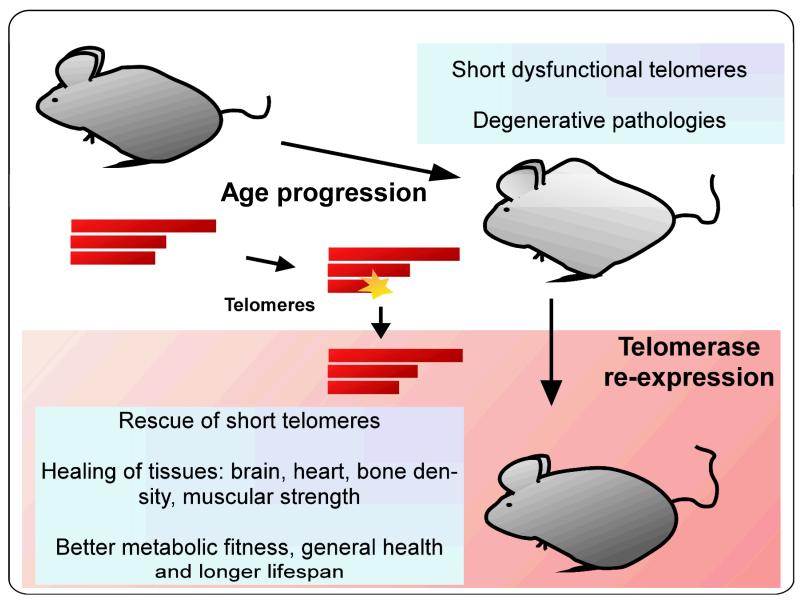
The cancer promoting activity of telomerase observed in the transgenic mouse models, however, is not apparent when telomerase is re-activated late in life. In particular, we have recently shown by using a gene therapy strategy with non-integrative adeno associated virus (AAV), that re-activation of telomerase in adult or old mice results in delayed aging and significant lifespan extension in the absence of increased cancer susceptibility [21]. A single telomerase (TERT) treatment of WT mice with these vectors was sufficient to rescue the age-dependent decline and to delay normal mouse physiological aging (Fig. 1). In this experimental setting, median lifespan was extended by up to 24% in 1-year-old mice, and by 13% in animals of 2 years of age. This study confirms that telomerase expression, by means of a gene therapy, could be considered a feasible approach to extend lifespan without increasing cancer incidence. Old mice treated with TERT showed a better skin and metabolic fitness and less bone loss after treatment, which are well characterized indicators of ageing progression. Moreover, TERT-treated mice showed an improved age-related impairment of balance and coordination and interestingly, a tendency for memory improvement. Telomerase has been proposed to have telomere-independent roles (independent of its catalytic activity) as a cofactor on the promoter of Wnt targeted genes [46], although questions have been raised about the relevance of this activation [42,47]. In this regard, when mice were treated with a catalytically dead TERT (TERT-DN [48]) the beneficial effects of TERT could not be reproduced and longevity was not increased, demonstrating that healthspan amelioration requires telomerase reverse transcriptase activity [21].
Fig.1. Rescue of age-dependent tissue degeneration in adult mice.
Therapies involving telomerase expression in adult tissues have demonstrated a potential impact in rescuing of age-associated degenerative pathologies [21,49]. Extension of short telomeres is one of the outcomes, but we cannot dismiss novel roles for telomerase in distinct networks [55].
Importantly, the safety of this type of strategy is illustrated by the fact that adult mice expressing TERT did not develop more cancer. This could be related to the fact that AAV vectors are non-integrative, leading to a loss of TERT expression in highly proliferating cells or tissues, such as cancer cells. Other explanation, could be the fact that AAV preferentially targets post-mitotic cells from peripheral tissues (of adult mice in that case), which are considered more refractory to cancer than the highly proliferative tissues. In this regard, telomerase re-introduction in an accelerated model of ageing involving accelerated telomerase loss (G4TERT-ER model) rescues the “ageing-phenotype” [49] without increasing cancer incidence. This could be related to the fact that cells lacking telomerase are resistant to cancer initiation [50], mimicking the tumor suppressor situation and, somehow, can preserve this characteristic after a telomerase pulse, even in the presence of a higher genomic instability [49]. These studies validate that telomerase could play important roles in tissue regeneration of adult organisms and are a proof-of-principle that ageing can be reversed and retarded. Moreover, normal aging comprises similar changes to those observed in aging produced by accelerated telomere shortening further linking telomere biology to the aging process. Novel therapeutic strategies involving telomerase expression could unveil potential mechanisms against tissue deterioration.
