Abstract
Reactive carbonyls are widespread species in living organisms and mainly known for their damaging effects. The most abundant reactive carbonyl species (RCS) are derived from oxidation of carbohydrates, lipids, and amino acids. Chemical modification of proteins, nucleic acids, and aminophospholipids by RCS results in cytotoxicity and mutagenicity. In addition to their direct toxicity, modification of biomolecules by RCS gives rise to a multitude of adducts and cross links that are increasingly implicated in aging and pathology of a wide range of human diseases. Understanding of the relationship between metabolism of RCS and the development of pathological disorders and diseases may help to develop effective approaches to prevent a number of disorders and diseases. On the other hand, constant persistence of RCS in cells suggests that they perform some useful role in living organisms. The most beneficial effects of RCS are their establishment as regulators of cell signal transduction and gene expression. Since RCS can modulate different biological processes, new tools are required to decipher the precise mechanisms underlying dual effects of RCS.
1. Introduction
Reactive carbonyl species (RCS) include a large number of biological compounds with one or more carbonyl groups that are continuously produced in various groups of organisms, from bacteria to man, and mainly known for their damaging effects. The steady-state concentration of RCS is maintained in a certain range and, according to homeostasis theory, fluctuates in the cell similar to other parameters. However, RCS level may leave the range due to changes occurring in RCS production and/or elimination. An increase in steady-state level of reactive carbonyls is the key cause of the phenomenon called carbonyl stress, a contributing factor to aging, pathogenesis of metabolic syndrome, chronic complications associated with diabetes and renal failure, neurodegenerative, and other disorders [1–9]. On the other hand, constant persistence of RCS in the cells at low concentrations can be considered to be the emergence of RCS as an important part of immune response, regulators of gene expression, and cellular signaling messengers [2, 8, 10]. Therefore, like other reactive species, RCS play a dual role in vivo which appears to be dose- and time-dependent [10–15].
2. Generation of Reactive Carbonyls In Vivo
Reactive carbonyls are compounds found widespread throughout biological life and can be endogenous or exogenously derived. More than 20 RCS have been identified in biological samples [10]. Figure 1 demonstrates most common saturated and unsaturated RCS detected in living organisms. Some reactive carbonyls (e.g., acrolein, crotonaldehyde, glyoxal, acetone, and formaldehyde) are ubiquitous industrial pollutants which can readily enter the cell from the environment [16–18]. Other exogenous sources of RCS are products of organic pharmaceutical chemistry, cigarette smoke, food additives, and browned food [19–24].
Figure 1.
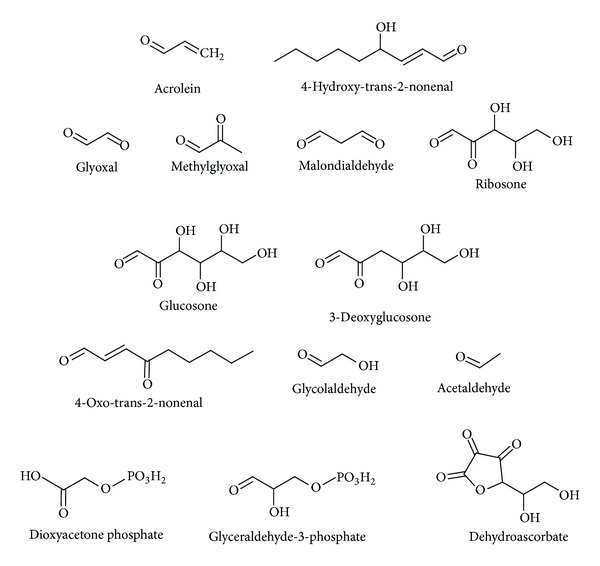
The structures of most common biological reactive carbonyl species.
There is increasing evidence that RCS are produced endogenously [10, 15, 25, 26]. Table 1 demonstrates most widespread biological reactive carbonyls generated during nonenzymatic or enzymatic reactions in vivo. A wide diversity of intracellular unstable RCS is readily produced by such nonenzymatic processes as lipid peroxidation, amino acid oxidation, and glycation [2, 9, 10, 27–31].
Table 1.
Carbonyl compounds and sources of their generation in vivo.
| Nonenzymatic | Enzymatic | |||
|---|---|---|---|---|
| Peroxidation of lipids | Glycation | Oxidation of amino acids | Polyol pathway | Glycolysis |
| Malondialdehyde 4-Hydroxy-trans-2-nonenal 4-Oxo-trans-2-nonenal Glyoxal Methylglyoxal Acrolein Crotonaldehyde Hexanal |
Glyoxal Methylglyoxal Glucosone 3-Deoxyglucosone Acrolein |
Glyoxal Methylglyoxal Acrolein Glycolaldehyde 2-Hydroxypropanal |
3-Deoxyglucosone 3-Deoxyfructose |
Acetaldehyde Glyceraldehyde-3-phosphate Dioxyacetone phosphate Methylglyoxal |
A key feature of lipid peroxidation is the free radical chain breakdown of polyunsaturated fatty acid residues in cholesterol esters, phospholipids, and triglycerides that yields a broad array of RCS, including malondialdehyde (MDA), glyoxal (GO), 4-hydroxy-2-nonenal (4-HNE), and 4-oxo-trans-2-nonenal [2, 10, 32, 33]. Such amino acids as threonine and glycine can be converted to RCS (e.g., methylglyoxal (MGO)) or their precursors (e.g., aminoacetone and succinylacetone) during oxidative modification [34].
Glycation, a nonenzymatic process involved reducing carbohydrates (e.g., glucose and fructose), attracts considerable attention during the last decades [15, 35–37]. This could be attributed to either an excessive consumption of carbohydrate sweeteners in the modern human diet [37] or their opposite dual effects in vivo [11, 14, 38–45]. Potential mechanisms underlying both detrimental and beneficial effects of reducing carbohydrates are under debate. Recently we suggested the involvement of RCS and reactive oxygen species (ROS) in both the cytotoxic and defensive effects of such reducing carbohydrate as fructose [14, 15].
