Abstract
In the present study, the ability of metformin to inhibit skin tumor promotion by 12-O- tetradecanoylphorbol-13-acetate (TPA) was analyzed in mice maintained on either an overweight control diet or an obesity inducing diet. Rapamycin was included for comparison, and a combination of metformin and rapamycin was also evaluated. Metformin (given in the drinking water) and rapamycin (given topically) inhibited development of both papillomas and squamous cell carcinomas in overweight and obese mice in a dose-dependent manner. A low dose combination of these two compounds displayed an additive inhibitory effect on tumor development. Metformin treatment also reduced the size of papillomas. Interestingly, all treatments appeared to be at least as effective for inhibiting tumor formation in obese mice and both metformin and rapamycin were more effective at reducing tumor size in obese mice compared to overweight control mice. The effect of metformin on skin tumor development was associated with a significant reduction in TPA-induced epidermal hyperproliferation. Furthermore, treatment with metformin led to activation of epidermal AMPK and attenuated signaling through mTORC1 and p70S6K. Combinations of metformin and rapamycin were more effective at blocking epidermal mTORC1 signaling induced by TPA consistent with the greater inhibitory effect on skin tumor promotion. Collectively, the current data demonstrate that metformin given in the drinking water effectively inhibited skin tumor promotion in both overweight and obese mice and that the mechanism involves activation of epidermal AMPK and attenuated signaling downstream of mTORC1.
Keywords: metformin, rapamycin, dietary energy balance, tumor promotion, mTORC1
Introduction
Metformin, a drug widely used for the treatment of type-II diabetes, is effective through its ability to inhibit gluconeogenesis via activation of the LKB1/AMP-activated protein kinase (AMPK) pathway in the liver (1). Population-based studies have provided evidence that patients with type II diabetes treated with metformin have reduced cancer incidence as well as reduced mortality (2, 3). In vitro and in vivo studies have provided evidence in support of this association (4-7). Metformin, in part through its ability to activate AMPK, impacts multiple signaling pathways involved in regulating intracellular energy levels, especially signaling through mTORC1 (8). AMPK is a central metabolic sensor involved in the regulation of cellular energy homeostasis and is activated in response to cellular stressors that increase the AMP/ATP ratio including hypoxia, low glucose, or oxidative stress (9). In addition to inhibiting mTORC1, activation of AMPK has been shown to activate autophagy pathways in multiple cell types (10, 11). Activation of AMPK also leads to inhibition of lipid biosynthesis in multiple tissues via inhibition of acetyl CoA carboxylase (ACC1 and ACC2), fatty acid synthase, SREBP-1, as well as through activation of mitochondrial biosynthesis regulator PGC-1α (12-16). Thus, the consequences of AMPK activation make activators such as metformin attractive agents for both the prevention and treatment of a variety of cancers.
Recent evidence strongly supports an important role for Akt/mTORC1 signaling in tumor development in the mouse skin model of epithelial carcinogenesis (17). Epidermal Akt activation is sustained throughout two-stage skin carcinogenesis in mice (18) and treatment with diverse skin tumor promoters resulted in alteration (phosphorylation) of epidermal Akt downstream effectors including GSK3β, BAD, and mTORC1 (19). Transgenic mice overexpressing IGF-1 in the epidermis have increased susceptibility to two-stage skin carcinogenesis and spontaneous tumor formation as well as increased signaling through the PI3K/Akt/mTOR pathway (20, 21). Dysregulated expression of Akt in epidermis of transgenic mice led to alterations in mTORC1 downstream signaling and significantly heightened susceptibility to tumor promotion and two- stage skin carcinogenesis (22). Recently, we demonstrated that inhibition of mTORC1 using rapamycin dramatically inhibited skin tumor promotion by TPA primarily through blocking epidermal hyperproliferation and mTORC1 downstream signaling through p70S6K (23).
In the current study, the ability of metformin to inhibit skin tumor promotion by TPA in both overweight (modified AIN76A control diet with 10 Kcal% fat) and obese mice (60 Kcal% fat diet) was evaluated. Rapamycin and a low dose combination of metformin and rapamycin were included in these experiments. There are very few studies in the literature to date that have evaluated host metabolic status as a variable to study the anticancer effects of metformin. Previously published data from our lab as well as others, has demonstrated that mice receiving a 60 Kcal % fat diet have BMI values and demonstrate a metabolic profile similar to that seen in obese humans (24-27). Metformin significantly inhibited skin tumor promotion by TPA in both diet groups. Inhibition of tumor development by metformin was dose-dependent and correlated with activation of epidermal AMPK and attenuation of TPA-induced activation of mTORC1 signaling in epidermal keratinocytes. Interestingly, a low dose combination of metformin and rapamycin was significantly more effective at blocking skin tumor promotion and tumor development than either agent alone. Collectively, the data suggest that metformin is an effective inhibitor of skin tumor promotion by TPA and that its mechanism displays similarities to that of calorie restriction and rapamycin in the mouse skin carcinogenesis model system.
Materials and Methods
Animals and diets
FVB/N female mice (7-8 weeks of age, NCI) were fed ad libitum and group housed for all experiments. Mice were weighed at study onset and then every two weeks for the duration of the experiments in accordance with institutional guidelines. For short-term studies, mice received a regular chow diet containing 10 Kcal% fat. For the tumor experiments, pelleted diets of varying caloric density [10 Kcal% fat (D12450B) and 60 Kcal% fat (D12492); Research Diets Inc.] were administered.
Two-stage skin carcinogenesis
Mice were placed on a 10 Kcal% fat diet and initiated with 25 nmol of 7,12- dimethylbenz[a]anthracene (DMBA, Sigma-Aldrich) or acetone. Two weeks after initiation, mice were randomized and received one of the two experimental diets. Six weeks later, mice were treated topically with various doses of rapamycin (2, 5, or 20 nmol) in 0.2 ml acetone (vehicle) or with metformin administered in the drinking water [50 or 250 mg/kg body weight (BW) per day; n =15-20/group at the beginning of the study], which was replaced twice weekly and adjusted for changes in BW every two weeks. At this time, mice also received twice-weekly topical treatments with 6.8 nmol of TPA (LC Laboratories) for the duration of the experiments. Rapamycin (administered bi-weekly) was applied 30 minutes prior to TPA in all experiments. Tumor multiplicity (average number of papillomas per mouse) and tumor incidence (percent of mice with papillomas) were recorded each week until multiplicity plateaued (week 25). Papillomas were measured at week 23 of tumor promotion by digital calipers, and tumor surface area was calculated. The incidence of squamous cell carcinomas (SCCs) and average SCCs per mouse were determined weekly from initial detection until week 49. All SCCs were verified histologically as previously described (28-31).
