Abstract
The tetrapod auditory system transmits sound through the outer and middle ear to the organ of Corti or other sound pressure receivers of the inner ear where specialized hair cells translate vibrations of the basilar membrane into electrical potential changes that are conducted by the spiral ganglion neurons to the auditory nuclei. In other systems, notably the vertebrate limb, a detailed connection between the evolutionary variations in adaptive morphology and the underlying alterations in the genetic basis of development has been partially elucidated. In this review, we attempt to correlate evolutionary and partially characterized molecular data into a cohesive perspective of the evolution of the mammalian organ of Corti out of the tetrapod basilar papilla. We propose a stepwise, molecularly partially characterized transformation of the ancestral, vestibular developmental program of the vertebrate ear. This review provides a framework to decipher both discrete steps in development and the evolution of unique functional adaptations of the auditory system. The combined analysis of evolution and development establishes a powerful cross-correlation where conclusions derived from either approach become more meaningful in a larger context not possible through exclusively evolution or development centered perspectives.
Selection may explain the survival of the fittest auditory system, but only developmental genetics can explain the arrival of the fittest auditory system.
[Modified after (Wagner 2011)]
Introduction
Among the many novel features that characterize mammals, few are as distinct as the auditory system that is so intricately associated with the evolution of social interactions, including human language. One of the striking features of the therian auditory system compared to (almost) all other vertebrates is that modern therians evolved a unique high frequency sensitivity in a range inaudible to nearly all non-mammalian vertebrates that have limited sensitivity above 10kHz (Fay 1988; Vater et al. 2004; Manley 2010). In addition to this high frequency perception, therian mammals evolved a sophisticated topological frequency distribution (Manley and Jones 2011) that provides the basis for both absolute pitch resolution and the sophisticated sonar system of bats. These abilities depend in part on the ordered cellular patterning of the organ of Corti, the therian hearing organ, with its arrangement of one row of inner hair cells (IHCs) and three rows of outer hair cells (OHCs). Each of these two hair cell (HC) types translate, together with the equally specialized surrounding set of supporting cells, a basilar membrane vibration into resting potential changes of the HCs. Therian HCs have special evolutionary novelties like a voltage related contraction of OHCs to function as a cochlear amplifier (Liberman et al. 2002) utilizing a highly derived molecular machinery that evolved rapidly in mammalian ancestors (Okoruwa et al. 2008). Adding to these mammalian novelties is the mammalian organization of the middle ear with three ossicles to transmit a wide range of frequencies from the tympanic membrane to the oval window (Reichert 1837; Hopson and Crompton 1969; Clack 1993; Voss et al. 2000). Equally unique are the central nervous system structures that process sound and show only a limited topological and structural similarity with the auditory neuronal structures of non-mammals (Grothe et al. 2004). In summary, the mammalian auditory system is one of the most outstanding examples of morpho-functional innovation among vertebrates, ranging from the classic paradigm of functional loss combined with transformation into a novel structure [the transformation of jaw bones to middle ear ossicles] to molecular transformation [a channel protein evolves into an electrically driven motor embedded in the cell membrane (Okoruwa et al. 2008)]. Moreover, the auditory system likely provided an essential basis for human social evolution via sound based language (Normile 2012), ensuring the evolution of social structures that laid the foundation for the success of the human species.
Unfortunately, despite its obvious importance, there are very limited integrative studies analyzing how genes and their expression changes affect development leading to anatomically and functionally different structures in the inner ear to parallel, for example, comparisons performed on the evolution and development of limbs (Shubin et al. 2009) and eyes (Gehring 2011). Despite nearly 200 years of interest in the subject of auditory evolution and great progress in recent years on multiple aspects pertaining to the molecular, cellular, and morphological evolution of the auditory system, major unanswered questions remain. This review aims to rectify this situation by going beyond previous attempts that established cellular evolution and some aspects of auditory system evolution and development (Fekete and Sienknecht 2007; Fritzsch et al. 2007). This review provides an intellectual platform to integrate the various disparate findings in the literature into a cohesive hypothesis of mammalian and tetrapod auditory system evolution and development. Our premise is that auditory system evolution can only be logically deduced if we have a starting point for the perplexing variations in the middle ear, inner ear, and central auditory system (Fritzsch 1992; Clack 2002; Grothe et al. 2004). We first need an understanding of the evolution of the auditory sensory organ(s) and sensory neurons of the inner ear of tetrapods before the rich diversity of fossil findings on the middle ear, or the modern data on physiology of divergent brain areas involved in auditory processing, can be interpreted in an evolutionary context. In essence, we propose a process of evolutionary events that starts with the auditory sensory epithelia evolution inside the inner ear to guide insights into auditory system evolution, including the impedance matching function of the middle ear. Following our premise, we will concentrate on the evolution of the organ of Corti and its tetrapod precursor, the basilar papilla, and associated neurons without dealing with the middle ear evolution. Specifically, we will review primary data on the evolutionary history of the basilar papilla/organ of Corti of the inner ear followed with what we currently understand about the molecular basis of its development through analysis of mouse mutants. The evolution of mechanosensory hair cells and their molecular basis has been treated elsewhere and is not part of this review (Fekete and Sienknecht 2007; Fritzsch et al. 2010)
1. The therian organ of Corti evolved out of the amniote basilar papilla
Comparative data agree that the mammalian organ of Corti is derived from the amniote basilar papilla situated in the lagenar recess (Fig. 1) and already evolved into its current shape early in therian evolution (Luo et al. 2011). This can be surmised through findings in monotremes (egg-laying mammals; prototherians) that illustrate an organization of their auditory system that is comparable to that of non-mammalian amniotes such as birds and crocodiles (Vater et al. 2004). Like these non-mammalian amniotes, monotremes have a basilar papilla-like structure in the basal part of the lagenar recess that, at its tip, contains the lagenar macula, an ancestral gravistatic sense organ already present in the sarcopterygian lineage (Ladhams and Pickles 1996; Manley and Koppl 1998; Fritzsch et al. 2011). While the basilar papilla has been recognized for over 100 years as being homologous across all tetrapods (Retzius 1884), it is important to clearly state what makes the basilar papilla homologous to avoid confusion as current literature somewhat confounds the issue. In essence, some claims of parallel evolution of the basilar papilla in different tetrapod lineages are formulated primarily around physiological considerations. Unfortunately, these claims are comparable to suggesting that bat and horse limbs are not homologous as they differ too much in their detailed anatomy as an adjustment to the specific physiological needs (flying compared to running on a single digit, respectively). In contrast to the contentious issue around the basilar papilla, the homology of the lagenar macula has never been questioned despite its even more unusual change in position from the saccular recess to the lagenar recess in the sarcopterygian lineage (Lewis et al. 1985).