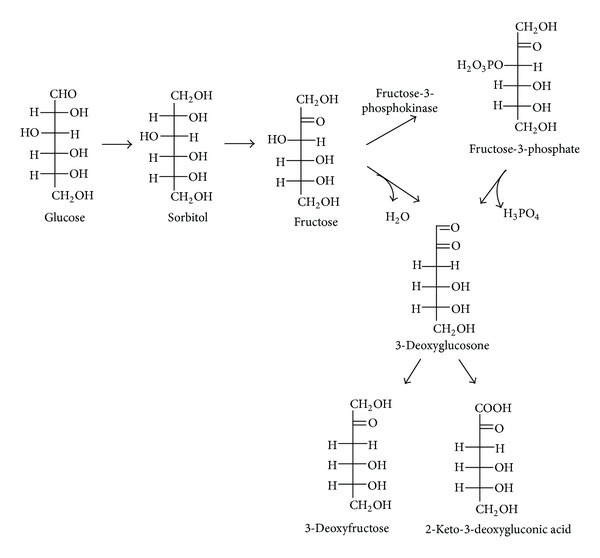
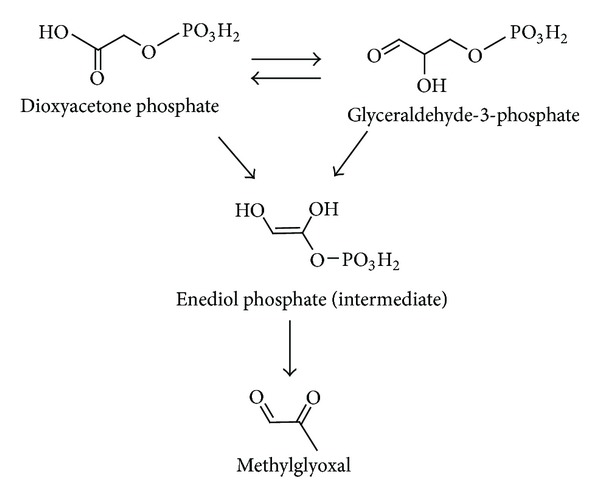
In several enzymatic pathways involving carbohydrates MGO, GO, and 3-deoxyglucosone (3-DG) are generated as side products (Table 1). The polyol pathway is a two-step metabolic pathway in which glucose is reduced to sorbitol, which is then converted to fructose (Figure 2). Generally, polyol pathway is associated with the production of 3-DG [46, 47]. Glycolysis is probably the most thoroughly studied metabolic pathway, the major enzymatic source of MGO in vivo [48–51]. Figure 3 demonstrates the mechanisms of MGO generation in glycolysis. Enediol phosphate, an intermediate of triosophosphate isomerase reaction, may escape from the active site of the enzyme and be rapidly decomposed to MGO and inorganic phosphate. MGO can also be formed from the intermediates in the enzymatic oxidation of ketone bodies [29, 31]. Different RCS are generated in vivo by activated human phagocytes. It has been found that stimulated neutrophils employed the myeloperoxidase-H2O2-chloride system produce α-hydroxy- and α,β-unsaturated aldehydes from hydroxy-amino acids in high yield [52].
Figure 2.
Formation of methylglyoxal as a by product of glycolysis.
Figure 3.
Polyol pathway as a source of formation of reactive carbonyl species.
The steady-state concentration of such carbonyl metabolic intermediates as acetaldehyde, glyceraldehyde-3-phosphate, and dioxyacetone phosphate are typically low in the cell because of their rapid utilization by the next step of the pathway. However, the concentration of reactive carbonyl by products in enzymatic reactions is not so tightly controlled in vivo. Therefore, under certain conditions, biological effects of these carbonyl side products may be more potent than the effect caused by carbonyl metabolic intermediates.
3. Deleterious Effects of Reactive Carbonyls
Like most intermediates and by products of metabolism, RCS are electrophilic and therefore highly reactive toward different cellular constituents majority of which are nucleophiles [32]. It should be noted that unsaturated RCS are usually an order of magnitude more reactive than their saturated counterparts. Therefore, most of biological damages caused by RCS are related to α, β-unsaturated aldehydes, dialdehydes and keto-aldehydes [2, 53]. Such strong nucleophilic sites as thiol, imidazole, and hydroxyl groups of biomolecules are the most attractive targets for electrophilic attacks. MDA, GO, MGO, 3-DG, glucosone, and ribosone are highly reactive α- or β-dicarbonyl compounds (Figure 1). Dicarbonyls react with nucleophilic groups of macromolecules like proteins, nucleic acids, and aminophospholipids, resulting in their irreversible modification and formation of a variety of adducts and cross links collectively named advanced glycation or lipoxidation end products (AGEs, ALEs) [53–59].
In general, ALEs and AGEs are poorly degraded complexes, accumulation of which increases with age. These adducts have been detected in various tissues and peripheral blood and considered to be pathogenic. Carboxymethyl phosphatidylethanolamine and carboxymethylguanosine represent the ALEs/AGEs derived from GO and MGO interaction with nucleic acids and phospholipids, respectively (Figure 4). Adducts such as GO-lysine dimmer, MGO-lysine dimmer, carboxymethyllysine, carboxymethylcysteine, and argpyrimidine are the most common ALEs/AGEs resulted from protein modification (Figure 4). RCS react preferentially with arginine, cysteine, and lysine residues with high reaction rates [35]. Physiological RCS may play important role in pathogenesis because of high abundance of the amino acid residues within protein active sites [60–63]. Carboxymethyllysine was the first AGE isolated from glycated proteins in vivo and together with pentosidine and glucosepane (Figure 4) was later recognized as one of the most important indicator of glycation in living organisms [55, 57, 64]. RCS as well as ALEs/AGEs are found to induce most features of the metabolic syndrome, including glucose intolerance and hyperglycemia, abdominal obesity, elevated blood pressure, inflammation, and renal injury [57, 59]. It should be noted that ALEs/AGEs may continue covalent interactions with biomolecules giving more complex cross-links. In addition, ALEs and AGEs are efficient sources of RCS and ROS in vivo [1, 28, 58, 65–67].
Figure 4.