Serum analysis
Blood was collected by cardiac puncture following CO2 asphyxiation (n=4-7/group), held at room temperature for 2 hours, spun at 7,500 rpm for 7 min (twice) and the serum (supernatant) was flash frozen in liquid nitrogen and stored at −80°C until analysis (10 μl sample/analysis). Serum levels of insulin and leptin were measured with a Milliplex MAP Mouse Serum Adipokine panel multiplex Luminex Assay (Millipore), adiponectin levels were measured with a Milliplex MAP Mouse Serum singleplex Luminex Assay, and IGF-1 levels were determined with the Quantikine ELISA Mouse/Rat IGF-1 Immunoassay (R&D Systems).
Epidermal hyperproliferation
The dorsal skin of mice was shaved and treated twice weekly for 2 weeks topically with either acetone (vehicle), 6.8 nmol of TPA, or metformin (50, 250, and 350 mg/kg BW) administered in the drinking water (n=3/group). BrdU was injected (IP, 100 μg/g BW) in 0.9% NaCl 30 min prior to sacrifice. Mice were sacrificed 48 hours after the last treatment. Dorsal skin samples were fixed in 10% neutral buffered formalin, stored in 70% ethanol, embedded in paraffin and then sectioned for staining with H&E and anti-BrdU. Epidermal thickness and labeling index were determined as described previously (23).
Preparation of epidermal lysates and Western blot analysis
Mouse dorsal skin was shaved and treated twice weekly for 2 weeks with either acetone (0.2 ml) or TPA (6.8 nmol), or rapamycin, metformin or combinations of the two compounds (n=5/group). Rapamycin (2 nmol) was applied topically 30 min prior to TPA. Metformin was administered in the drinking water at the start of the two-week treatment period at doses of 50 or 250 mg/kg BW per day. Mice were sacrificed 6 hours after the final treatment (acetone, TPA), epidermis was scraped, and protein lysates were prepared as previously described (23). Western blot analyses were performed as previously described (23, 32). Antibodies were obtained from Cell Signaling Technology, Inc.
Statistical Analysis
An assumption of a normal distribution for comparison of tumor multiplicity, serum levels, BrdU labeling index and epidermal thickness between each treatment group could not be made and therefore a non-parametric statistical method (the one-tailed Mann-Whitney U-test) was used for tests of statistical significance between treatment groups. To compare tumor incidence between treatment groups, the one-tailed Fisher's exact test was used. To compare time to first tumor between groups, a one-tailed log-rank (Mantel-Cox) test was used, assigning a time point of 25 weeks to any mouse that did not develop a papilloma over the course of the study. Dose- response trend analyses were conducted using the Kruskal-Wallis test. To compare the percent decrease in papillomas per mouse and percent decrease in tumor size, the one-tailed Mann- Whitney U-test was used. All comparisons were planned and the P values were not corrected for multiple comparisons. GraphPad Prism 5 software (GraphPad Software, La Jolla, CA) was used for all statistical tests, and significance was set at P≤0.05.
Results
Effect of metformin and rapamycin on skin tumor promotion and progression in overweight mice
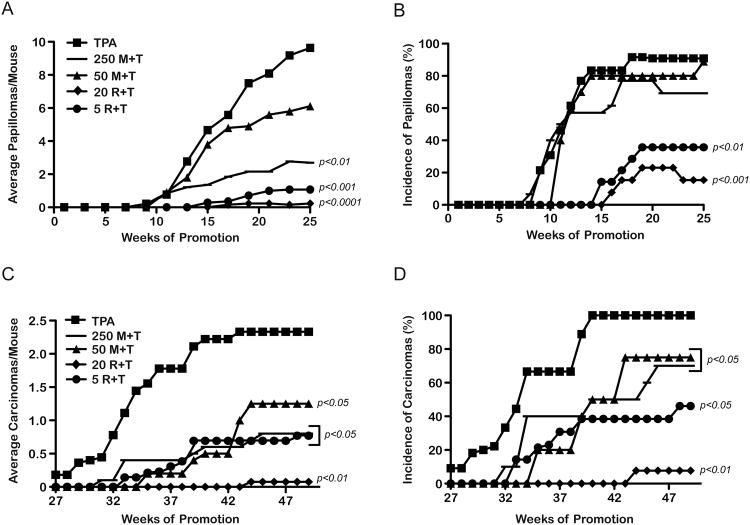
A two-stage skin carcinogenesis experiment was conducted using mice maintained on the 10 Kcal% fat diet fed ad libitum (overweight control diet) (25, 33). Eight weeks following initiation with DMBA, mice began receiving twice weekly treatments with TPA. Certain groups began receiving metformin in the drinking water (50 or 250 mg/kg BW/day) or were treated topically with 5 or 20 nmol of rapamycin 30 min prior to treatment with TPA similar to our recent study (23). Metformin significantly inhibited skin tumor promotion by TPA in a dose-dependent manner (Figure 1A). There was 36% and 72% inhibition of papilloma development in the 50 mg/kg and 250 mg/kg BW dose groups, respectively. Although the reduction in papilloma response with the 50 mg/kg BW/day dose of metformin was not statistically significant, the reduction with the 250 mg/kg BW/day dose was highly significant (p<0.01, Mann-Whitney U- test). Furthermore, additional statistical analyses of these data revealed a significant dose- dependent trend for reduction in papillomas per mouse with metformin (p<0.05; Kruskal Wallis test). There were no significant differences in papilloma incidence observed at either dose of metformin in this experiment (Figure 1B). Rapamycin inhibited skin tumor promotion by TPA as expected. The group receiving 5 nmol of rapamycin prior to TPA had a significant reduction (88%) in papilloma development at week 25 as compared to the TPA control group (p<0.001; Mann-Whitney U-test). The group receiving 20 nmol rapamycin prior to TPA had a 97% reduction in papilloma development (p<0.001; Mann-Whitney U-test). Similar to metformin, there was a significant dose-dependent trend for inhibition of papillomas with rapamycin (p<0.001; Kruskal Wallis test). As shown in Figure 1B, the incidence of papillomas in the 5 and 20 nmol rapamycin treatment groups was significantly reduced (to 36% and 15%, respectively), at 25 weeks of promotion (p<0.01 and p<0.001, respectively, Fischer's exact test). Metformin at either dose did not significantly affect tumor latency (Figure 1B). In contrast, both doses of rapamycin significantly increased tumor latency (p<0.001, Mantel-Cox test). Finally, there were no significant differences in BW gain over the course of this experiment with any of the treatment regimens (data not shown).