Chemical activators of telomerase are promising strategies nowadays. Some telomerase activators were assessed in the literature. TA-65, a single chemical compound extracted from Astragalus Membranaceus, was shown to activate telomerase in vitro and in vivo [51,52]. Adult mice supplemented with this compound presented an improved healthspan, in particular at the metabolic level. Previously (to the study in mice), data in humans demonstrated a better dynamics of the immune system (CD8+ T lymphocytes) from HIV-infected humans [51] and a similar increase in health-span of aged healthy costumers [53]. Recently, new compounds activators of telomerase have been described, for instance a novel telomerase activator (AGS-499) was demonstrated to play neuroprotective effects after NMDA treatment in mice and delayed the progression of amyotrophic lateral sclerosis (ALS) in SOD1 transgenic mice increasing their survival, further supporting a role for telomerase in tissue functionality [54].
These new findings open a new door in ageing research and degenerative healing. The modulation of telomerase and/or its associated “ageing-network” [55] in adult tissues establishes an important basis for ageing research and demonstrates that age-associated degeneration is a potential target of biomedical intervention. Further studies; in particular long-term follow ups, should be carried to assess adverse effects and to discriminate changes at the tissue-level.
Perspectives for a healthy life
The increase in the worldwide life expectancy was accompanied by intensification in age-associated diseases. Characterization of biomarkers and modulation of different pathways are candidates for a faster characterization of disease and discovery of novel therapeutics, respectively. In this aspect telomerase has been recently scrutinized as an anti-ageing factor. Several independent works demonstrated that telomerase expression through genetic modifications, viral delivery or chemical activation result in a significant rescue of age-related pathologies. These new results are exciting however additional efforts will be needed to translate these findings into actual therapies. This opens an unprecedented door for anti-aging research.
Acknowledgments
Funding Work at the Blasco lab was funded by Spanish Ministry of Science and Innovation Projects SAF2008-05384 and CSD2007-00017, European Union FP7 Projects 2007-A-201630 (GENICA) and 2007-A-200950 (TELOMARKER), European Research Council Advanced Grant GA#232854, Körber Foundation, Fundación Botín and Fundación Lilly.
Footnotes
Disclosures MAB is a co-founder of Life Length, S.L. a biotechnology company that commercializes telomere length tests.