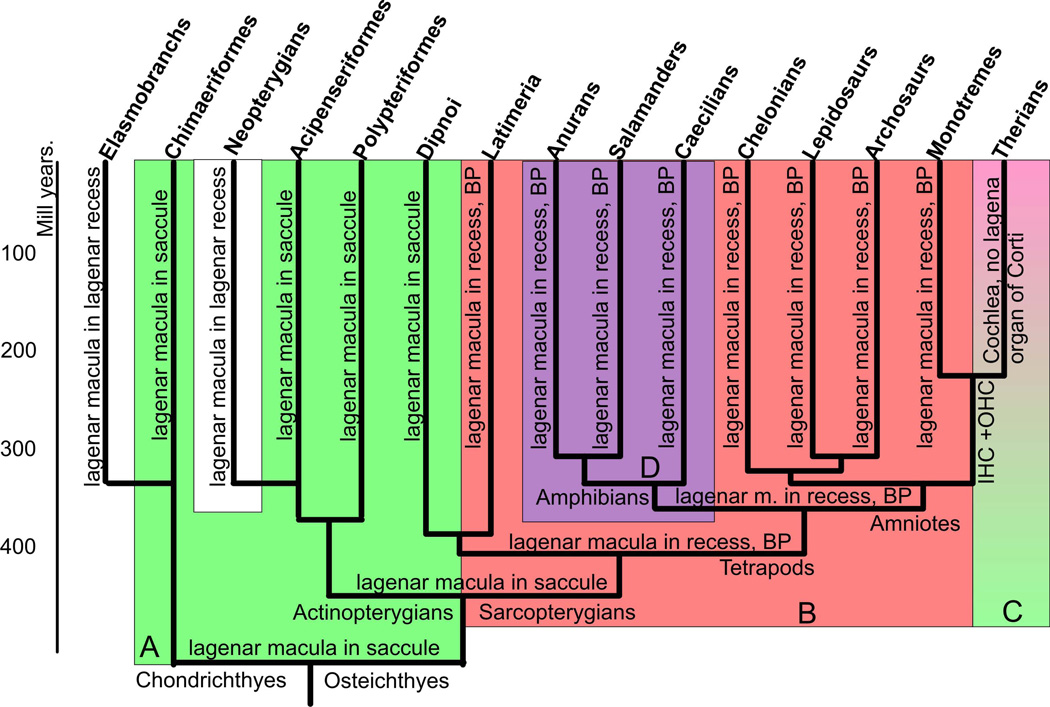
Figure 1.
This figure displays a consensus diagram of multiple cladistic analyses pointing out the likely relationship between different sarcopterygian and actinopterygian taxa and in different shades the major morphological changes. Note that lungfish resemble basal actinopterygians and Chondrichthyes, in particular ratfish, in great detail in that the lagenar macula is together with the saccular macula in the saccular recess (highlighted in light green, A). The derived conditions of tetrapods, apparently shared with the coelacanth Latimeria, is the possession of a lagenar recess with the lagenar macula at the tip, the formation of a basilar papilla and a perilymphatic sac to a round window in the posterior wall of the otocyst (highlighted in light red, B). Some derived Chondrichthyes and actinopterygians also have a lagenar recess with a lagenar macula in it (shown in white). All amphibians have a shared derived character, the amphibian papilla in its own recess (highlighted in light lilac, D). Salamanders and frogs have lost the neglected papilla and some salamanders and caecilians have lost the basilar papilla which may be in its own recess that comes off the lagenar recess. Some caecilians have lost the lagenar macula but retain the lagenar recess devoid of any hair cells or innervation. Ancestral mammals evolved a basilar papilla with two types of hair cells arranged in multiple rows, inner and outer hair cells (IHC, OHC). Therian mammals either lost or transformed the lagenar macula into the apex of the organ of Corti in a greatly elongated lagenar recess, now referred to as the cochlea or cochlear duct (shown in shades of light green to light red, C). Cladistic relationships compiled from (Shubin et al. 2009; Amemiya et al. 2010; Raincrow et al. 2011; Shan and Gras 2011).