Figure 1.
Effect of metformin on tumor development and progression. Two-stage skin carcinogenesis studies were conducted using mice fed the 10 Kcal% fat diet (AIN76A; overweight control diet). A, At 25 weeks, the tumor multiplicity in the 250 mg/kg (−) BW/day metformin+TPA treated groups and the 5 nmol (●) and 20 nmol (◆) rapamycin+TPA treated groups was significantly lower than the 6.8 nmol TPA (■) control group (Mann-Whitney U-test). B, The 5 nmol (●) and 20 nmol (◆) rapamycin+TPA treated groups had a significantly reduced papilloma incidence compared to the 6.8 nmol TPA (■) group (Fisher's exact test). C, Both doses of metformin and rapamycin significantly reduced the average number of carcinomas/mouse compared to the TPA control group (Mann-Whitney U-test). D, All treatments significantly reduced the incidence of carcinomas (Fisher's exact test).
All treatments in this experiment were continued for an additional 24 weeks to evaluate the effects of metformin and rapamycin on formation of SCCs. As shown in Figures 1C and 1D, both doses of metformin and rapamycin significantly decreased the number and incidence of SCCs in a dose-dependent manner consistent with the inhibition of papilloma development.
Effect of metformin, rapamycin and their combination on skin tumor promotion in obese mice
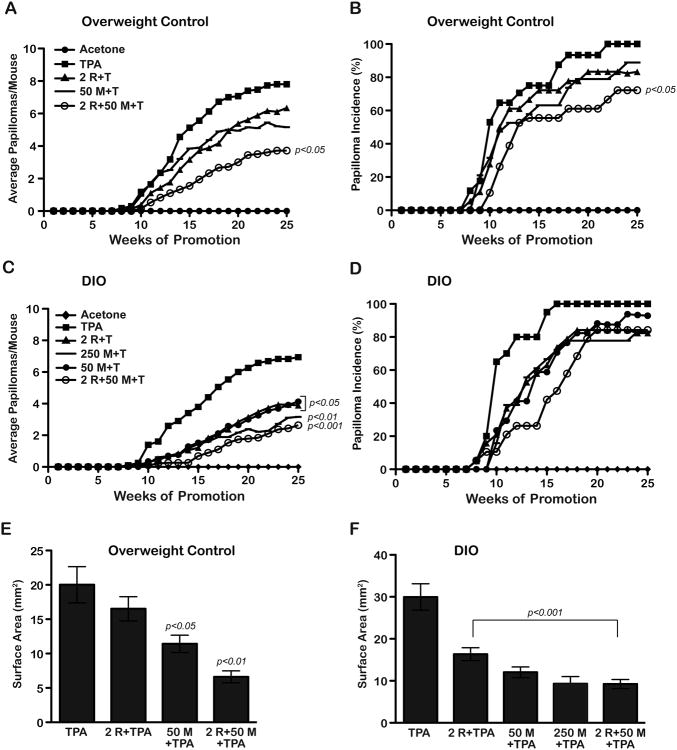
A second two-stage skin carcinogenesis experiment was conducted to compare the effect of metformin in overweight versus obese mice. Mice were maintained on the modified AIN76A diet and initiated with DMBA. Two weeks following initiation, mice were randomized to receive either the obesity inducing diet (60 Kcal% fat) or the overweight control diet (10 Kcal% fat) for the duration of the study. Tumor promotion with TPA began after an additional 6 weeks on each experimental diet. Metformin was given in the drinking water at doses of either 50 or 250 mg/kg BW/day in the obese diet groups and 50 mg/kg BW/day in the overweight control diet groups. An additional group of mice received a low dose of topical rapamycin (2 nmol) administered prior to each TPA treatment and another group received a combination of rapamycin (2 nmol) and metformin (50 mg/kg). As shown in Figure 2A, metformin treatment (50 mg/kg dose) inhibited papilloma development by 34% whereas rapamycin treatment (2 nmol dose) inhibited papilloma development by only 19%. Neither of these reductions in tumor response was statistically significant. However, there was a 52% inhibition of papilloma development in the metformin + rapamycin treatment group compared to the TPA control group that was statistically significant (p<0.05, Mann-Whitney U-test). The combination treatment group also exhibited a statistically significant reduction in papilloma incidence (p<0.05; Fischer's exact test) (Figure 2B). In this experiment, the groups treated with metformin and metformin + rapamycin also had significantly increased tumor latency compared to the DMBA-TPA only control group (p<0.05 and p<0.01, respectively, Mantel-Cox test).
Figure 2.
Effect of metformin alone and in combination with rapamycin on tumor promotion in overweight control and obese mice. Two-stage skin carcinogenesis studies were conducted using mice on an overweight control (10 Kcal% fat) diet (A, B) or an obese (Diet-induced obesity, DIO, 60 Kcal% fat) diet (C, D). Papilloma number and incidence were recorded weekly. Tumor surface area was determined from a subset of tumors from each treatment group after 23 weeks of promotion. A, The combination treatment group (○) (2 nmol rapamycin, 50 mg/kg metformin+TPA) had significantly reduced tumor multiplicity at 25 weeks, (Mann-Whitney U - test). B, The combination treatment group (○) had a significant reduction in tumor incidence (Fisher's exact test). C, All treatment groups had significantly reduced tumor multiplicity (Mann- Whitney U-test). D, No differences were observed in tumor incidence in any treatment group (Fisher's exact test). E, Graphs represent the average surface area ± SEM in overweight control mice. The 50 mg/kg metformin+TPA group and the combination treatment group displayed a significant reduction in tumor size compared to the TPA control group (Mann- Whitney U-test). F, Graphs represent the average surface area ± SEM in obese mice. All treatment groups had statistically significant decreases in skin tumor size compared to the TPA control group (Mann-Whitney U-test).
Notably, both the individual treatments as well as the combination treatment produced statistically significant reductions in papilloma numbers in mice on the obesity-inducing diet (see Figure 2C for specific p-values). Metformin at 50 mg/kg produced a 44% inhibition in papilloma development in the obese diet group compared with 34% in the overweight group (Figures 2C and 2A, respectively). In addition, there was a 42% inhibition of papilloma development in the obese diet group with rapamycin (Figure 2C) compared to 19% inhibition in the overweight mice (Figure 2A). The combination of metformin + rapamycin inhibited papilloma formation in the obese diet group by 62% compared to 52% inhibition in the overweight group. No significant decreases in final tumor incidence were observed in any of the obese diet treatment groups (Figure 2D). However, significant increases in tumor latency were observed in all treatment groups on the DIO diet compared to the DMBA-TPA only control group (p<0.01 for all treatment groups; Mantel-Cox test).