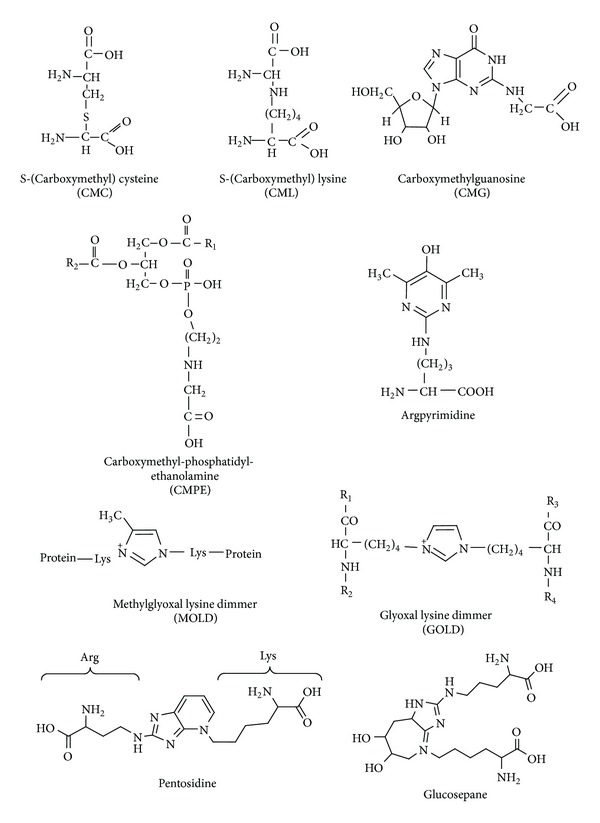
The structures of most common biological advanced lipoxidation and glycation end products.
Generally, biological effects by RCS seems somewhat similar to those by ROS thus it can be expected that physicochemical properties of both reactive groups should be similar as well. However, RCS have a relatively long half-life time and higher stability, in contrast to ROS. For instance, reactive carbonyls have average half-life from minutes to hours [2, 53]. At the same time, half-life of some ROS ranges from 10−9 to 10−6 s [68, 69]. It is well known that such uncharged ROS as H2O2 and HO2 • are able to cross biological membranes and diffuse for relatively long distances in the intracellular environment. Higher stability of uncharged RCS allows them even to escape from the cell and interact with targets far from the sites of their generation.
4. Beneficial Impacts of Reactive Carbonyl Species
Although excessive RCS may lead to pathological disorders and accelerate aging, the reactive species may also exert beneficial effects at low levels. An obvious question arises: what are the “excessive” and “low” concentrations of RCS in the cell? A measurement of physiological concentration of RCS is often problematic due to (i) a vast variety of RCS generated in vivo by different mechanisms; (ii) simultaneous production, degradation, and excretion of RCS; (iii) dependence of the above processes on different factors (intensity of metabolism, oxygen concentration, temperature, etc.); and (iv) since the cell is not homogenous structure, RCS concentrations may differ to large extent in different cellular compartments. In addition, there are no standard techniques to measure RCS concentration in vivo, therefore controversial results can be obtained in different laboratories. Nonetheless, numerous studies report the RCS levels in biological samples. For instance, in plasma of healthy individuals the total concentration of RCS derived from lipid peroxidation is found below 1 μM [10]. The physiological concentration of 4-HNE and MGO in plasma ranges from 0.3 to 0.7 μM and from 0.12 to 0.65 μM, respectively [8, 34, 70–72]. So, if the concentration of RCS does not exceed “normal” level, RCS involved in many of the cellular functions may have beneficial effects.
Phagocytic white blood cells that are of central importance in host defense mechanisms implicate RCS against invading pathogens. It is demonstrated that, besides certain ROS, myeloperoxidase generates such RCS as glycolaldehyde, 2-hydroxypropanal, acetone, and acrolein [52, 73]. Being highly reactive and toxic, some RCS are found to be potent anticancer agents. In the 1960s, it was proposed and then provided strong experimental evidence that MGO acted as an anticancer agent [72, 74–77]. Subsequent studies had indicated that MGO inhibited both glycolysis and mitochondrial respiration of specifically malignant cells [76, 78, 79]. Besides anticancer effect, RCS demonstrate antibacterial, antiprotozoal, antifungal, and antiviral activity [72].
5. Reactive Carbonyls in Signaling/Transcription Regulation
Understanding of the roles of RCS in intracellular signaling has evolved during the last decades. This was preceded by a discussion of RCS ability to participate in signaling/transcription regulation. The main question was how RCS meet the requirements for signaling molecules? Regardless of the nature of molecules, they can be recognized as signal if: (i) their level is tightly controlled in vivo; (ii) they are sufficiently stable, small, and hydrophobic to diffuse across biological membranes; (iii) they bind to specific receptors, triggering a chain of events within the cell; and (iv) their signaling effects are reversible. The enzymatic control of RCS production/elimination, RCS ability to cross biological membranes and diffuse for relatively long distances are the undoubted arguments for signaling role of reactive carbonyls. Recent studies from several laboratories show that RCS activate specific receptors [8, 11, 33]. It is also supposed that degradation and resynthesis of RCS-modified proteins are involved in the reversible aspect of RCS signaling [8]. For all the above-mentioned reasons, RCS seem among the best candidates for signaling purposes.
Accumulating evidence from the last decades has shown that such RCS as 4-HNE, MDA, MGO, and GO can function as messengers that activate or inhibit signaling pathways under physiologic or pathologic conditions (Figure 5). They affect signaling mechanisms in a concentration- and time-dependent manner [10–13]. It has been shown, for example, that low levels of 4-HNE promote proliferation [80], but at higher concentrations it induces differentiation and apoptosis [81–83]. The underlying mechanisms by which RCS act as signaling messengers have been discussed extensively [8, 11, 80, 84–87]. Several cell signaling pathways, including the stress responses, proapoptotic events, kinase/phosphatase activities, and nuclear transcription factor function can be modulated by RCS in microorganisms, plant, and animals [85, 86, 88].
Figure 5.
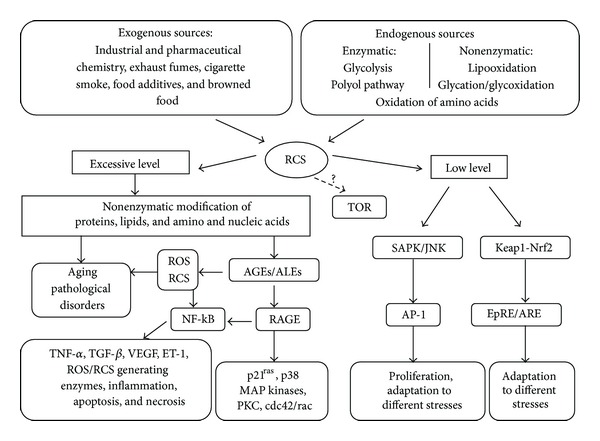
Involvement of reactive carbonyl species in signaling/transcription regulation.