References
- 1.Moslehi J, Depinho RA, Sahin E. Telomeres and mitochondria in the aging heart. Circ Res. 2012;110:1226–1237. doi: 10.1161/CIRCRESAHA.111.246868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vijg J, Campisi J. Puzzles, promises and a cure for ageing. Nature. 2008;454:1065–1071. doi: 10.1038/nature07216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 5.Hao LY, Armanios M, Strong MA, Karim B, Feldser DM, Huso D, Greider CW. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Armanios M, Alder JK, Parry EM, Karim B, Strong MA, Greider CW. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am J Hum Genet. 2009;85:823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 8.de Jesus BB, Blasco MA. Assessing cell and organ senescence biomarkers. Circ Res. 2012;111:97–109. doi: 10.1161/CIRCRESAHA.111.247866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DePinho RA. The age of cancer. Nature. 2000;408:248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg RA, Chin L, Femino A, Lee KH, Gottlieb GJ, Singer RH, Greider CW, DePinho RA. Short dysfunctional telomeres impair tumorigenesis in the INK4a(delta2/3) cancer-prone mouse. Cell. 1999;97:515–525. doi: 10.1016/s0092-8674(00)80761-8. [DOI] [PubMed] [Google Scholar]
- 11.Feldser DM, Greider CW. Short telomeres limit tumor progression in vivo by inducing senescence. Cancer Cell. 2007;11:461–469. doi: 10.1016/j.ccr.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Begus-Nahrmann Y, Hartmann D, Kraus J, Eshraghi P, Scheffold A, Grieb M, Rasche V, Schirmacher P, Lee HW, Kestler HA, et al. Transient telomere dysfunction induces chromosomal instability and promotes carcinogenesis. J Clin Invest. 2012;122:2283–2288. doi: 10.1172/JCI61745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding Z, Wu CJ, Jaskelioff M, Ivanova E, Kost-Alimova M, Protopopov A, Chu GC, Wang G, Lu X, Labrot ES, et al. Telomerase reactivation following telomere dysfunction yields murine prostate tumors with bone metastases. Cell. 2012;148:896–907. doi: 10.1016/j.cell.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matheu A, Maraver A, Klatt P, Flores I, Garcia-Cao I, Borras C, Flores JM, Vina J, Blasco MA, Serrano M. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375–379. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 15.Serrano M, Blasco MA. Cancer and ageing: convergent and divergent mechanisms. Nat Rev Mol Cell Biol. 2007;8:715–722. doi: 10.1038/nrm2242. [DOI] [PubMed] [Google Scholar]
- ** 16.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. The authors demonstrated that late-in-life clearance of senescent cells attenuate progression of already established age-related disorders. These results demonstrate the implication of cellular senescence in generating age-related phenotypes and that removal of senescent cells can prevent or delay tissue dysfunction.
- 17.Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:1–8. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 18.Ortega-Molina A, Efeyan A, Lopez-Guadamillas E, Munoz-Martin M, Gomez-Lopez G, Canamero M, Mulero F, Pastor J, Martinez S, Romanos E, et al. Pten positively regulates brown adipose function, energy expenditure, and longevity. Cell Metab. 2012;15:382–394. doi: 10.1016/j.cmet.2012.02.001. Here, the authors uncover a role of PTEN in promoting energy expenditure, thus decreasing nutrient storage and its associated damage. Mice overexpressing Pten present increased energy expenditure, protection from metabolic pathologies, protection from cancer and a significant extension of life span.
- * 19.Garcia-Cao I, Song MS, Hobbs RM, Laurent G, Giorgi C, de Boer VC, Anastasiou D, Ito K, Sasaki AT, Rameh L, et al. Systemic elevation of PTEN induces a tumor-suppressive metabolic state. Cell. 2012;149:49–62. doi: 10.1016/j.cell.2012.02.030. The authors described how PTEN elevation orchestrates a metabolic switch negatively impacting two of the most pronounced metabolic features of tumor cells: glutaminolysis and the Warburg effect.
- ** 20.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. The authors demonstrate that telomere dysfunction represses PGC-1α and PGC-1β promoters, thereby linking telomere and mitochondrial biology. They propose that this contributes to organ and metabolic failure and to the diminishing organismal fitness in the setting of telomere dysfunction, which is observed across many tissues including more quiescent systems.
- ** 21.Bernardes de Jesus B, Vera E, Schneeberger K, Tejera AM, Ayuso E, Bosch F, Blasco MA. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med. 2012;4:1–14. doi: 10.1002/emmm.201200245. Using a gene-therapy approach to express telomerase in adult and old mice, the authors describe a role for telomerase in delaying physiological aging and extending longevity in wt mice. This is the first study using a gene-therapy approach to counteract age decline.
