Abstract
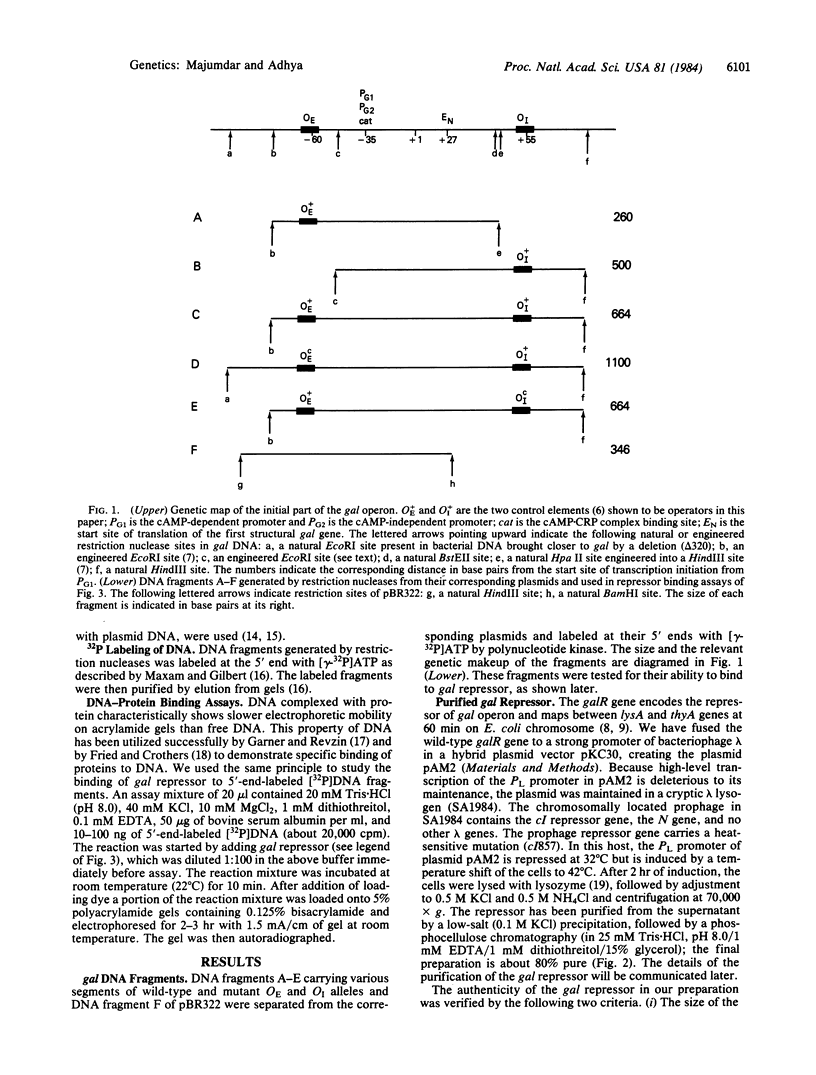
Genetic and DNA base sequence analyses of cis-dominant mutations that derepress the gal operon of Escherichia coli suggested the existence of two operator loci needed for gal repression. One (OE) is located immediately upstream to the two overlapping gal promoters and the other (OI) is inside the first structural gene. We have investigated the ability of wild-type and mutant OE and OI DNA sequences to bind to gal repressor. The repressor has been purified from cells containing a multicopy plasmid in which the repressor gene is brought under the control of phage lambda PL promoter. The DNA-repressor interactions are detected by the change in electrophoretic mobility of labeled DNA that accompanies its complex formation with repressor protein. The purified repressor shows concentration-dependent binding to both O+E and O+I but not to OEc and OIc sequences. These results authenticate the proposed operator role of the two homologous gal DNA control elements and thereby establish that the negative control of the gal operon requires repressor binding at both OE and OI, which are separated by greater than 90 base pairs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Echols H. Glucose effect and the galactose enzymes of Escherichia coli: correlation between glucose inhibition of induction and inducer transport. J Bacteriol. 1966 Sep;92(3):601–608. doi: 10.1128/jb.92.3.601-608.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhya S., Miller W. Modulation of the two promoters of the galactose operon of Escherichia coli. Nature. 1979 Jun 7;279(5713):492–494. doi: 10.1038/279492a0. [DOI] [PubMed] [Google Scholar]
- Arai K., McMacken R., Yasuda S., Kornberg A. Purification and properties of Escherichia coli protein i, a prepriming protein in phi X174 DNA replication. J Biol Chem. 1981 May 25;256(10):5281–5286. [PubMed] [Google Scholar]
- BUTTIN G. M'ECANISMES R'EGULATEURS DANS LA BIOSYNTH'ESE DES ENZYMES DU M'ETABOLISME DU GALACTOSE CHEZ ESCHERICHIA COLI K12. I. LA BIOSYNTH'ESE INDUITE DE LA GALACTOKINASE ET L'INDUCTION SIMULTAN'EE DE LA S'EQUENCE ENZYMATIQUE. J Mol Biol. 1963 Aug;7:164–182. doi: 10.1016/s0022-2836(63)80044-3. [DOI] [PubMed] [Google Scholar]
- BUTTIN G. M'ECANISMES R'EGULATEURS DANS LA BIOSYNTH'ESE DES ENZYMES DU M'ETABOLISME DU GALACTOSE CHEZ ESCHERICHIA COLI K12. II. LE D'ETERMINISME G'EN'ETIQUE DE LA R'EGULATION. J Mol Biol. 1963 Aug;7:183–205. doi: 10.1016/s0022-2836(63)80045-5. [DOI] [PubMed] [Google Scholar]
- Busby S., Irani M., Crombrugghe B. Isolation of mutant promoters in the Escherichia coli galactose operon using local mutagenesis on cloned DNA fragments. J Mol Biol. 1982 Jan 15;154(2):197–209. doi: 10.1016/0022-2836(82)90060-2. [DOI] [PubMed] [Google Scholar]
- Culard F., Maurizot J. C. Lac repressor - lac operator interaction. Circular dichroism study. Nucleic Acids Res. 1981 Oct 10;9(19):5175–5184. doi: 10.1093/nar/9.19.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLauro R., Taniguchi T., Musso R., de Crombrugghe B. Unusual location and function of the operator in the Escherichia coli galactose operon. Nature. 1979 Jun 7;279(5713):494–500. doi: 10.1038/279494a0. [DOI] [PubMed] [Google Scholar]