Numerous studies from different laboratories using a variety of mammalian cell lines have shown that 4-HNE induces SAPK/JNK signaling pathway [11, 81, 89, 90]. SAPK/JNK is stress-activated protein kinase/c-Jun NH(2)-terminal kinase, a member of MAPK family, activated by different types of stress and extracellular signals. SAPK/JNK activation plays essential role in organogenesis during mammal's development by regulating cell survival, apoptosis, and proliferation [91]. In hepatic cells, 4-HNE activates JNK through direct binding [89]. In other cells, 4-HNE activates JNK through the redox-sensitive MAPK kinase cascade [90]. It is suggested that 4-HNE-induced JNK activation promotes its translocation in the nucleus where JNK-dependent phosphorylation of c-Jun and the transcription factor activator protein (AP-1) binding take place [81, 92]. The AP-1 proteins are highly conserved among eukaryotes and belong to unspecific group of transcription factors controlling gene response to different signals. In mammalian cells, AP-1 proteins regulate the transcription of a number of genes involved in proliferation, differentiation, immune response, and adaptation to different stresses [88].
The vast majority of RCS, including 4-HNE and MDA, modulate transcription through the Keap1-Nrf2 pathway, which regulates the electrophile response element/antioxidant response element (EpRE/ARE) [33, 88, 93]. The activity of transcription factor Nrf2 (NF-E2-related factor 2) is dependent on its redox-sensitive inhibitor Keap1 (kelch-like ECH-associated protein 1). Under nonstressful conditions, the transcription factor Nrf2 is bound to Keap1. This complex promotes the ubiquitination of Nrf2 that followed by proteasomal degradation [93, 94]. Under cell exposure to RCS due to change of its conformation Keap1 becomes unable to form the complex with Nrf2 that results in the increased Nrf2 concentration. Further Nrf2 migrates into the nucleus, where it upregulates the transcription of target genes encoding superoxide dismutase, catalase, peroxiredoxin, glutathione peroxidase, thioredoxin reductase, γ-glutamylcysteine synthase, glutathione reductase thioredoxine reductase, heme oxygenase, quinone reductase, glutathione S-transferases, glutathione reductase, and other defensive proteins [12, 84, 87, 93, 95]. Interestingly, the Arabidopsis thaliana genome does not appear to encode Nrf2 homologues, although there are genes showing similarity to Keap1 that are considered to be involved in RCS signaling in plants [84]. The strong parallels in RCS stimulated gene expression are found in plants and animals (e.g., glutathione S-transferases, glutamylcysteine ligase, glutathione reductase, thioredoxin reductase, quinone reductase, heme oxygenase, and epoxide hydrolase). In Saccharomyces cerevisiae, some of these genes are under control of the yeast AP-1, called Yap1p transciptional factor that can be activated by MGO [96].
In the middle of the 1980s, it was demonstrated that macrophages could specifically recognize, uptake, and degrade AGEs/ALEs-modified proteins in vitro [97]. This observation led to an active search for high affinity AGEs/ALEs receptors on various cells. The first discovered multiligand receptor able to bind AGEs/ALEs-modified proteins with high affinity was RAGE (the receptor for AGE, member of the immunoglobulin superfamily of cell surface molecules) [98]. RAGE interacts with distinct molecules implicated in homeostasis, inflammation, and certain diseases [55, 99].
In the presence of extracellular AGEs/ALEs, susceptible cells can rapidly upregulate expression of RAGE on their membranes (Figure 5). Engagement of RAGE by a ligand triggers activation of key cell signalling pathways such as p21ras, protein kinase C, MAP kinases, cdc42/rac, and NF-kB, thereby reprogramming cellular properties [2, 99, 100]. For example, activation of nuclear factor NF-kB due to AGEs/ALEs and RAGE interaction was shown to be involved in the regulation of the gene transcription for various factors: endothelin-1 (ET-1), vascular endothelial growth factor (VEGF), transforming growth factor β (TGF-β), and tumor necrosis factor α (TNF-α) [55, 101]. Also, NF-kB controls the expression of almost 100 proinflammatory genes encoding cytokines, adhesion molecules, and ROS/RCS generating enzymes such as NADPH-oxidase, superoxide dismutase, inducible nitric oxide synthase, and myeloperoxidase [100–103].
Search for new AGEs/ALEs receptors has resulted in the identification of macrophage scavenger receptors (MSR) types A and B1 (CD36), oligosaccharyl transferase-48 termed AGE receptor 1 (AGE-R1), 80K-H phosphoprotein (AGE-R2), and galectin-3 (AGE-R3) [100, 101], but the best studied is the RAGE receptor.
The TOR (target of rapamycin) signaling pathway integrates a large number of environmental changes and regulates cell growth and aging through control of certain anabolic and catabolic processes [104]. In clinical biology, TOR is implicated in many diseases. Although there is no information on the relationship between RCS and TOR pathway, it has been suggested that rapamycin decreases MGO generation in vivo by inhibiting TOR activity [105]. In our preliminar experiments, yeast parental strain and its isogenic derivatives defective in TOR demonstrated significantly different intracellular levels of RCS and susceptibilities to RCS-induced stress. Therefore, potential interplay between certain reactive carbonyls and TOR signaling cascade cannot be excluded.
6. Conclusions
There is sufficient experimental evidence that reactive species, and RCS in particular, have the ability to modulate homeostasis at various levels, probably by both damaging biological molecules and participating in signaling/transcription regulation. Different signaling networks are involved in both deleterious and beneficial effects of reactive carbonyls. This dual nature of RCS biological effects appears to be dose- and time-dependent. Since RCS can modulate such biological processes as proliferation, differentiation, reproduction, maintenance of metabolic equilibrium, immune response, adaptation to different stresses, apoptosis, necrosis, aging and development of certain pathologies, new tools are required to decipher the mechanisms underlying the dual effects. Understanding of the relationship between metabolism of RCS and the development of pathological disorders and diseases will also make a contribution not only to our knowing of how RCS cause biological effects, but also on how to define effective therapeutic approaches to prevent them.