Following previous suggestions (Lewis et al. 1985; Fritzsch 1987), we define the term ‘basilar papilla’ to describe the following structure of the vertebrate ear: the basilar papilla is a non-otoconia bearing sensory organ that is innervated by a branch of the eighth nerve that also extends to the lagenar macula and is situated near the orifice of the lagenar recess in proximity to a perilymphatic sac that extends to the round window. Homology is thus established using classical criteria such as topology inside the ear with respect to clearly homologous neighboring structures [saccular macula, lagenar macula, lagenar recess], the connectivity criterion [or hodological argument (Fritzsch and Glover 2007)], possibly uniquely derived function (no otoconia and proximity to a perilymphatic space, thus possibly functioning to measure pressure differences between the ear and the perilymphatic space leading to the round window), and unique association with nerve fibers establishing a connection with the brain that is distinct from the lagenar macula (Fritzsch 1988; Fischer et al. 1994). All amniotes have such a ‘basilar papilla’, including monotremes (Vater et al. 2004). It follows that the most parsimonious explanation for the presence of this basilar papilla in all amniotes is that it evolved only once in the amniote ancestor (Fig. 1). Modern amniotes have evolved different organizations as an adaptation to various functions, including different ranges of sensitivity to frequencies (Manley and Koppl 1998, 2008; Manley 2011).
Among mammals, only the monotreme basilar papilla fits these homology criteria. However, in contrast to other amniotes (Lewis et al. 1985; Manley and Koppl 2008), the monotreme basilar papilla shows a distinct organization into IHCs and OHCs, separated by multiple rows of pillar cells (Ladhams and Pickles 1996). This cellular organization is reminiscent of the organ of Corti in the cochlear duct of therian mammals (placental and marsupial mammals). These data suggest that the therian cochlea/organ of Corti evolved out of a basilar papilla of amniote ancestors in a three-step process:
Evolution of a lagenar recess with a basilar papilla near its orifice adjacent to the sound conducting perilymphatic space connecting to the round window (Fritzsch 1992).
Transformation of the basilar papilla HCs into the distinct IHCs and OHCs found only in monotreme therians (a derived or autapomorphic feature shared only by mammals among amniotes).
Transformation of the monotreme-like basilar papilla and hair cell organization into the arrangement of one row of IHCs and three rows of OHCs of the therian organ of Corti. This appears to come about through convergent extension movements perhaps made possible through the loss of the lagenar macula at the tip of the lagenar recess (Fritzsch et al. 2011) thus turning the lagenar recess into the coiled cochlear duct characteristic of therian mammals (Vater et al. 2004; Luo et al. 2011).
In essence, evolution transformed an amniotic sensory epithelium, the basilar papilla, presumably already dedicated to hearing in the common amniote ancestor, into the therian organ of Corti. This was achieved through cellular reorganizations, specification of novel cell types, and apparent loss of the ancestral otoconia bearing organ, the lagenar macula (Fig. 1), converting the lagenar recess into the coiled cochlear duct. While these data provide a clear perspective of the morphological changes and their progressive stepwise transformation among amniotes, neither the origin of the lagenar macula, nor of the lagenar recess, or the basilar papilla of amniotes is completely resolved (Fritzsch 1992). Here we will describe the evidence for the origin of these structures by comparing the monophyletic group of amphibians with amniotes, followed by data on the tetrapod outgroup, sarcopterygian and actinopterygian fish. We will then provide the most parsimonious interpretation of all available data.
1.a. Ancestral tetrapods had a lagenar macula in its own recess with a basilar papilla near the orifice
Amphibians have long been grouped together based on the appearance of a unique inner ear structure known as the amphibian papilla (Retzius 1881; Carroll 2007). This organ, used for hearing middle frequency ranges in frogs (Fritzsch et al. 1988; Smotherman and Narins 2004), is a distinctively displaced amphibian derivative of the neglected papilla found in all vertebrate lineages (Fritzsch and Wake 1988). Apparently, the bipartite neglected papilla in the utricle moved, during development, across the utriculo-saccular foramen to generate the amphibian papilla in the postero-dorsal aspect of the saccular recess (Fig. 1,2). The innervation to the amphibian papilla is always from the posterior canal nerve and is conserved between the neglected papilla and the amphibian papilla (Will and Fritzsch 1988; Fritzsch and Neary 1998). Only caecilians, currently considered the least derived amphibian taxon (Carroll 2007), retain both papillae whereas the more derived amphibians, salamanders and frogs, have lost the neglected papilla. In frogs, the basal taxa have a single patch of the amphibian papilla whereas derived frogs have two patches comprising the amphibian papilla. The most simplistic explanation for this derived condition is that the anlage for two patches of the ancestral neglected papilla may have shifted in derived frogs into the saccule, possibly by altering the timing of sensory anlage formation and constriction of the utriculo-saccular foramen (Fritzsch and Wake 1988).
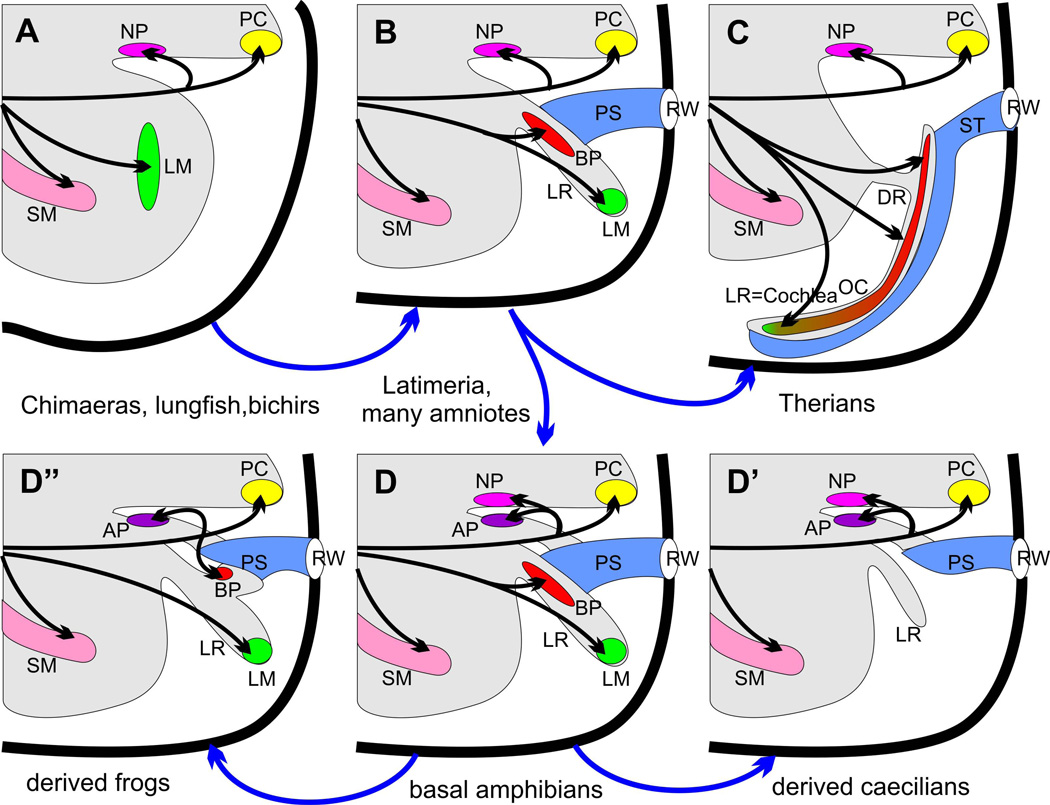
Figure 2.