- Fiethen L., Starlinger P. Mutations in the galactose-operator. Mol Gen Genet. 1970;108(4):322–330. doi: 10.1007/BF00267769. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz H. J., Bicknäse H., Gleumes B., Heibach C., Rosahl S., Ehring R. Characterization of two mutations in the Escherichia coli galE gene inactivating the second galactose operator and comparative studies of repressor binding. EMBO J. 1983;2(12):2129–2135. doi: 10.1002/j.1460-2075.1983.tb01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani M. H., Orosz L., Adhya S. A control element within a structural gene: the gal operon of Escherichia coli. Cell. 1983 Mar;32(3):783–788. doi: 10.1016/0092-8674(83)90064-8. [DOI] [PubMed] [Google Scholar]
- Irani M., Orosz L., Busby S., Taniguchi T., Adhya S. Cyclic AMP-dependent constitutive expression of gal operon: use of repressor titration to isolate operator mutations. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4775–4779. doi: 10.1073/pnas.80.15.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso R. E., Di Lauro R., Adhya S., de Crombrugghe B. Dual control for transcription of the galactose operon by cyclic AMP and its receptor protein at two interspersed promoters. Cell. 1977 Nov;12(3):847–854. doi: 10.1016/0092-8674(77)90283-5. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Adhya S., Gottesman M. E., Pastan I. In vitro repression of the transcription of gas operon by purified gal repressor. Proc Natl Acad Sci U S A. 1973 Feb;70(2):334–338. doi: 10.1073/pnas.70.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gorman R. B., Dunaway M., Matthews K. S. DNA binding characteristics of lactose repressor and the trypsin-resistant core repressor. J Biol Chem. 1980 Nov 10;255(21):10100–10106. [PubMed] [Google Scholar]
- Parks J. S., Gottesman M., Shimada K., Weisberg R. A., Perlman R. L., Pastan I. Isolation of the gal repressor. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1891–1895. doi: 10.1073/pnas.68.8.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saedler H., Gullon A., Fiethen L., Starlinger P. Negative control of the galactose operon in E. coli. Mol Gen Genet. 1968;102(1):79–88. doi: 10.1007/BF00341872. [DOI] [PubMed] [Google Scholar]
- Saint-Girons I., Fritz H. J., Shaw C., Tillmann E., Starlinger P. Integration specificity of an artificial kanamycin transposon constructed by the in vitro insertion of an internal Tn5 fragment into IS2. Mol Gen Genet. 1981;183(1):45–50. doi: 10.1007/BF00270136. [DOI] [PubMed] [Google Scholar]
- Takanami M. RNA polymerase nascent product analysis. Methods Enzymol. 1980;65(1):497–499. doi: 10.1016/s0076-6879(80)65058-7. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., O'Neill M., de Crombrugghe B. Interaction site of Escherichia coli cyclic AMP receptor protein on DNA of galactose operon promoters. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5090–5094. doi: 10.1073/pnas.76.10.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wilcken-Bergmann B., Müller-Hill B. Sequence of galR gene indicates a common evolutionary origin of lac and gal repressor in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2427–2431. doi: 10.1073/pnas.79.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]