Abbreviations
- AP-1:
Activator protein 1
- 3-DG:
3-deoxyglucosone
- 4-HNE:
4-hydroxy-2-nonenal
- AGEs:
Advanced glycation end products
- ALEs:
Advanced lipoxidation end products
- EpRE/ARE:
Electrophile response element/antioxidant response element
- GO:
Glyoxal
- Keap1:
Kelch-like ECH-associated protein 1
- MDA:
Malondialdehyde
- MGO:
Methylglyoxal
- Nrf2:
NF-E2-related factor 2
- RAGE:
Receptor for AGE; RCS, reactive carbonyl species
- ROS:
Reactive oxygen species
- SAPK/JNK:
Stress-activated protein kinase/c-Jun NH(2)-terminal kinase.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Gugliucci A. Glycation as the glucose link to diabetic complications. The Journal of the American Osteopathic Association. 2000;100(10):621–634. [PubMed] [Google Scholar]
- 2.Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free Radical Biology and Medicine. 2000;28(12):1685–1696. doi: 10.1016/s0891-5849(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 3.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 4.Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer's disease brain and is toxic to primary hippocampal cultures. Neurobiology of Aging. 2001;22(2):187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- 5.Picklo MJ, Sr., Montine TJ, Amarnath V, Neely MD. Carbonyl toxicology and Alzheimer's disease. Toxicology and Applied Pharmacology. 2002;184(3):187–197. doi: 10.1006/taap.2002.9506. [DOI] [PubMed] [Google Scholar]
- 6.Bourajjaj M, Stehouwer CDA, Van Hinsbergh VWM, Schalkwijk CG. Role of methylglyoxal adducts in the development of vascular complications in diabetes mellitus. Biochemical Society Transactions. 2003;31(6):1400–1402. doi: 10.1042/bst0311400. [DOI] [PubMed] [Google Scholar]
- 7.Chang T, Wu L. Methylglyoxal, oxidative stress, and hypertension. Canadian Journal of Physiology and Pharmacology. 2006;84(12):1229–1238. doi: 10.1139/y06-077. [DOI] [PubMed] [Google Scholar]
- 8.Forman HJ, Fukuto JM, Miller T, Zhang H, Rinna A, Levy S. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Archives of Biochemistry and Biophysics. 2008;477(2):183–195. doi: 10.1016/j.abb.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semchyshyn HM, Lushchak VI. Interplay between oxidative and carbonyl stresses: molecular mechanisms, biological effects and therapeutic strategies of protection. In: Lushchak VI, Semchyshyn HM, editors. Oxidative Stress—Molecular Mechanisms and Biological Effects. Rijeka, Croatia: InTech; 2012. pp. 15–46. [Google Scholar]
- 10.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radical Biology and Medicine. 2009;47(5):469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Sharma R, Sharma A, Awasthi S, Awasthi YC. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochimica Polonica. 2003;50(2):319–336. [PubMed] [Google Scholar]
- 12.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radical Biology and Medicine. 2009;47(9):1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forman HJ. Reactive oxygen species and α,β-unsaturated aldehydes as second messengers in signal transduction. Annals of the New York Academy of Sciences. 2010;1203:35–44. doi: 10.1111/j.1749-6632.2010.05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semchyshyn HM, Lozinska LM. Fructose protects baker’s yeast against peroxide stress: potential role of catalase and superoxide dismutase. FEMS Yeast Research. 2012;12(7):761–773. doi: 10.1111/j.1567-1364.2012.00826.x. [DOI] [PubMed] [Google Scholar]
- 15.Semchyshyn HM. Fructation in vivo: detrimental and protective effects of fructose. BioMed Research International. 2013;2013:9 pages. doi: 10.1155/2013/343914.343914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trotter EW, Collinson EJ, Dawes IW, Grant CM. Old yellow enzymes protect against acrolein toxicity in the yeast Saccharomyces cerevisiae . Applied and Environmental Microbiology. 2006;72(7):4885–4892. doi: 10.1128/AEM.00526-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X-Y, Zhu M-X, Xie J-P. Mutagenicity of acrolein and acrolein-induced DNA adducts. Toxicology Mechanisms and Methods. 2010;20(1):36–44. doi: 10.3109/15376510903530845. [DOI] [PubMed] [Google Scholar]
- 18.Seo Y-K, Baek S-O. Characterization of carbonyl compounds in the ambient air of an industrial city in Korea. Sensors. 2011;11(1):949–963. doi: 10.3390/s110100949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uribarri J, Cai W, Peppa M, et al. Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging. The Journals of Gerontology A. 2007;62(4):427–433. doi: 10.1093/gerona/62.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birlouez-Aragon I, Morales F, Fogliano V, Pain J-P. The health and technological implications of a better control of neoformed contaminants by the food industry. Pathologie Biologie. 2010;58(3):232–238. doi: 10.1016/j.patbio.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Colombo G, Aldini G, Orioli M, et al. Water-soluble α,β-unsaturated aldehydes of cigarette smoke induce carbonylation of human serum albumin. Antioxidants and Redox Signaling. 2010;12(3):349–364. doi: 10.1089/ars.2009.2806. [DOI] [PubMed] [Google Scholar]
- 22.Dini L. Phagocytosis of dying cells: influence of smoking and static magnetic fields. Apoptosis. 2010;15(9):1147–1164. doi: 10.1007/s10495-010-0490-z. [DOI] [PubMed] [Google Scholar]
- 23.Robert L, Robert A-M, Labat-Robert J. The Maillard reaction—illicite (bio)chemistry in tissues and food. Pathologie Biologie. 2011;59(6):321–328. doi: 10.1016/j.patbio.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Sticozzi C, Belmonte G, Pecorelli A, et al. Cigarette smoke affects keratinocytes SRB1 expression and localization via H2O2 production and HNE protein adducts formation. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033592.e33592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semchyshyn HM, Lozinska LM, Miedzobrodzki J, Lushchak VI. Fructose and glucose differentially affect aging and carbonyl/oxidative stress parameters in Saccharomyces cerevisiae cells. Carbohydrate Research. 2011;346(7):933–938. doi: 10.1016/j.carres.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Lozinska LM, Semchyshyn HM. Fructose as a factor of carbonyl and oxidative stress development and accelerated aging in the yeast Saccharomyces cerevisiae . Ukrainian Biochemical Journal. 2011;83(4):67–76. [PubMed] [Google Scholar]