This diagram displays the basic changes in the posterior part of the ear in the sarcopterygian lineage. The plesiomorphic organization or shared primitive pattern (A) is found in lungfish, basal actinopterygians (polypteriformes, holocephalens, chondrosteans, holosteans) and basal Chondrichthyes (chimaeras). The characteristic features are the presence of a nerve branch of the fibers to the posterior canal crista (PC) extending to the neglected papilla (NP), a separate nerve twig going to the distinct lagenar macula (LM) in the saccular recess posterior to the saccular macula (SM). Note that there appears to be no opening other than for nerves in the otic capsule (bold line) and no specialization of the perilymphatic space enabling pressure differences to generate relative movements between sensory epithelia and their extracellular covering (otoconia, cupula). The derived condition (B) is found in Latimeria and amniotes, excluding therian mammals. The derived condition is characterized by the formation of a lagenar recess (LR), translocation of the lagenar macula (LM) into the lagenar recess, formation of the basilar papilla (BP) at the orifice of the lagenar recess, and the formation of a perilymphatic sac (PS) that connects the basilar papilla functionally with an opening in the otocyst wall, the round window (RW). Note that the innervation of the basilar papilla is via a nerve branch coming off the lagenar macula innervation. Further derived from this basic tetrapod condition are basic amphibians (many caecilians) through the formation of a recess for the amphibian papilla (AP) and the translocation of a part of the neglected papilla into this unique recess as an amphibian papilla (D). Salamanders and frogs have all lost the neglected papilla (D”) and most salamanders and all frogs have evolved a second recess from the lagenar recess that contains the basilar papilla thus generating the derived conditions of two saccular recesses (in addition to the lagenar recess) each with its own sensory epithelium (amphibian and basilar papilla). Note also that the derived condition of innervation for the basilar papilla is via a nerve coming off the nerve to the posterior canal crista that can be reconciled through intermediates. Finally, loss of the lagenar macula occurred independently in therian mammals where the anlage might have become incorporated into the elongated basilar papilla now referred to as the organ of Corti (green tip of the red basilar papilla) due to its unique organization of hair cells (C), which is connected via narrow canal, the ductus reuniens (DR) to the saccular recess (D’). Loss of lagenar macula also occurred in some caecilians where both the basilar papilla and the lagenar macula are lost (D’) but a short lagenar recess is retained. Modified after (Lewis et al. 1985; Fritzsch and Wake 1988; Fritzsch 1992).
In addition to the amphibian papilla, all basal caecilians, basal salamanders, and all frogs have a basilar papilla at the orifice of the lagenar recess (Lewis et al. 1985; Fritzsch and Wake 1988; Smotherman and Narins 2004). While present in all amphibian lineages, derived caecilians and lungless salamanders have lost the basilar papilla. Absence of a basilar papilla in plethodontid (lungless) salamanders, may indicate that vibration elicited from the lungs (Ehret et al. 1994) reaching the ear through the round window facing toward the lungs (Fritzsch 1999) may suffice to maintain some sound pressure reception epithelia in amphibians even in the absence of a middle ear. Several derived caecilians, which typically are limbless, have lost not only the basilar papilla but also the lagenar macula, but nevertheless retain a lagenar recess. This indicates that limblessness is associated with a reduction of the third gravistatic receptor, the lagenar macula, in amphibians. Combined, these data suggest that amphibians have a ‘basilar papilla’ comparable to that found in amniotes and that such a basilar papilla was, by logical extension, the primitive condition for tetrapods. Following our basic assumption that evolution changes the ear at the level of the receptor organs in the ear first, one must evolve a sound pressure receptor before middle ear evolution can adjust the hyomandibular bone, freed of its ancestral function to brace the upper and lower jaw, to a novel, sound conducting middle ear function (Fritzsch 1999). It is almost impossible to assume that the rich variety of middle ears found in fossil tetrapods (Clack 1997) evolved to ultimately provide an impedance mismatch of sound to an ear that lacked a sound pressure receiver. In fact, it is more likely that the ear evolved pressure reception abilities in water (van Bergeijk 1966; Fritzsch 1999) and that the middle ears of the fossil record provide a rich variety of partial or incomplete adaptations to serve as impedance matching devices to adjust an aquatic inner ear to sound reception in air (Fritzsch 1992). Most modern reviews on this subject agree that the evolution of the basilar papilla started with the formation of a lagenar recess in ancestral tetrapods (Fritzsch 1992; Clack and Allin 2004), but differences exist in which early tetrapod group this might have happened.