During week 23 of the study shown in Figure 2, a representative subset of papillomas (n= 18-24) from each treatment/diet group was measured to determine average surface area. All treatments led to a reduced tumor size regardless of diet (Figures 2E, F). Except for the rapamycin only treatment group in overweight control mice, all other decreases in tumor size were statistically significant (Mann Whitney U-test, p-values shown in Figure 2). Interestingly, the effect of the individual compounds on tumor size appeared to be more pronounced in the obese mice. In this regard, the decrease in tumor size in obese mice was significantly greater than the decrease in tumor size in mice on the overweight control diet for the metformin and rapamycin treatment groups (p<0.05 for both groups; Mann Whitney U-test) but not for the metformin + rapamycin group. Notably, the combination treatment dramatically reduced the size of tumors in both diet groups to similar levels, and this effect appeared to be additive in both cases.
Influence of dietary manipulation and treatments on BW and circulating levels of insulin, IGF-1, leptin and adiponectin
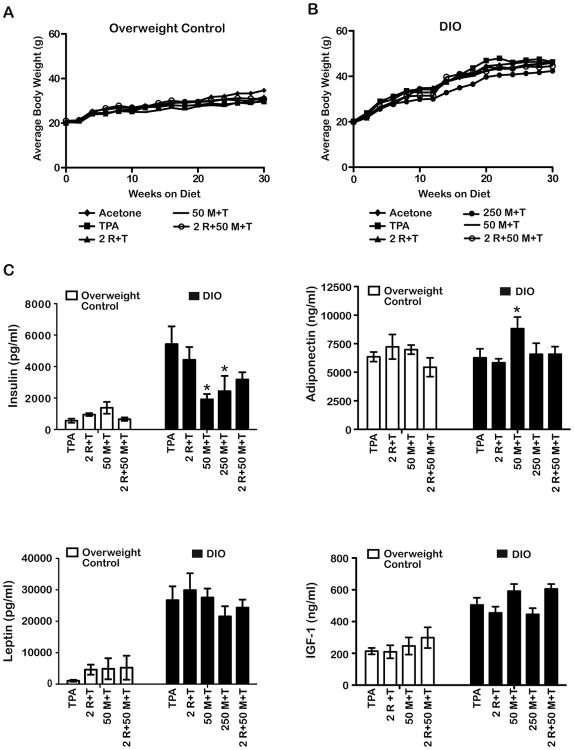
Figures 3A and 3B show the BW gain for the various treatment groups maintained on the overweight control and DIO diets, respectively, over the course of the first 30 weeks of this study. Promotion and treatment began after the first 6 weeks represented on these figures. After 30 weeks on diet, the mean BW (including all treatment groups) of the DIO diet group was 45.2 ± 0.63 g, and the mean BW (including all treatment groups) of the overweight control diet group was 31.6 ± 0.86 g. These differences were statistically significant (p<0.05; Mann-Whitney U- test). In contrast, there were no differences in mean BW in any of the treatment groups maintained on a given diet.
Figure 3.
Influence of metformin and diet on body weight (BW) and serum hormones. Mice undergoing the carcinogenesis protocol described in Figure 2 were weighed every two weeks once groups were switched to either the 10 Kcal% fat (overweight control) or 60 Kcal% fat (Diet- induced obesity, DIO) diet. Serum was collected at the end of the 25-week promotion period (30 weeks total on the different diets). A, Treatment with metformin, rapamycin, or combinations of these compounds prior to TPA had no significant effect on BW gain in the overweight control groups. B, None of the treatment regimens affected weight gain in the obese groups compared to the TPA only group. The differences in average BW between the overweight control and DIO diet groups (including all treatment groups combined) were statistically significant (31.6 ± 0.86 vs. 45.2 ± 0.63, respectively, p<0.05, Mann-Whitney U-test). C, Fasting serum levels (mean ± SEM) of insulin, adiponectin, leptin, and IGF-1 in overweight or obese mice receiving TPA, metformin+TPA, rapamycin+TPA, or metformin+rapamycin+TPA at the doses indicated (n=4-7). * Indicates significantly different from the TPA only group, (p<0.05; Mann-Whitney U-test).
Obese mice receiving TPA only had an ∼8-fold increase in fasting serum insulin levels, an ∼2.4-fold increase in serum IGF-1 levels and an ∼25-fold increase in serum leptin levels relative to the overweight control mice receiving the same treatment (Figure 3C; p<0.05, Mann- Whitney U-test). Levels of serum adiponectin were not statistically significant in these two diet groups. Metformin at the 50 mg/kg dose significantly reduced serum insulin levels in the obese mice (5,427.67 ± 1,131.5 versus 1,914.7 ± 338.8 pg/ml, respectively; p<0.05, Mann-Whitney U- test) (Figure 3C). Similar decreases in serum insulin were observed at the 250 mg/kg dose in obese mice (5,427.67 ± 1,131.5 versus 2,435.5 ± 977.2 pg/ml, respectively; p< 0.05, Mann- Whitney U-test). In contrast, metformin (50 mg/kg) had no effect on insulin levels in the overweight control mice. Serum adiponectin levels were not altered by any treatments in the overweight control mice. In mice on the obese diet, serum adiponectin levels were slightly elevated in the 50 mg/kg metformin treatment group (p<0.05) but not in any of the other treated groups. Finally, leptin and IGF-1 levels were not significantly altered by metformin in mice on either dietary regimen and topical rapamycin (2 nmol per mouse) had no effect on any serum parameter analyzed in mice in either diet group.