- 27.Metz TO, Alderson NL, Thorpe SR, Baynes JW. Pyridoxamine, an inhibitor of advanced glycation and lipoxidation reactions: a novel therapy for treatment of diabetic complications. Archives of Biochemistry and Biophysics. 2003;419(1):41–49. doi: 10.1016/j.abb.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 28.Thornalley PJ. Dicarbonyl intermediates in the Maillard reaction. Annals of the New York Academy of Sciences. 2005;1043:111–117. doi: 10.1196/annals.1333.014. [DOI] [PubMed] [Google Scholar]
- 29.Turk Z. Glycotoxines, carbonyl stress and relevance to diabetes and its complications. Physiological Research. 2010;59(2):147–156. doi: 10.33549/physiolres.931585. [DOI] [PubMed] [Google Scholar]
- 30.Onyango AN. Small reactive carbonyl compounds as tissue lipid oxidation products, and the mechanisms of their formation thereby. Chemistry and Physics of Lipids. 2012;165(7):777–786. doi: 10.1016/j.chemphyslip.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Kalapos MP. Where does plasma methylglyoxal originate from? Diabetes Research and Clinical Practice. 2013;99(3):260–271. doi: 10.1016/j.diabres.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Zimniak P. Relationship of electrophilic stress to aging. Free Radical Biology and Medicine. 2011;51(6):1087–1105. doi: 10.1016/j.freeradbiomed.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yadav UCS, Ramana KV. Regulation of NF-κB-induced inflammatory signaling by lipid peroxidation-derived aldehydes. Oxidative Medicine and Cellular Longevity. 2013;2013:11 pages. doi: 10.1155/2013/690545.690545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalapos MP. The tandem of free radicals and methylglyoxal. Chemico-Biological Interactions. 2008;171(3):251–271. doi: 10.1016/j.cbi.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Rabbani N, Thornalley PJ. Glycation research in amino acids: a place to call home. Amino Acids. 2012;42(4):1087–1096. doi: 10.1007/s00726-010-0782-1. [DOI] [PubMed] [Google Scholar]
- 36.Bray GA. Energy and fructose from beverages sweetened with sugar or high-fructose corn syrup pose a health risk for some people. Advances in Nutrition. 2013;4(2):220–225. doi: 10.3945/an.112.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White JS. Challenging the fructose hypothesis: new perspectives on fructose consumption and metabolism. Advances in Nutrition. 2013;4(2):246–256. doi: 10.3945/an.112.003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bogdanović J, Mojović M, Milosavić N, Mitrović A, Vucinić Z, Spasojević I. Role of fructose in the adaptation of plants to cold-induced oxidative stress. European Biophysics Journal. 2008;37(7):1241–1246. doi: 10.1007/s00249-008-0260-9. [DOI] [PubMed] [Google Scholar]
- 39.González-Siso MI, García-Leiro A, Tarrío N, Cerdán ME. Sugar metabolism, redox balance and oxidative stress response in the respiratory yeast Kluyveromyces lactis . Microbial Cell Factories. 2009;8(article 46) doi: 10.1186/1475-2859-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong Q, Yang K, Wong SM, O'Brien PJ. Hepatocyte or serum albumin protein carbonylation by oxidized fructose metabolites: glyceraldehyde or glycolaldehyde as endogenous toxins? Chemico-Biological Interactions. 2010;188(1):31–37. doi: 10.1016/j.cbi.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Yang K, Feng C, Lip H, Bruce WR, O'Brien PJ. Cytotoxic molecular mechanisms and cytoprotection by enzymic metabolism or autoxidation for glyceraldehyde, hydroxypyruvate and glycolaldehyde. Chemico-Biological Interactions. 2011;191(1–3):315–321. doi: 10.1016/j.cbi.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 42.Valeri F, Boess F, Wolf A, Göldlin C, Boelsterli UA. Fructose and tagatose protect against oxidative cell injury by iron chelation. Free Radical Biology and Medicine. 1996;22(1-2):257–268. doi: 10.1016/s0891-5849(96)00331-0. [DOI] [PubMed] [Google Scholar]
- 43.Frenzel J, Richter J, Eschrich K. Fructose inhibits apoptosis induced by reoxygenation in rat hepatocytes by decreasing reactive oxygen species via stabilization of the glutathione pool. Biochimica et Biophysica Acta. 2002;1542(1–3):82–94. doi: 10.1016/s0167-4889(01)00169-0. [DOI] [PubMed] [Google Scholar]
- 44.Spasojević I, Bajić A, Jovanović K, Spasić M, Andjus P. Protective role of fructose in the metabolism of astroglial C6 cells exposed to hydrogen peroxide. Carbohydrate Research. 2009;344(13):1676–1681. doi: 10.1016/j.carres.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 45.MacAllister SL, Choi J, Dedina L, O'Brien PJ. Metabolic mechanisms of methanol/formaldehyde in isolated rat hepatocytes: carbonyl-metabolizing enzymes versus oxidative stress. Chemico-Biological Interactions. 2011;191(1–3):308–314. doi: 10.1016/j.cbi.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Chung SSM, Ho ECM, Lam KSL, Chung SK. Contribution of polyol pathway to diabetes-induced oxidative stress. Journal of the American Society of Nephrology. 2003;14(3):S233–S236. doi: 10.1097/01.asn.0000077408.15865.06. [DOI] [PubMed] [Google Scholar]
- 47.Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Experimental Diabesity Research. 2007;2007:10 pages. doi: 10.1155/2007/61038.61038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martins AMTBS, Cordeiro CAA, Freire AMJP. In situ analysis of methylglyoxal metabolism in Saccharomyces cerevisiae . FEBS Letters. 2001;499(1-2):41–44. doi: 10.1016/s0014-5793(01)02519-4. [DOI] [PubMed] [Google Scholar]
- 49.Phillips SA, Thornalley PJ. The formation of methylglyoxal from triose phosphates: investigation using a specific assay for methylglyoxal. European Journal of Biochemistry. 1993;212(1):101–105. doi: 10.1111/j.1432-1033.1993.tb17638.x. [DOI] [PubMed] [Google Scholar]
- 50.Pompliano DL, Peyman A, Knowles JR. Stabilization of a reaction intermediate as a catalytic device: definition of the functional role of the flexible loop in triosephosphate isomerase. Biochemistry. 1990;29(13):3186–3194. doi: 10.1021/bi00465a005. [DOI] [PubMed] [Google Scholar]
- 51.Richard JP. Mechanism for the formation of methylglyoxal from triosephosphates. Biochemical Society Transactions. 1993;21(2):549–553. doi: 10.1042/bst0210549. [DOI] [PubMed] [Google Scholar]