Alternative views suggest that while the basilar papilla of amphibians is homologous, it is not homologous to that of amniotes as in many amphibians it sits in its own recess at the orifice of the lagenar recess and is only indirectly connected to the perilymphatic space (Wever 1974; Lewis et al. 1985). However, the simple fact that some basal caecilians and basal salamanders have a basilar papilla situated on a basilar membrane without a recess indicates that the condition in frogs is a derived feature (Fritzsch and Wake 1988; Fritzsch and Neary 1998). It is more likely that the basilar papilla of amphibians is as much homologous to the basilar papilla of amniotes as are various tetrapod limbs homologous as limbs despite a rich variation in form and functional adaptation to running, swimming, digging, etc. However, it is entirely possible that the use of the basilar papilla for sound pressure reception in air evolved independently in amphibians and amniotes as much as wings evolved independently in birds, pterosaurs and bats (Shubin et al. 2009). If the assumption of homology of the basilar papilla across tetrapods were true, either of the two sarcopterygian outgroups of tetrapods, Latimeria and lungfish, could have had a basilar papilla. More recent data suggest that the coelacanth Latimeria and the lungfish are either a monophyletic group (Shan and Gras 2011) or that their true relationship is unresolved (Takezaki et al. 2004). No matter this unresolved taxonomic problem, the presence of a basilar papilla-like structure would strongly suggest that the evolution of the basilar papilla started in sarcopterygian ancestors in water in parallel to structurally different adaptations of the actinopterygian ear to pressure reception (Fritzsch 1999).
1.b. The sarcopterygian ancestor of tetrapods had a basilar papilla
The ear of lungfish have been described by Retzius (Retzius 1881) who suggested the presence of a lagenar macula in the saccular recess comparable to basal actinopterygian fish and ratfish among Chondrichthyes, but he found no lagenar recess. In fact, based on the ear anatomy and the pattern of innervation, he suggested that the ear of lungfish most closely resembles that of ratfish and basal group of actinopterygians, the polypteriformes (Fig. 1). Recent data confirm this finding and show that the large saccular recess of lungfish has two separated sensory epithelia, suggested to represent the saccular and lagenar sensory epithelia (Platt et al. 2004). Moreover, the separate lagenar macula in the common saccular recess is a primitive feature of lungfish shared with basal actinopterygians such as the polytperiformes (Retzius 1881; Lewis et al. 1985; Fritzsch and Wake 1988) including the detailed pattern of the HC polarity (Lewis et al. 1985; Platt et al. 2004) that differs from the hair cell polarity of the tetrapod lagenar macula (Fig. 2). In lungfish, the lagenar macula is innervated by a distinct posterior nerve branch that follows a trajectory across the saccule (Fig. 2) consistent with the lagenar macula innervation in tetrapods and Latimeria (Fritzsch 1987; Fritzsch and Neary 1998). While lungfish have a neglected papilla, they have no amphibian or basilar papilla, no lagenar recess and no perilymphatic space anywhere near their otic sensory epithelia. In conclusion, a lagenar macula together with a saccular macula in the same saccular recess without the formation of a lagenar recess is the basal feature of both actinopterygian and sarcopterygian fish and appears to reflect the generalized (plesiomorphic) condition of osteichthyes.
In contrast to lungfish, the coelacanth Latimeria chalumnae has a basilar papilla situated at the orifice of the lagenar recess on a basilar membrane separating a perilymphatic space from the endolymph-filled inner ear (Fritzsch 1987). The perilymphatic space extends through a ‘round’ window in the otic capsule to the occipital region (Fritzsch 1992; Bernstein 2003; Fritzsch 2003). The basilar papilla is not covered by otoconia but instead is covered by a tectorial-like membrane. The innervation is via the lagenar nerve (Fig. 2) with multiple fibers extending to this structure (Fritzsch 1987). While the function of the basilar membrane is unclear, it appears that in all adult coelacanths, this membrane is ruptured, allowing the endolymph of the inner ear to freely communicate with the perilymph surrounding the ear, a fact already noted in the original description of the Latimeria ear (Millot and Anthony 1965). It is possible that the decompression these fish go through when they are elevated from their depth of several hundred meters causes a rupture of the membrane, rendering the ear functionless due to endolymph/perilymph mixing and the animals unable to swim coordinately. It should be noted that sound will be reflected at any surface of differing density such as water temperature and salinity and that the lung of Latimeria is filled with fat and serves as an organ for buoyancy. Sound could thus reach the ear from the lungs using the same basic principle as in larval and adult frogs. This could be related to the unusual canal leading form the basilar papilla to the belly (Millot and Anthony 1965; Fritzsch 1992; Bernstein 2003). Whatever the function of this structure at the lagenar orifice is, the topological, structural and innervation similarities make it extremely akin to the tetrapod and in particular the amniote basilar papilla (Figs. 1, 2). These detailed similarities argue strongly against the assumption that this organ evolved in parallel to the tetrapod basilar papilla. As will be apparent below, multiple genes have already been identified that play a role in the development of the mammalian organ of Corti, indicating that the sarcopterygian basilar papilla may have required an as yet undisclosed multitude of genes to evolve, rendering it unlikely to be duplicating in detail the tetrapod basilar papilla. Combined, these facts make a parallel evolution of the Latimeria and tetrapod basilar papilla, with their detailed anatomical similarities driven likely by the same developmental gene module, as unlikely as the parallel evolution of a pentadactyl limb.