- 22.Tomas-Loba A, Flores I, Fernandez-Marcos PJ, Cayuela ML, Maraver A, Tejera A, Borras C, Matheu A, Klatt P, Flores JM, et al. Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell. 2008;135:609–622. doi: 10.1016/j.cell.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Suarez E, Samper E, Ramirez A, Flores JM, Martin-Caballero J, Jorcano JL, Blasco MA. Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. EMBO J. 2001;20:2619–2630. doi: 10.1093/emboj/20.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 25.Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol. 2007;3:640–649. doi: 10.1038/nchembio.2007.38. [DOI] [PubMed] [Google Scholar]
- 26.Jiang H, Ju Z, Rudolph KL. Telomere shortening and ageing. Z Gerontol Geriatr. 2007;40:314–324. doi: 10.1007/s00391-007-0480-0. [DOI] [PubMed] [Google Scholar]
- 27.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canela A, Vera E, Klatt P, Blasco MA. High-throughput telomere length quantification by FISH and its application to human population studies. Proc Natl Acad Sci U S A. 2007;104:5300–5305. doi: 10.1073/pnas.0609367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flores I, Cayuela ML, Blasco MA. Effects of telomerase and telomere length on epidermal stem cell behavior. Science. 2005;309:1253–1256. doi: 10.1126/science.1115025. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 31.Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, Dokal I. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 32.Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell. 2010;143:1059–1071. doi: 10.1016/j.cell.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parry EM, Alder JK, Qi X, Chen JJ, Armanios M. Syndrome complex of bone marrow failure and pulmonary fibrosis predicts germline defects in telomerase. Blood. 2011;117:5607–5611. doi: 10.1182/blood-2010-11-322149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blasco MA. Telomeres in cancer and aging: lessons from the mouse. Cancer Lett. 2003;194:183–188. doi: 10.1016/s0304-3835(02)00705-x. [DOI] [PubMed] [Google Scholar]
- 35.Martinez P, Thanasoula M, Munoz P, Liao C, Tejera A, McNees C, Flores JM, Fernandez-Capetillo O, Tarsounas M, Blasco MA. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev. 2009;23:2060–2075. doi: 10.1101/gad.543509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tejera AM, Stagno d’Alcontres M, Thanasoula M, Marion RM, Martinez P, Liao C, Flores JM, Tarsounas M, Blasco MA. TPP1 is required for TERT recruitment, telomere elongation during nuclear reprogramming, and normal skin development in mice. Dev Cell. 2010;18:775–789. doi: 10.1016/j.devcel.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flores I, Canela A, Vera E, Tejera A, Cotsarelis G, Blasco MA. The longest telomeres: a general signature of adult stem cell compartments. Genes Dev. 2008;22:654–667. doi: 10.1101/gad.451008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beier F, Foronda M, Martinez P, Blasco MA. Conditional TRF1 knockout in the hematopoietic compartment leads to bone marrow failure and recapitulates clinical features of Dyskeratosis congenita. Blood. 2012 Aug 29; doi: 10.1182/blood-2012-03-418038. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korbling M, Estrov Z. Adult stem cells for tissue repair - a new therapeutic concept? N Engl J Med. 2003;349:570–582. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- 40.Jung P, Sato T, Merlos-Suarez A, Barriga FM, Iglesias M, Rossell D, Auer H, Gallardo M, Blasco MA, Sancho E, et al. Isolation and in vitro expansion of human colonic stem cells. Nat Med. 2011;17:1225–1227. doi: 10.1038/nm.2470. [DOI] [PubMed] [Google Scholar]
- 41.Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strong MA, Vidal-Cardenas SL, Karim B, Yu H, Guo N, Greider CW. Phenotypes in mTERT+/− and mTERT−/− Mice Are Due to Short Telomeres, Not Telomere-Independent Functions of Telomerase Reverse Transcriptase. Mol Cell Biol. 2011;31:2369–2379. doi: 10.1128/MCB.05312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 43.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. Telomere length in early life predicts lifespan. Proc Natl Acad Sci U S A. 2012;109:1743–1748. doi: 10.1073/pnas.1113306109. In this study authors tracked from early in life telomere length in zebra finches, and correlate it with the actual lifespan they survived. The authors found telomere length to be a very strong predictor of realized lifespan where those individuals living longest had relatively long telomeres. These results provide the strongest evidence of the relationship between telomere length and lifespan.