- 52.Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and beta-unsaturated aldehydes by phagocytes at sites of inflammation. Journal of Clinical Investigation. 1997;99(3):424–432. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pamplona R. Advanced lipoxidation end-products. Chemico-Biological Interactions. 2011;192(1-2):14–20. doi: 10.1016/j.cbi.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Miyata T, Saito A, Kurokawa K, De Strihou CVY. Advanced glycation and lipoxidation end products: reactive carbonyl compounds-related uraemic toxicity. Nephrology Dialysis Transplantation. 2001;16(4):8–11. doi: 10.1093/ndt/16.suppl_4.8. [DOI] [PubMed] [Google Scholar]
- 55.Lapolla A, Traldi P, Fedele D. Importance of measuring products of non-enzymatic glycation of proteins. Clinical Biochemistry. 2005;38(2):103–115. doi: 10.1016/j.clinbiochem.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 56.Ellis EM. Reactive carbonyls and oxidative stress: potential for therapeutic intervention. Pharmacology and Therapeutics. 2007;115(1):13–24. doi: 10.1016/j.pharmthera.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 57.Tessier FJ. The Maillard reaction in the human body. The main discoveries and factors that affect glycation. Pathologie Biologie. 2010;58(3):214–219. doi: 10.1016/j.patbio.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 58.Peng X, Ma J, Chen F, Wang M. Naturally occurring inhibitors against the formation of advanced glycation end-products. Food & Function. 2011;2(6):289–301. doi: 10.1039/c1fo10034c. [DOI] [PubMed] [Google Scholar]
- 59.Voziyan P, Brown KL, Chetyrkin S, Hudson B. Site-specific AGE modifications in the extracellular matrix: a role for glyoxal in protein damage in diabetes. Clinical Chemistry and Laboratory Medicine. 2013;52(1):1–7. doi: 10.1515/cclm-2012-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thornalley PJ. Glyoxalase I—structure, function and a critical role in the enzymatic defence against glycation. Biochemical Society Transactions. 2003;31(6):1343–1348. doi: 10.1042/bst0311343. [DOI] [PubMed] [Google Scholar]
- 61.Lesgards J-F, Gauthier C, Iovanna J, Vidal N, Dolla A, Stocker P. Effect of reactive oxygen and carbonyl species on crucial cellular antioxidant enzymes. Chemico-Biological Interactions. 2011;190(1):28–34. doi: 10.1016/j.cbi.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 62.Desai KM, Wu L. Free radical generation by methylglyoxal in tissues. Drug Metabolism and Drug Interactions. 2008;23(1-2):151–173. doi: 10.1515/dmdi.2008.23.1-2.151. [DOI] [PubMed] [Google Scholar]
- 63.Chetyrkin S, Mathis M, Pedchenko V, et al. Glucose autoxidation induces functional damage to proteins via modification of critical arginine residues. Biochemistry. 2011;50(27):6102–6112. doi: 10.1021/bi200757d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miyata T, De Strihou CVY, Kurokawa K, Baynes JW. Alterations in nonenzymatic biochemistry in uremia: origin and significance of “carbonyl stress” in long-term uremic complications. Kidney International. 1999;55(2):389–399. doi: 10.1046/j.1523-1755.1999.00302.x. [DOI] [PubMed] [Google Scholar]
- 65.Yim MB, Yim H-S, Lee C, Kang S-O, Chock PB. Protein glycation: creation of catalytic sites for free radical generation. Annals of the New York Academy of Sciences. 2001;928:48–53. [PubMed] [Google Scholar]
- 66.Takamiya R, Takahashi M, Myint T, et al. Glycation proceeds faster in mutated Cu, Zn-superoxide dismutases related to familial amyotrophic lateral sclerosis. The FASEB Journal. 2003;17(8):938–940. doi: 10.1096/fj.02-0768fje. [DOI] [PubMed] [Google Scholar]
- 67.Shumaev KB, Gubkina SA, Kumskova EM, Shepelkova GS, Ruuge EK, Lankin VZ. Superoxide formation as a result of interaction of L-lysine with dicarbonyl compounds and its possible mechanism. Biochemistry. 2009;74(4):461–466. doi: 10.1134/s0006297909040154. [DOI] [PubMed] [Google Scholar]
- 68.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford, UK: Clarendon Press; 1989. [Google Scholar]
- 69.Demple B. Regulation of bacterial oxidative stress genes. Annual Review of Genetics. 1991;25(1):315–337. doi: 10.1146/annurev.ge.25.120191.001531. [DOI] [PubMed] [Google Scholar]
- 70.Esterbauer H, Schaur RJ, Zollner H. Chemistry and Biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology and Medicine. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 71.Strohmaier H, Hinghofer-Szalkay H, Schaur RJ. Detection of 4-hydroxynonenal (HNE) as a physiological component in human plasma. Journal of Lipid Mediators and Cell Signalling. 1995;11(1):51–61. doi: 10.1016/0929-7855(94)00027-a. [DOI] [PubMed] [Google Scholar]
- 72.Talukdar D, Chaudhuri BS, Ray M, Ray S. Critical evaluation of toxic versus beneficial effects of methylglyoxal. Biochemistry. 2009;74(10):1059–1069. doi: 10.1134/s0006297909100010. [DOI] [PubMed] [Google Scholar]
- 73.Cilento G. Generation of triplet carbonyl compounds during peroxidase catalysed reactions. Journal of Bioluminescence and Chemiluminescence. 1989;4(1):193–199. doi: 10.1002/bio.1170040128. [DOI] [PubMed] [Google Scholar]
- 74.Együd LG, Szent-Györgyi A. On the regulation of cell division. Proceedings of the National Academy of Sciences of the United States of America. 1966;56(1):203–207. doi: 10.1073/pnas.56.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Együd LG, Szent-Györgyi A. Cancerostatic action of methylglyoxal. Science. 1968;160(3832):p. 1140. doi: 10.1126/science.160.3832.1140. [DOI] [PubMed] [Google Scholar]
- 76.Ghosh M, Talukdar D, Ghosh S, Bhattacharyya N, Ray M, Ray S. In vivo assessment of toxicity and pharmacokinetics of methylglyoxal: augmentation of the curative effect of methylglyoxal on cancer-bearing mice by ascorbic acid and creatine. Toxicology and Applied Pharmacology. 2006;212(1):45–58. doi: 10.1016/j.taap.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 77.Talukdar D, Ray S, Ray M, Das S. A brief critical overview of the biological effects of methylglyoxal and further evaluation of a methylglyoxal-based anticancer formulation in treating cancer patients. Drug Metabolism and Drug Interactions. 2008;23(1-2):175–210. doi: 10.1515/dmdi.2008.23.1-2.175. [DOI] [PubMed] [Google Scholar]