Based on our current understanding of the interrelationship of lungfish, Latimeria and tetrapods (Takezaki et al. 2004; Shan and Gras 2011) and the genomic data suggesting Latimeria as an important outgroup for tetrapod genomic evolution (Amemiya et al. 2010; Smith et al. 2012), it is most parsimonious to assume that the basilar papilla evolved only once in the aquatic sarcopterygian ancestor of tetrapods, the common ancestor of Latimeria and lungfish. The lagenar macula without a lagenar recess found in lungfish could possibly represent a reversion back to the ancestral condition of both a lagenar and saccular macula being present in a undivided saccular recess (Retzius 1881; Lewis et al. 1985; Fritzsch and Wake 1988). Lungfish show a uniquely derived condition of the middle ear. Unlike Latimeria, basal actinopterygians and tetrapods (Clack 1997; Fritzsch 1999), lungfish have fused the hyomandibular bone to the brain case. The complete lack of a functional middle ear or of a perilymphatic space leading to any of the sensory epithelia relates nicely to the lack of higher frequency sound perception in lungfish (Christensen-Dalsgaard et al. 2011) also known for non-frequency-specialists among amphibians (Fritzsch et al. 1988; Fritzsch and Neary 1998). It is noteworthy, that lungfish differ radically from the caecilian regression of the lagena: while the latter loses the lagenar macula but retains the recess (Fritzsch and Wake 1988) lungfish loses the lagenar recess but retain the lagenar macula with a detailed similarity in hair cell organization to basal actinopterygians (Lewis et al. 1985; Platt et al. 2004). These similarities in lungfish and basal actinopterygians and Chondrichthyes could be most parsimoniously explained by assuming that the lungfish ear is as closely related to the ancestral actinopterygian/sarcopterygian ear as are those found in basal actinopterygians and Chondrichthyes (Fig. 1), a point already made by Retzius over 100 years ago (Retzius 1881). If one adds the unique position of the utricular macula in its own recess in Chondrichthyes and lungfish, it is understandable that Retzius grouped lungfish together with Chondrichthyes, not with tetrapods.
In summary, based on the detailed ear anatomy and the more recent confirmation of the initial finding on Latimeria (Fritzsch 1987; Bernstein 2003) combined with the partially resolved systematic affinity of lungfish, Latimeria and tetrapods (Takezaki et al. 2004; Shan and Gras 2011), it appears most parsimonious to propose that the tetrapod basilar papilla in the lagenar recess evolved in the sarcopterygian ancestor of tetrapods as a pressure receiver measuring pressure differences between the belly (lungs) and a middle ear that is covered by a tympanic membrane. As originally proposed on theoretical grounds (van Bergeijk 1966), inner ear (sound) pressure receiver evolution may have predated the middle ear evolution. The middle ear fossils may reflect multiple attempts to adapt the middle ear of early tetrapods to function as a sound impedance matching system, among other things (Fritzsch 1992; Clack 1997). In fact, many actinopterygian species apparently have evolved independently a sound pressure hearing that is structurally so different that they share only one common feature, an association of sorts with the swim bladder through various means (Fritzsch 1999; Ladich and Popper 2004). As already proposed for the evolution of the basilar papilla of amniotes, it is most parsimonious to assume that the sarcopterygian basilar papilla evolved in the context of formation of a lagenar recess when an ancestral lagenar macula became displaced from the saccular recess to the tip of the lagenar recess as a consequence of an altered development. In the following, we will analyze mouse mutants that bear on this hypothesis, showing that a cochlear recess can grow without formation of a sensory epithelium and that multiple genes are necessary to segregate sensory epithelia during development and most likely also during evolution.
2. Mutational analysis of the development of the mouse organ of Corti and cochlea identifies genes needed for distinct steps in basilar papilla/organ of Corti evolution
No matter the current interpretation of the specific occurrence of evolutionary events in certain vertebrate lineages, the evolution of a tetrapod basilar papilla to become the organ of Corti of therian mammals has to track the following steps:
The anlage of the lagenar macula has to move from the saccule into the lagenar recess that forms independently of the presence or absence of a lagenar macula.
The lagenar macula and the basilar papilla have to segregate from a common saccular/lagenar/basilar papilla anlage to form three distinct sensory epithelia that can evolve to serve novel functions.
The basilar papilla hair cell (HC) organization needs to be transformed into two types of HCs with their stereotyped distribution to form the organ of Corti.
The lagenar macula formation has to be either suppressed or transformed in the therian lineage to allow the coiling of the cochlear duct and the formation of a continuous organ of Corti for uninterrupted frequency presentations with the lowest frequency coinciding with the apical tip, the area of the cochlear duct formerly housing the lagenar macula.
A novel pattern of innervation dedicated to connect the basilar papilla/organ of Corti to the auditory nuclei of the hindbrain has to evolve and be molecularly distinct from the innervation of the adjacent lagenar macula.
In the following we will present data on several mouse mutants that show developmental alterations that are meaningful for the predicted ontogenetic transformations underlying the evolution of the basilar papilla/organ of Corti. While these induced atavisms are indicative of the involvement of these proteins in the development of these morphological alterations, the ultimate test for their functions would be to transform the development of the frog basilar papilla into that of the mouse organ of Corti through expansion of the basilar papilla, growth of the lagenar recess, and loss of the lagenar macula. Technically, this is now possible with the advent of genetic manipulations of frog development (Dagle et al. 2000; Abu-Daya et al. 2012) in combination with surgical ear manipulation (Elliott and Fritzsch 2010).
