Abstract
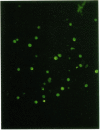
In this investigation, we studied the ability of human cytomegalovirus to infect peripheral blood mononuclear cells. With monoclonal antibody technology, we demonstrated that cytomegalovirus could infect human lymphocytes of T- and B-cell lineage, natural killer cells, and monocytes. Furthermore, virus expression was limited to the synthesis of immediate-early cytomegalovirus polypeptides. These peripheral blood mononuclear cells did not produce infectious virus, nor were mature virions visualized by electron microscopy. This abortive infection of mononuclear cells was most convincingly shown with stocks of cytomegalovirus that had been recently isolated from infected patients and passaged minimally in fibroblasts. This argues for an increased lymphotropic effect of some isolates of cytomegalovirus, compared to strains of virus that are extensively adapted to growth in fibroblasts. Furthermore, immunocompetent cells that were shown to be abortively infected with cytomegalovirus lost selected differentiated functions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casali P., Rice G. P., Oldstone M. B. Viruses disrupt functions of human lymphocytes. Effects of measles virus and influenza virus on lymphocyte-mediated killing and antibody production. J Exp Med. 1984 May 1;159(5):1322–1337. doi: 10.1084/jem.159.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarchi J. M. Correlation between stimulation of host cell DNA synthesis by human cytomegalovirus and lack of expression of a subset of early virus genes. Virology. 1983 Sep;129(2):274–286. doi: 10.1016/0042-6822(83)90167-8. [DOI] [PubMed] [Google Scholar]
- DeMarchi J. M. Nature of the block in the expression of some early virus genes in cells abortively infected with human cytomegalovirus. Virology. 1983 Sep;129(2):287–297. doi: 10.1016/0042-6822(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Diosi P., Moldovan E., Tomescu N. Latent cytomegalovirus infection in blood donors. Br Med J. 1969 Dec 13;4(5684):660–662. doi: 10.1136/bmj.4.5684.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn L., Ost A. Cytomegalovirus infection of human blood cells. J Infect Dis. 1984 Feb;149(2):207–214. doi: 10.1093/infdis/149.2.207. [DOI] [PubMed] [Google Scholar]
- Fiala M., Payne J. E., Berne T. V., Moore T. C., Henle W., Montgomerie J. Z., Chatterjee S. N., Guze L. B. Epidemiology of cytomegalovirus infection after transplantation and immunosuppression. J Infect Dis. 1975 Oct;132(4):421–433. doi: 10.1093/infdis/132.4.421. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Thompson L. F., Huddlestone J. R. T gamma cells express T lymphocyte-associated antigens. J Immunol. 1981 May;126(5):2062–2063. [PubMed] [Google Scholar]
- Furukawa T., Yoshimura N., Jean J. H., Plotkin S. A. Chronically persistent infection with human cytomegalovirus in human lymphoblasts. J Infect Dis. 1979 Feb;139(2):211–214. doi: 10.1093/infdis/139.2.211. [DOI] [PubMed] [Google Scholar]
- Glenn J. Cytomegalovirus infections following renal transplantation. Rev Infect Dis. 1981 Nov-Dec;3(6):1151–1178. doi: 10.1093/clinids/3.6.1151. [DOI] [PubMed] [Google Scholar]
- Goldstein L. C., McDougall J., Hackman R., Meyers J. D., Thomas E. D., Nowinski R. C. Monoclonal antibodies to cytomegalovirus: rapid identification of clinical isolates and preliminary use in diagnosis of cytomegalovirus pneumonia. Infect Immun. 1982 Oct;38(1):273–281. doi: 10.1128/iai.38.1.273-281.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby D. R., Oldstone M. B. Delineation of suppressor and helper activity within the OKT4-defined T lymphocyte subset in human newborns. J Immunol. 1983 Oct;131(4):1765–1770. [PubMed] [Google Scholar]
- Joncas J. H., Alfieri C., Leyritz-Wills M., Brochu P., Jasmin G., Boldogh I., Huang E. S. Simultaneous congenital infection with Epstein-Barr virus and cytomegalovirus. N Engl J Med. 1981 Jun 4;304(23):1399–1403. doi: 10.1056/NEJM198106043042306. [DOI] [PubMed] [Google Scholar]