- 44.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Suarez E, Geserick C, Flores JM, Blasco MA. Antagonistic effects of telomerase on cancer and aging in K5-mTert transgenic mice. Oncogene. 2005;24:2256–2270. doi: 10.1038/sj.onc.1208413. [DOI] [PubMed] [Google Scholar]
- 46.Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, Chang W, Meng Z, Cheung P, Ji H, et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature. 2009;460:66–72. doi: 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greider CW. Molecular biology. Wnt regulates TERT--putting the horse before the cart. Science. 2012;336:1519–1520. doi: 10.1126/science.1223785. [DOI] [PubMed] [Google Scholar]
- 48.Sachsinger J, Gonzalez-Suarez E, Samper E, Heicappell R, Muller M, Blasco MA. Telomerase inhibition in RenCa, a murine tumor cell line with short telomeres, by overexpression of a dominant negative mTERT mutant, reveals fundamental differences in telomerase regulation between human and murine cells. Cancer Res. 2001;61:5580–5586. [PubMed] [Google Scholar]
- ** 49.Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadinanos J, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. Using a novel knock-in model carrying an allele encoding a 4-hydroxytamoxifen (4-OHT)-inducible telomerase reverse transcriptase-oestrogen receptor (TERT-ER) under transcriptional control of the endogenous TERT promoter, the authors demonstrated that telomerase reactivation in late generation TERT-ER mice (accelerated aged mice) extends telomeres, reduces DNA damage signaling and results in a marked reversal of systemic degenerative phenotypes characteristic of this adult mice model.
- 50.Gonzalez-Suarez E, Samper E, Flores JM, Blasco MA. Telomerase-deficient mice with short telomeres are resistant to skin tumorigenesis. Nat Genet. 2000;26:114–117. doi: 10.1038/79089. [DOI] [PubMed] [Google Scholar]
- 51.Fauce SR, Jamieson BD, Chin AC, Mitsuyasu RT, Parish ST, Ng HL, Kitchen CM, Yang OO, Harley CB, Effros RB. Telomerase-based pharmacologic enhancement of antiviral function of human CD8+ T lymphocytes. J Immunol. 2008;181:7400–7406. doi: 10.4049/jimmunol.181.10.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 52.de Jesus BB, Schneeberger K, Vera E, Tejera A, Harley CB, Blasco MA. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell. 2011;10:604–621. doi: 10.1111/j.1474-9726.2011.00700.x. Here, the authors describe that a small-molecule activator of telomerase (TA-65) is capable of increasing average telomere length and decreasing the percentage of critically short telomeres and of DNA damage in vitro and in vivo. TA-65 supplementation result in an improvement of certain health-span indicators. This study, together with references 21, 48 and 53 support the development of regenerative strategies designed to restore telomere integrity.
- 53.Harley CB, Liu W, Blasco M, Vera E, Andrews WH, Briggs LA, Raffaele JM. A natural product telomerase activator as part of a health maintenance program. Rejuvenation Res. 2011;14:45–56. doi: 10.1089/rej.2010.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 54.Eitan E, Tichon A, Gazit A, Gitler D, Slavin S, Priel E. Novel telomerase-increasing compound in mouse brain delays the onset of amyotrophic lateral sclerosis. EMBO Mol Med. 2012;4:313–329. doi: 10.1002/emmm.201200212. The authors described a novel compound (AGS-499) that increases telomerase activity and expression in the mouse brain and spinal cord (SC). They demonstrated that telomerase re-expression exerts neuroprotective effects in NMDA-injected CD-1 mice and delays the onset and progression of the amyotrophic lateral sclerosis (ALS) disease in SOD1 transgenic mice. This work shows that a transient increase in telomerase expression and activity in the brain may exert neuroprotective properties.
- 55.Sahin E, Depinho RA. Axis of ageing: telomeres, p53 and mitochondria. Nat Rev Mol Cell Biol. 2012 doi: 10.1038/nrm3352. [DOI] [PMC free article] [PubMed] [Google Scholar]