2.a. A cochlear duct forms in the absence of sensory epithelium development
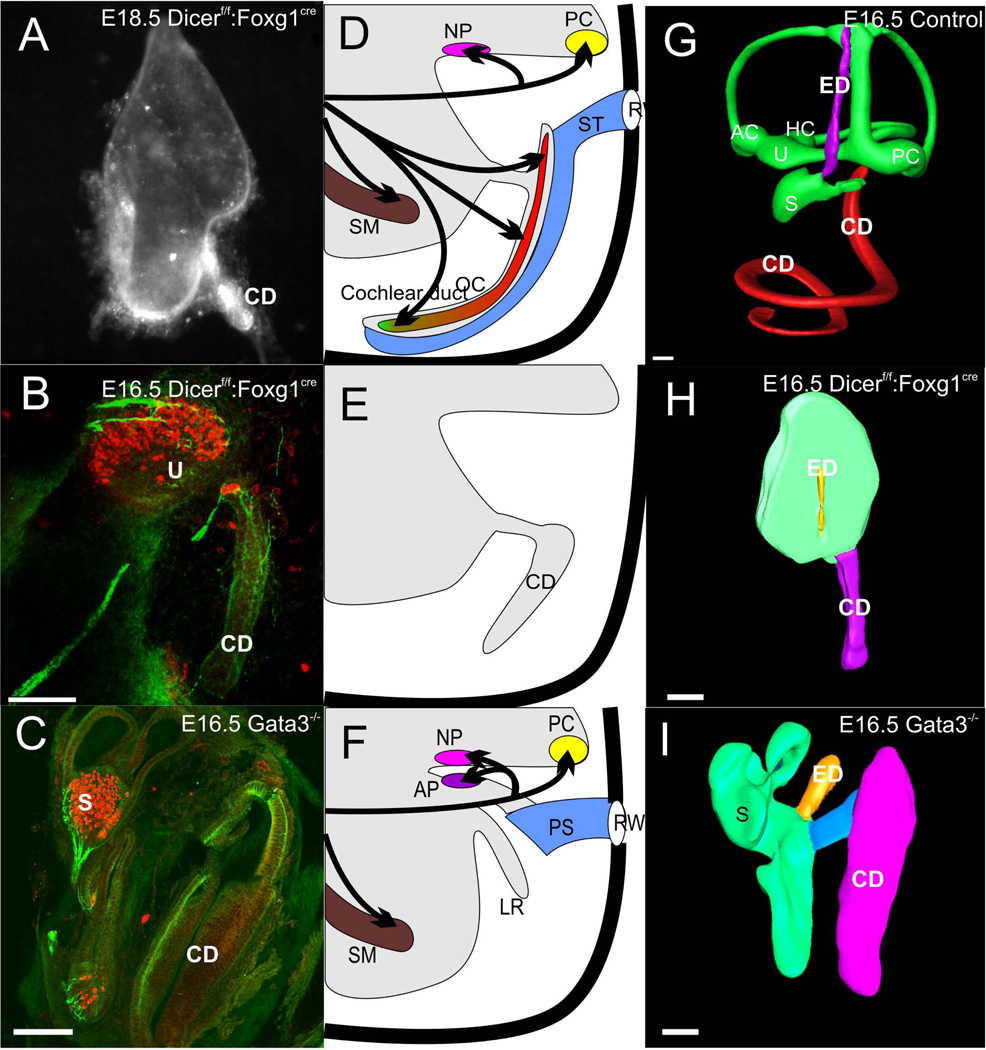
The evolutionary data suggest that a lagenar macula can exist in the saccular recess without a lagenar recess whereas a lagenar recess (or even basilar papilla recess in some amphibians) can form without a sensory epithelium. This is in striking contrast to the vertical semicircular canal formation of the ear which is clearly driven by the anterior and posterior canal cristae and the expression of diffusible factors such as Fgf10 and Bmp4 (Pauley et al. 2003; Bok et al. 2007). However, despite the clear correlation of sensory epithelia with the formation of the anterior and posterior semicircular canal, the horizontal canal shows that to a certain degree the formation of the canal can be molecularly uncoupled from the formation of the canal crista. For example, a crista without a canal forms in Otx1 and N-Myc conditional knockout (CKO) mutants (Morsli et al. 1999; Fritzsch et al. 2001; Hammond and Whitfield 2006; Kopecky et al. 2011) whereas a canal forms with at most a transient development of a crista in the Foxg1 null mutant (Pauley et al. 2006; Koo et al. 2009). Apparently, it is the latter model of independence of sensory epithelium formation on morphogenesis that applies to the cochlear duct. For example, a cochlear duct forms in mutants null for the microRNA producing enzyme Dicer1 (Kersigo et al. 2011) or the transcription factor Gata3 (Duncan et al. 2011), both of which never develop organ of Corti hair cells (Fig. 3). Indeed, even without prosensory formation as after the loss of the transcription factors Sox2 (Kiernan et al. 2005) or Atoh1 (Pan et al. 2011) there is a near normal development of the cochlear duct, suggesting that elongation of the cochlea is possible in the absence of sensory epithelia differentiation (Fritzsch et al. 2011). This is not to say that the convergent extension movement of the prosensory cells is not adding to the cochlear duct extension (Kelly and Chen 2007) as there is a clear truncation of the cochlear duct when the proliferation is cut short in Foxg1 (Pauley et al. 2006) and N-Myc mutants (Kopecky et al. 2011). Despite the likely more complex interactions, these data make it clear that one can experimentally uncouple cochlear duct extension from neurosensory development of the organ of Corti, indicating that both are to a certain degree independent as expected based on the evolutionary uncoupling of lagenar sensory epithelium formation from lagenar recess formation outlined above.
Figure 3.