Effect of metformin on TPA-induced epidermal hyperproliferation and hyperplasia
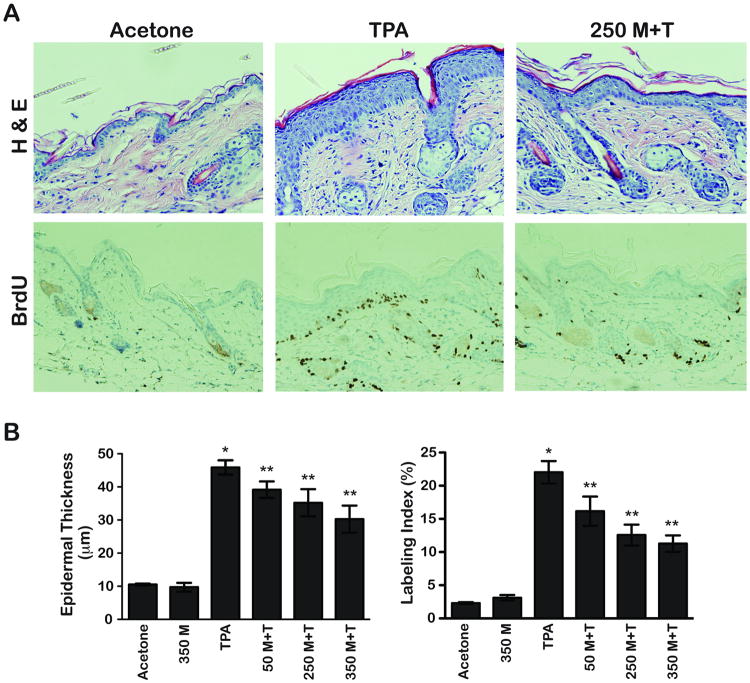
Figure 4A displays representative H&E and BrdU stained skin sections from groups of mice treated with acetone, TPA, and 250 mg/kg metformin + TPA as described in Materials and Methods. Metformin treatment produced statistically significant decreases in both epidermal thickness and BrdU labeling index at all three doses used. Quantitative evaluation (Figure 4B) revealed that metformin significantly reduced each of these parameters compared to the TPA control group (p<0.05, Mann-Whitney U-test) in a dose-dependent manner.
Figure 4.
Effect of metformin on TPA-induced epidermal hyperproliferation and hyperplasia. A, Representative H&E and BrdU stained sections of dorsal skin collected from female FVB/N mice (4/group) at 48h after the last of 4 treatments with either acetone or 6.8 nmol of TPA alone or with metformin in the drinking water (250 mg/kg BW/day). B, Quantitative evaluation (mean ± SEM) of the effects of metformin on TPA-induced epidermal hyperproliferation (labeling index) and hyperplasia (epidermal thickness). * Indicates significantly different from the acetone and metformin only (350 mg/kg) treatment groups (p<0.05, Mann-Whitney U-test). ** Significantly different from the TPA-treated group (p<0.05, Mann-Whitney U-test).
Effect of metformin on epidermal AMPK and TPA-induced mTORC1 signaling
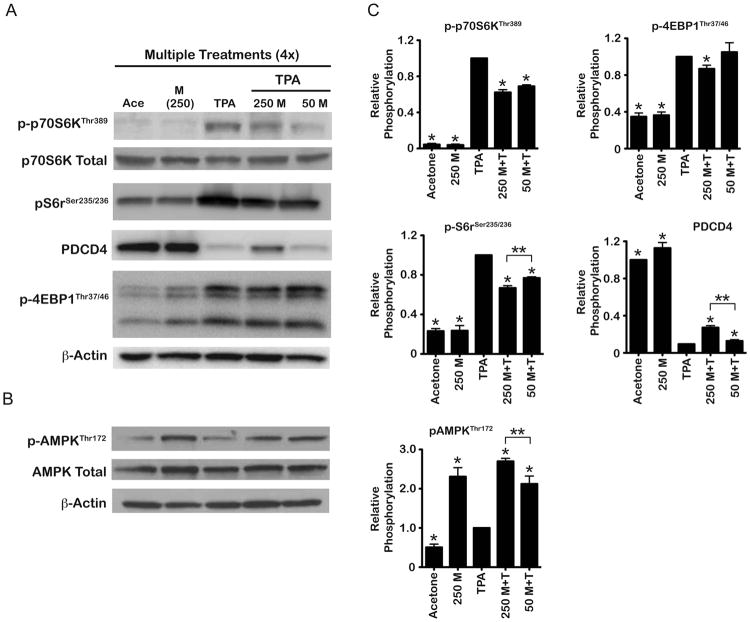
As reported previously (19, 23), topical treatment with TPA resulted in activation of epidermal mTORC1 as seen through increases in p-p70S6KThr389, p-S6rSer235/236, and p- 4EBP1Thr37/46, as well as degradation of the mTORC1 downstream target and translational repressor, PDCD4 (Figure 5A). In treatment groups receiving metformin, there was a dose- dependent reduction in TPA-induced phosphorylation of p70S6KThr389 as well as S6rSer235/236 (Figure 5A). In addition, PDCD4 was partially protected from TPA-mediated degradation with a greater effect seen at the higher dose of metformin (250 mg/kg). Metformin had no apparent effect on mTORC1-mediated phosphorylation of 4EBP1 following TPA treatment at the 50 mg/kg dose but did produce a small, statistically significant decrease at the 250 mg/kg dose. Metformin (250 mg/kg) given alone had no apparent effects on mTORC1 signaling. Analyses of AMPK activation (Figure 5B) as measured by phosphorylation at Thr172 revealed that metformin alone increased activation of epidermal AMPK compared to the acetone control group. Both doses of metformin increased activation of AMPK compared to the TPA and acetone control groups (Figure 5B). Figure 5C displays the quantification and statistical analyses of three independent experiments whereas the Western blots shown in Figure 5A and 5B are from a single representative experiment. As a further confirmation that metformin activated epidermal AMPK, the phosphorylation of ULK1 was also analyzed. As shown in Supplemental Figure 1, phosphorylation of ULK1 at Ser555 was increased ∼two-fold in epidermis of mice treated with 250 mg/kg metformin compared to the acetone control group. In contrast, TPA treatment reduced phosphorylation at this site compared to the acetone control group. Metformin at 250 mg/kg partially reversed the effect of TPA on ULK1 phosphorylation.
Figure 5.
Effect of metformin on epidermal mTORC1 and AMPK in the absence or presence of TPA treatment. Pooled protein lysates were prepared from epidermal scrapings of FVB/N mice (n=5/group) undergoing a multiple treatment regimen of either acetone (vehicle), metformin alone (250 mg/kg), 6.8 nmol TPA alone, or metformin+TPA (50 or 250 mg/kg BW/day). A, Representative Western blot analyses of p-p70S6K, p-S6 ribosomal, PDCD4 and p-4EBP1. B, Representative Western blot analyses of epidermal p-AMPK. C, Quantification of Western blots from three independent experiments. Expression levels were normalized to β-actin and averaged. * Indicates significantly different from the TPA-treated group (p<0.05, Mann Whitney U-test). ** Indicates significantly different values between the indicated treatment groups (p<0.05, Mann Whitney U-test).