- Jordan M. C. Latent infection and the elusive cytomegalovirus. Rev Infect Dis. 1983 Mar-Apr;5(2):205–215. doi: 10.1093/clinids/5.2.205. [DOI] [PubMed] [Google Scholar]
- Jordan M. C. Latent infection and the elusive cytomegalovirus. Rev Infect Dis. 1983 Mar-Apr;5(2):205–215. doi: 10.1093/clinids/5.2.205. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LaFemina R. L., Hayward G. S. Replicative forms of human cytomegalovirus DNA with joined termini are found in permissively infected human cells but not in non-permissive Balb/c-3T3 mouse cells. J Gen Virol. 1983 Feb;64(Pt 2):373–389. doi: 10.1099/0022-1317-64-2-373. [DOI] [PubMed] [Google Scholar]
- Lang D. J., Ebert P. A., Rodgers B. M., Boggess H. P., Rixse R. S. Reduction of postperfusion cytomegalovirus-infections following the use of leukocyte depleted blood. Transfusion. 1977 Jul-Aug;17(4):391–395. doi: 10.1046/j.1537-2995.1977.17477216868.x. [DOI] [PubMed] [Google Scholar]
- Olding L. B., Jensen F. C., Oldstone M. B. Pathogenesis of of cytomegalovirus infection. I. Activation of virus from bone marrow-derived lymphocytes by in vitro allogenic reaction. J Exp Med. 1975 Mar 1;141(3):561–572. doi: 10.1084/jem.141.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Fujinami R. S., Tishon A., Finney D., Powell H. C., Lampert P. W. Mapping of the major histocompatibility complex and viral antigens on the plasma membrane of a measles virus-infected cell. Virology. 1983 Jun;127(2):426–437. doi: 10.1016/0042-6822(83)90155-1. [DOI] [PubMed] [Google Scholar]
- Perussia B., Fanning V., Trinchieri G. A human NK and K cell subset shares with cytotoxic T cells expression of the antigen recognized by antibody OKT8. J Immunol. 1983 Jul;131(1):223–231. [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Rook A. H., Manischewitz J. F., Jackson L., Moreschi G., Santos G. W., Saral R., Burns W. H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982 Jul 1;307(1):7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Black P. H., Hirsch M. S. Interaction of cytomegalovirus with leukocytes from patients with mononucleosis due to cytomegalovirus. J Infect Dis. 1977 Nov;136(5):667–678. doi: 10.1093/infdis/136.5.667. [DOI] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Richter B. S., Black P. H., Callery R., Chess L., Hirsch M. S. Replication of herpes simplex virus and cytomegalovirus in human leukocytes. J Immunol. 1978 Jan;120(1):130–136. [PubMed] [Google Scholar]
- Sixbey J. W., Vesterinen E. H., Nedrud J. G., Raab-Traub N., Walton L. A., Pagano J. S. Replication of Epstein-Barr virus in human epithelial cells infected in vitro. Nature. 1983 Dec 1;306(5942):480–483. doi: 10.1038/306480a0. [DOI] [PubMed] [Google Scholar]
- St Jeor S., Rapp F. Cytomegalovirus replication in cells pretreated with 5-iodo-2'-deoxyuridine. J Virol. 1973 Jun;11(6):986–990. doi: 10.1128/jvi.11.6.986-990.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl R. E., Friedman-Kien A., Dubin R., Marmor M., Zolla-Pazner S. Immunologic abnormalities in homosexual men. Relationship to Kaposi's sarcoma. Am J Med. 1982 Aug;73(2):171–178. doi: 10.1016/0002-9343(82)90174-7. [DOI] [PubMed] [Google Scholar]
- Thomas Y., Rogozinski L., Irigoyen O. H., Shen H. H., Talle M. A., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. V. Suppressor cells within the activated OKT4+ population belong to a distinct subset. J Immunol. 1982 Mar;128(3):1386–1390. [PubMed] [Google Scholar]
- Tocci M. J., St Jeor S. C. Susceptibility of lymphoblastoid cells to infection with human cytomegalovirus. Infect Immun. 1979 Feb;23(2):418–423. doi: 10.1128/iai.23.2.418-423.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren B., Robèrt K. H., Nordlund S. Conditions for cytomegalovirus stimulation of lymphocytes. Scand J Immunol. 1981;13(6):581–586. doi: 10.1111/j.1365-3083.1981.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Weller T. H. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. I. N Engl J Med. 1971 Jul 22;285(4):203–214. doi: 10.1056/NEJM197107222850406. [DOI] [PubMed] [Google Scholar]