- 78.Ghosh A, Bera S, Ray S, Banerjee T, Ray M. Methylglyoxal induces mitochondria-dependent apoptosis in sarcoma. Biochemistry. 2011;76(10):1164–1171. doi: 10.1134/S0006297911100105. [DOI] [PubMed] [Google Scholar]
- 79.Ghosh A, Bera S, Ghosal S, Ray S, Basu A, Ray M. Differential inhibition/inactivation of mitochondrial complex I implicates its alteration in malignant cells. Biochemistry. 2011;76(9):1051–1060. doi: 10.1134/S0006297911090100. [DOI] [PubMed] [Google Scholar]
- 80.Dubinina EE, Dadali VA. Role of 4-hydroxy-trans-2-nonenal in cell functions. Biochemistry. 2010;75(9):1069–1087. doi: 10.1134/s0006297910090014. [DOI] [PubMed] [Google Scholar]
- 81.Cheng J-Z, Singhal SS, Sharma A, et al. Transfection of mGSTA4 in HL-60 cells protects against 4-hydroxynonenal-induced apoptosis by inhibiting JNK-mediated signaling. Archives of Biochemistry and Biophysics. 2001;392(2):197–207. doi: 10.1006/abbi.2001.2452. [DOI] [PubMed] [Google Scholar]
- 82.Soh Y, Jeong K-S, Lee IJ, Bae M-A, Kim Y-C, Song BJ. Selective activation of the c-Jun N-terminal protein kinase pathway during 4-hydroxynonenal-induced apoptosis of PC12 cells. Molecular Pharmacology. 2000;58(3):535–541. doi: 10.1124/mol.58.3.535. [DOI] [PubMed] [Google Scholar]
- 83.Awasthi YC, Sharma R, Cheng JZ, et al. Role of 4-hydroxynonenal in stress-mediated apoptosis signaling. Molecular Aspects of Medicine. 2003;24(4-5):219–230. doi: 10.1016/s0098-2997(03)00017-7. [DOI] [PubMed] [Google Scholar]
- 84.Farmer EE, Davoine C. Reactive electrophile species. Current Opinion in Plant Biology. 2007;10(4):380–386. doi: 10.1016/j.pbi.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 85.Mueller MJ, Berger S. Reactive electrophilic oxylipins: pattern recognition and signalling. Phytochemistry. 2009;70(13-14):1511–1521. doi: 10.1016/j.phytochem.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 86.Groeger AL, Freeman BA. Signaling actions of electrophiles: anti-inflammatory therapeutic candidates. Molecular Interventions. 2010;10(1):39–50. doi: 10.1124/mi.10.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Advances in Enzyme Regulation. 2006;46(1):113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 88.Lushchak VI. Adaptive response to oxidative stress: bacteria, fungi, plants and animals. Comparative Biochemistry and Physiology C. 2011;153(2):175–190. doi: 10.1016/j.cbpc.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 89.Parola M, Robino G, Marra F, et al. HNE interacts directly with JNK isoforms in human hepatic stellate cells. Journal of Clinical Investigation. 1998;102(11):1942–1950. doi: 10.1172/JCI1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation: 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. Journal of Biological Chemistry. 1999;274(4):2234–2242. doi: 10.1074/jbc.274.4.2234. [DOI] [PubMed] [Google Scholar]
- 91.Nishina H, Wada T, Katada T. Physiological roles of SAPK/JNK signaling pathway. Journal of Biochemistry. 2004;136(2):123–126. doi: 10.1093/jb/mvh117. [DOI] [PubMed] [Google Scholar]
- 92.Camandola S, Poli G, Mattson MP. The lipid peroxidation product 4-hydroxy-2,3-nonenal increases AP-1- binding activity through caspase activation in neurons. Journal of Neurochemistry. 2000;74(1):159–168. doi: 10.1046/j.1471-4159.2000.0740159.x. [DOI] [PubMed] [Google Scholar]
- 93.Calabrese V, Cornelius C, Dinkova-Kostova AT, et al. Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochimica et Biophysica Acta. 2012;1822(5):753–783. doi: 10.1016/j.bbadis.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 94.Botzen D, Grune T. Degradation of HNE-modified proteins—possible role of ubiquitin. Redox Report. 2007;12(1-2):63–67. doi: 10.1179/135100007X162130. [DOI] [PubMed] [Google Scholar]
- 95.Kaspar JW, Jaiswal AK. Antioxidant-induced phosphorylation of tyrosine 486 leads to rapid nuclear export of Bach1 that allows Nrf2 to bind to the antioxidant response element and activate defensive gene expression. Journal of Biological Chemistry. 2010;285(1):153–162. doi: 10.1074/jbc.M109.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maeta K, Izawa S, Okazaki S, Kuge S, Inoue Y. Activity of the Yap1 transcription factor in Saccharomyces cerevisiae is modulated by methylglyoxal, a metabolite derived from glycolysis. Molecular and Cellular Biology. 2004;24(19):8753–8764. doi: 10.1128/MCB.24.19.8753-8764.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vlassara H, Brownlee M, Cerami A. High-affinity-receptor-mediated uptake and degradation of glucose-modified proteins: a potential mechanism for the removal of senescent macromolecules. Proceedings of the National Academy of Sciences of the United States of America. 1985;82(17):5588–5592. doi: 10.1073/pnas.82.17.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schmidt AM, Vianna M, Gerlach M, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. Journal of Biological Chemistry. 1992;267(21):14987–14997. [PubMed] [Google Scholar]
- 99.Taguchi A, Blood DC, Del Toro G, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405(6784):354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 100.Bohlender JM, Franke S, Stein G, Wolf G. Advanced glycation end products and the kidney. American Journal of Physiology—Renal Physiology. 2005;289(4):F645–F659. doi: 10.1152/ajprenal.00398.2004. [DOI] [PubMed] [Google Scholar]
- 101.Peyroux J, Sternberg M. Advanced glycation endproducts (AGEs): pharmacological inhibition in diabetes. Pathologie Biologie. 2006;54(7):405–419. doi: 10.1016/j.patbio.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 102.Atanasiu V, Stoian I, Manolescu B, Lupescu O. The glyoxalase system—a link between carbonilic stress and human therapy. Revue Roumaine de Chimie. 2006;51(9):861–869. [Google Scholar]
- 103.Yamagishi S-I. Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Experimental Gerontology. 2011;46(4):217–224. doi: 10.1016/j.exger.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 104.Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189(4):1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hipkiss AR. Energy metabolism, proteotoxic stress and age-related dysfunction—protection by carnosine. Molecular Aspects of Medicine. 2011;32(4–6):267–278. doi: 10.1016/j.mam.2011.10.004. [DOI] [PubMed] [Google Scholar]