Effect of combination treatments with metformin and rapamycin on TPA-induced mTORC1 signaling in the epidermis
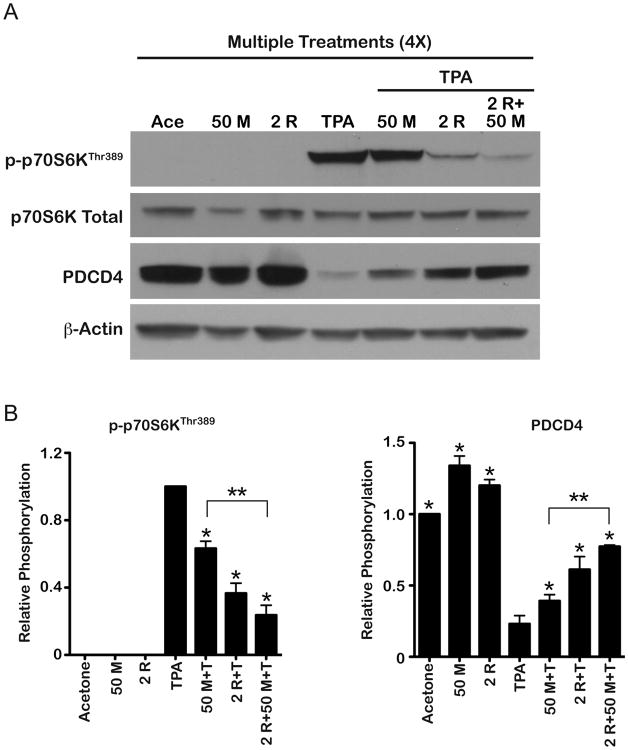
As shown in Figure 6A, topical application of TPA again led to the activation of p70S6K, a downstream effector of mTORC1, and degradation of translation repressor, PDCD4, as measured in epidermal protein lysates prepared 6 h after the last TPA treatment. Both metformin (50 mg/kg) and rapamycin (2 nmol) given alone inhibited these alterations. The combination of metformin + rapamycin produced a greater inhibition of the alterations in mTORC1 signaling seen following TPA treatment consistent with the greater inhibition of skin tumor promotion observed with this combination. Figure 6B displays the quantitation along with statistical analyses of combined data from three independent experiments whereas the Western blot shown in Figure 6A is from a single representative experiment. These data further confirm that metformin given in the drinking water at a dose of 50 mg/kg inhibited mTORC1 signaling as seen in the experiment presented in Figure 5 and that the combination of metformin + low dose rapamycin produced a greater inhibitory effect on mTORC1 signaling.
Figure 6.
Effect of metformin or rapamycin alone and in combination on TPA-induced mTORC1 signaling. Pooled protein lysates were prepared from epidermal scrapings of FVB/N mice (n=5/group) undergoing a multiple treatment regimen of acetone, metformin alone (50 mg/kg), 6.8 nmol of TPA alone, 50 mg/kg metformin+TPA, 2 nmol of rapamycin+TPA, or combinations of these two treatments prior to TPA. A, Representative Western blot analyses of mTORC1 downstream targets p-p70S6K and PDCD4. B, Quantification of Western blots from three independent experiments. Expression levels were normalized to p-actin and averaged. * Indicates significantly different from the TPA-treated group (p<0.05, Mann Whitney U-test). **Indicates significantly different values between the indicated treatment groups (p<0.05, Mann Whitney U-test).
Discussion
In the current study, metformin given in the drinking water at doses of 50 and 250 mg/kg BW/day effectively inhibited skin tumor promotion by TPA in mice on an overweight control diet. Metformin effectively inhibited formation of both premalignant papillomas as well as SCCs. In addition, treatment with metformin reduced the size of papillomas. Mechanistic studies also revealed that metformin decreased TPA-induced epidermal hyperplasia and hyperproliferation, activated epidermal AMPK, and reduced TPA-induced mTORC1 signaling. Furthermore, a low dose combination of metformin + rapamycin was more effective than either agent alone at the same doses. We also examined the effects of metformin, rapamycin, and the low dose combination of metformin + rapamycin in mice maintained on an obesity-inducing diet for comparison. Metformin again effectively inhibited skin tumor promotion as did rapamycin and the combination of metformin + rapamycin. Metformin and rapamycin also reduced the size of papillomas and this effect was greater in obese mice compared to that observed in overweight control mice. Overall, the data from this study demonstrate that metformin given in the drinking water is an effective inhibitor of skin tumor promotion in both overweight and obese mice.
The anti-tumorigenic properties of metformin have been recently explored in several in vitro and in vivo experimental systems. Studies in cancer cell lines using high concentrations have provided evidence that metformin can inhibit cell growth (6, 7). In HER-2/neu transgenic mice, metformin administered in the drinking water (100 mg/kg BW per day) reduced the size and incidence of mammary adenocarcinomas as well as prolonged lifespan (4). In addition, metformin administered in a basal powdered diet form (250 mg/kg BW per day) in APCmin/+ mice reduced polyp growth as well as activated AMPK and reduced signaling through mTORC1 in tumor tissue (5). Recently, it was reported that metformin inhibited benzo[a]pyrene (B[a]P) as well as UVB-induced skin carcinogenesis. In this regard, metformin administered in the drinking water to female and male SHR mice alone and in combination with melatonin significantly reduced the number and size of skin tumors induced by B[a]P (34, 35). Additionally, metformin administered topically as well as systemically, suppressed the growth of existing UVB-induced skin tumors and prevented the growth of new tumors (36). In this latter study, metformin treatment was shown to enhance disappearance of UV-photoproducts in mouse embryo fibroblasts. However, the effect of metformin on UV-photoproduct formation and disappearance was not examined in epidermal keratinocytes in culture or in vivo. Finally, metformin was also shown to inhibit growth of A431 SCC cells in a tumor xenograft model (37). Collectively, these data and our current data demonstrate the cancer preventive effects of metformin in a variety of animal model systems. Furthermore, the current data show that metformin primarily affects the tumor promotion stage of skin carcinogenesis in the two-stage chemical carcinogenesis model.
The anticancer activity of metformin has been attributed to both direct and indirect effects (38, 39). The primary direct mechanism of action occurs through inhibition of mitochondrial complex I and subsequent induction of cellular energy stress resulting in activation of AMPK (9). Activation of the LKB1/AMPK pathway modulates a host of downstream effectors that control cellular growth and metabolism to help regulate cellular energy balance during stress (40). Metformin has been shown to induce a growth-suppressive effect in a variety of transformed cells via inhibition of mTORC1 and reduced protein synthesis (6, 7, 41). We have shown that targeting mTORC1 effectively inhibits skin tumor promotion by TPA [(23); Figures 1 and 2, this study]. Inhibition of skin tumor development in response to metformin treatment correlated with activation of epidermal AMPK and a decrease in TPA-induced mTORC1 signaling assessed by decreased levels of p-p70S6KThr389 and p-S6r Ser235/236 protein. In addition, the translational repressor PDCD4, a downstream target of p70S6K, was partially protected from TPA-mediated degradation. Collectively, these data suggest that activation of epidermal AMPK and subsequent reduction of mTORC1 signaling contributed to the inhibition of skin tumor promotion by TPA. Since metformin treatment also modulated phosphorylation of ULK1 in epidermis it is possible that activation of autophagy signaling pathways may have also contributed to the effects of metformin on skin tumor promotion, however, further work will be required to determine this possibility.
Epidemiological data have provided evidence that patients with type II diabetes treated with metformin have reduced cancer incidence as well as reduced mortality compared to patients receiving other types of treatments for this disease (2, 3, 42). A few animal studies have evaluated metformin's anti-cancer effects in an obese/high calorie diet setting. In this regard, administration of metformin via the drinking water (50 mg/kg BW/per day) blocked the stimulatory effect of a high-energy diet on MC38 colon carcinoma cells growth in vivo but had no effect on tumor growth in mice on a control diet (12). Additionally, oral administration of metformin attenuated the effect of a high-energy diet on growth of Lewis lung LLC1 carcinoma cells in vivo but again had no effect on tumor growth in mice on a control diet (43). Thus, both of these studies showed that metformin was more effective in mice on a high-energy diet, with little or no effect on tumor growth in mice on a control diet. These latter studies examined the growth of existing cancer cells, whereas we examined the ability of metformin to prevent skin tumor promotion i.e., a chemopreventive effect. In addition, the experimental diets used in our studies differed in caloric density as well as nutritional composition, which may also account for some of the differences in efficacy observed. Thus, in our experimental model system, metformin was effective at inhibiting skin tumor promotion in both the overweight control and obese diet groups. Notably, metformin was more effective at reducing the size of papillomas in obese mice compared to mice on the overweight control diet (see Figures 2E and 2F).
The mechanism for the greater inhibitory effect of metformin on tumor size in obese mice is not clear at the present time. Metformin may influence tumor growth indirectly through impaired gluconeogenesis, which lowers glucose production and subsequent circulating insulin levels via activation of the LKB1/AMPK pathway in the liver (44) especially in an obese state. In the current study, serum analyses revealed a significant reduction in serum insulin levels in obese mice but no change in the overweight control mice in response to metformin treatment (Figure 3C). Metformin at the doses used in the tumor experiments did not alter circulating levels of IGF-1 or leptin in mice on either diet regimen. Analysis of adiponectin levels revealed no treatment related changes in overweight control mice and a slight but statistically significant increase in obese mice treated with 50 mg/kg metformin. No other treatment affected serum adiponectin levels in obese mice. Therefore, the most consistent systemic change observed at both doses of metformin was on circulating levels of insulin in the obese mice, which may have contributed to the overall action of metformin on tumor size in this diet group. However, the fact that rapamycin treatment was also more effective at reducing tumor size in obese mice argues against this hypothesis. Thus, additional studies are required to determine if reduction in serum insulin levels contributed to the greater inhibition of tumor growth by metformin in obese mice.
Overall, the current data support the hypothesis that elevation of mTORC1 and subsequent activation of downstream signaling pathways are highly important events during skin tumor promotion. We show for the first time that oral administration of metformin given in the drinking water effectively inhibits skin tumor promotion in a chemically induced model of multistage skin carcinogenesis. This effect of metformin was evident in both overweight control mice as well as obese mice. Furthermore, we have shown that combinations of metformin + rapamycin are more effective at inhibiting skin tumor promotion than either compound alone. Metformin appears to be an ideal candidate for further evaluation of its chemopreventive effectiveness against multiple cancers, including skin cancer.
Supplementary Material
Acknowledgments
The authors wish to thank Ms. Lauren Pascale for her assistance in the submission of this manuscript.
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–9. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–61. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 3.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anisimov VN, Berstein LM, Egormin PA, Piskunova TS, Popovich IG, Zabezhinski MA, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40:685–93. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Tomimoto A, Endo H, Sugiyama M, Fujisawa T, Hosono K, Takahashi H, et al. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci. 2008;99:2136–41. doi: 10.1111/j.1349-7006.2008.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rattan R, Giri S, Hartmann LC, Shridhar V. Metformin attenuates ovarian cancer cell growth in an AMP-kinase dispensable manner. J Cell Mol Med. 2011;15:166–78. doi: 10.1111/j.1582-4934.2009.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–73. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 8.Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F. Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Mol Cancer Ther. 2010;9:1092–9. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- 9.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JW, Park S, Takahashi Y, Wang HG. The association of AMPK with ULK1 regulates autophagy. PLoS One. 2010;5:e15394. doi: 10.1371/journal.pone.0015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Algire C, Amrein L, Zakikhani M, Panasci L, Pollak M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr Relat Cancer. 2010;17:351–60. doi: 10.1677/ERC-09-0252. [DOI] [PubMed] [Google Scholar]
- 13.Brunet J, Vazquez-Martin A, Colomer R, Grana-Suarez B, Martin-Castillo B, Menendez JA. BRCA1 and acetyl-CoA carboxylase: the metabolic syndrome of breast cancer. Mol Carcinog. 2008;47:157–63. doi: 10.1002/mc.20364. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–88. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suwa M, Egashira T, Nakano H, Sasaki H, Kumagai S. Metformin increases the PGC- 1alpha protein and oxidative enzyme activities possibly via AMPK phosphorylation in skeletal muscle in vivo. J Appl Physiol. 2006;101:1685–92. doi: 10.1152/japplphysiol.00255.2006. [DOI] [PubMed] [Google Scholar]
- 16.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rho O, Kim DJ, Kiguchi K, Digiovanni J. Growth factor signaling pathways as targets for prevention of epithelial carcinogenesis. Mol Carcinog. 2011;50:264–79. doi: 10.1002/mc.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segrelles C, Ruiz S, Perez P, Murga C, Santos M, Budunova IV, et al. Functional roles of Akt signaling in mouse skin tumorigenesis. Oncogene. 2002;21:53–64. doi: 10.1038/sj.onc.1205032. [DOI] [PubMed] [Google Scholar]
- 19.Lu J, Rho O, Wilker E, Beltran L, Digiovanni J. Activation of epidermal akt by diverse mouse skin tumor promoters. Mol Cancer Res. 2007;5:1342–52. doi: 10.1158/1541-7786.MCR-07-0115. [DOI] [PubMed] [Google Scholar]
- 20.DiGiovanni J, Bol DK, Wilker E, Beltran L, Carbajal S, Moats S, et al. Constitutive expression of insulin-like growth factor-1 in epidermal basal cells of transgenic mice leads to spontaneous tumor promotion. Cancer Res. 2000;60:1561–70. [PubMed] [Google Scholar]
- 21.Wilker E, Lu J, Rho O, Carbajal S, Beltran L, DiGiovanni J. Role of PI3K/Akt signaling in insulin-like growth factor-1 (IGF-1) skin tumor promotion. Mol Carcinog. 2005;44:137–45. doi: 10.1002/mc.20132. [DOI] [PubMed] [Google Scholar]
- 22.Segrelles C, Lu J, Hammann B, Santos M, Moral M, Cascallana JL, et al. Deregulated activity of Akt in epithelial basal cells induces spontaneous tumors and heightened sensitivity to skin carcinogenesis. Cancer Res. 2007;67:10879–88. doi: 10.1158/0008-5472.CAN-07-2564. [DOI] [PubMed] [Google Scholar]
- 23.Checkley LA, Rho O, Moore T, Hursting S, DiGiovanni J. Rapamycin is a potent inhibitor of skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Prev Res (Phila) 2011;4:1011–20. doi: 10.1158/1940-6207.CAPR-10-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nunez NP, Oh WJ, Rozenberg J, Perella C, Anver M, Barrett JC, et al. Accelerated tumor formation in a fatless mouse with type 2 diabetes and inflammation. Cancer Res. 2006;66:5469–76. doi: 10.1158/0008-5472.CAN-05-4102. [DOI] [PubMed] [Google Scholar]
- 25.Nunez NP, Carpenter CL, Perkins SN, Berrigan D, Jaque SV, Ingles SA, et al. Extreme obesity reduces bone mineral density: complementary evidence from mice and women. Obesity (Silver Spring) 2007;15:1980–7. doi: 10.1038/oby.2007.236. [DOI] [PubMed] [Google Scholar]
- 26.Yakar S, Nunez NP, Pennisi P, Brodt P, Sun H, Fallavollita L, et al. Increased tumor growth in mice with diet-induced obesity: impact of ovarian hormones. Endocrinology. 2006;147:5826–34. doi: 10.1210/en.2006-0311. [DOI] [PubMed] [Google Scholar]
- 27.Moore T, Beltran L, Carbajal S, Strom S, Traag J, Hursting SD, et al. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res (Phila) 2008;1:65–76. doi: 10.1158/1940-6207.CAPR-08-0022. [DOI] [PubMed] [Google Scholar]
- 28.Kruszewski FH, Conti CJ, DiGiovanni J. Characterization of skin tumor promotion and progression by chrysarobin in SENCAR mice. Cancer Res. 1987;47:3783–90. [PubMed] [Google Scholar]
- 29.Aldaz CM, Conti CJ, Chen A, Bianchi A, Walker SB, DiGiovanni J. Promoter independence as a feature of most skin papillomas in SENCAR mice. Cancer Res. 1991;51:1045–50. [PubMed] [Google Scholar]
- 30.Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc. 2009;4:1350–62. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim DJ, Kataoka K, Sano S, Connolly K, Kiguchi K, DiGiovanni J. Targeted disruption of Bcl-xL in mouse keratinocytes inhibits both UVB- and chemically induced skin carcinogenesis. Mol Carcinog. 2009;48:873–85. doi: 10.1002/mc.20527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore T, Beltran L, Carbajal S, Hursting SD, Digiovanni J. Energy Balance Modulates Mouse Skin Tumor Promotion through Altered IGF-1R and EGFR Crosstalk. Cancer Prev Res (Phila) 2012;5:1236–46. doi: 10.1158/1940-6207.CAPR-12-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nunez NP, Perkins SN, Smith NC, Berrigan D, Berendes DM, Varticovski L, et al. Obesity accelerates mouse mammary tumor growth in the absence of ovarian hormones. Nutr Cancer. 2008;60:534–41. doi: 10.1080/01635580801966195. [DOI] [PubMed] [Google Scholar]
- 34.Deriabina ON, Plotnikova NA, Anisimov VN. Melatonin and metformin inhibit skin carcinogenesis induced by benz(a)pyrene in mice. Vopr Onkol. 2010;56:583–7. [PubMed] [Google Scholar]
- 35.Man'cheva TA, Demidov DV, Plotnikova NA, Kharitonova TV, Pashkevich IV, Anisimov VN. Melatonin and metformin inhibit skin carcinogenesis and lipid peroxidation induced by benz(a)pyrene in female mice. Bull Exp Biol Med. 2011;151:363–5. doi: 10.1007/s10517-011-1331-y. [DOI] [PubMed] [Google Scholar]
- 36.Wu CL, Qiang L, Han W, Ming M, Viollet B, He YY. Role of AMPK in UVB-induced DNA damage repair and growth control. Oncogene. 2013;32:2682–9. doi: 10.1038/onc.2012.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhary SC, Kurundkar D, Elmets CA, Kopelovich L, Athar M. Metformin, an antidiabetic agent reduces growth of cutaneous squamous cell carcinoma by targeting mTOR signaling pathway. Photochem Photobiol. 2012;88:1149–56. doi: 10.1111/j.1751-1097.2012.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–69. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 39.Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov. 2012;2:778–90. doi: 10.1158/2159-8290.CD-12-0263. [DOI] [PubMed] [Google Scholar]
- 40.van Veelen W, Korsse SE, van de Laar L, Peppelenbosch MP. The long and winding road to rational treatment of cancer associated with LKB1/AMPK/TSC/mTORC1 signaling. Oncogene. 2011;30:2289–303. doi: 10.1038/onc.2010.630. [DOI] [PubMed] [Google Scholar]
- 41.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–12. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 42.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–8. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 43.Algire C, Zakikhani M, Blouin MJ, Shuai JH, Pollak M. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocr Relat Cancer. 2008;15:833–9. doi: 10.1677/ERC-08-0038. [DOI] [PubMed] [Google Scholar]
- 44.Dowling RJ, Niraula S, Stambolic V, Goodwin PJ. Metformin in cancer: translational challenges. J Mol Endocrinol. 2012;48:R31–43. doi: 10.1530/JME-12-0007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.