This figure displays the loss of neurosensory development in the cochlea duct (CD) of two mutant mouse lines, a conditional deletion of Dicer 1 with Foxg1-cre (A,B) and deletion of Gata3 (C). Note that in either case hair cells and innervation exists to vestibular epithelia interpreted as utricle (U) and saccule (S), identifiable by the endolymphatic duct (ED) emanating from it. The cochlear duct is devoid of both hair cells and innervation other than autonomic fibers. The middle panel shows a diagram of the normal innervation and organization of the organ of Corti and the cochlear duct with the scala tympani (ST) attached (D), the loss of any innervation and hair cell formation in the two mutant lines but retention of the cochlear duct (E) and the loss of the basilar papilla and lagenar macula in a caecilian (F). The right column shows 3D reconstruction of confocal images of an E16.5 wildtype ear with Amira software (G), the remaining ‘ear’ of a conditional deletion of Dicer with the cochlear duct extending from the undivided upper part (H) and the partially developed ear of a Gata3 null mouse with a cochlear duct connected via a ductus reunions to the vestibular part of the ear. For abbreviations see Fig. 2. Images taken from (Duncan et al. 2011; Kersigo et al. 2011).
2.b. Segregation of the organ of Corti from the saccule is essential for normal development
Logically, the transformation of vestibular-like HCs into basilar papilla/organ of Corti-like HCs requires specific cues driving cellular patterns and their differentiation. To achieve this, each of the sensory organs harboring a distinct type of epithelium is segregated from other epithelia to ensure individual development. In essence, to evolve unique features, epithelia have to become segregated to allow unique signaling that guide a given sensory epithelium’s specific development to evolve (Duncan and Fritzsch 2012). Several mutants have been described with partial or incomplete segregation of various sensory epithelia, but in only a few cases has there been a detailed analysis of sensory epithelia in the fused region. For example, in Otx1 null mutants there is a fusion of the utricular and saccular macula through an incompletely closed utriculo-saccular foramen (Morsli et al. 1999). These sensory epithelia seem to develop a contiguous striolar region but show a transitory blending of opposing HC polarity, resembling somewhat the undivided common macula of lampreys (Fritzsch et al. 2001).
A more profound fusion of the utricle, saccule and organ of Corti has been described in several mutant mice. In one of these mice (Nichols et al. 2008), the basal part of the organ of Corti blends into the caudal extension of the saccular macula (Fig. 4). The transitory region is characterized by hair cells that are neither vestibular nor organ of Corti-like in their appearance and show a blending of several features. Interestingly, the basal turn of the organ of Corti/saccular macula transition does not show the development of a tectorial membrane, has no spiral limbus, and is not on a basilar membrane separating the scala tympani from the scala media. While the basal half of the organ of Corti shows these remarkable changes, near the middle of the single turn the organ of Corti is transitioning into an apex that develops a near normal pattern of hair cells sitting on a basilar membrane (Fig. 4). Important for our evolutionary considerations, the simple fact that a single mutation can turn a part of the organ of Corti from an epithelium situated on a basilar membrane into an epithelium situated on limbic tissue renders the argument to use such structural differences and their implied functional differences as indicative for independent evolutionary origin (Wever 1974) all but obsolete. Otherwise one would need to argue that the basal turn of the organ of Corti is not homologous to the contiguous apical turn in certain null mutants, despite the fact that both are contiguous.
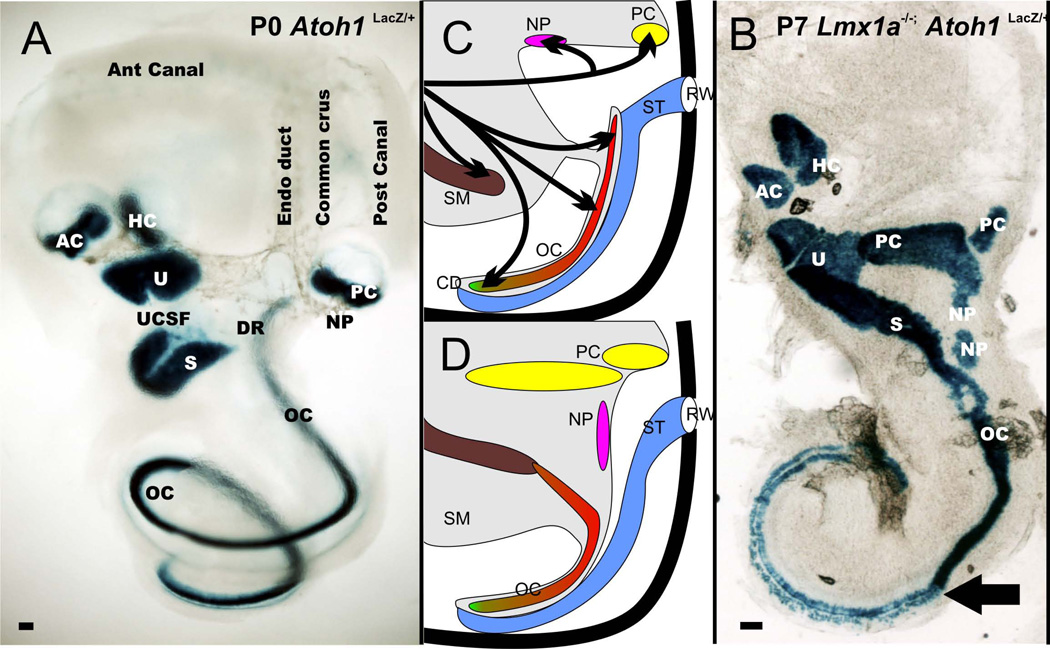
Figure 4.
The distribution of the six mammalian sensory epithelia and the neglected papilla (NP) is shown for a wildtype ear (A) and Lmx1a mutant ear (B). Note that there is continuity of utricle (U), saccule (S), and organ of Corti (OC) in the mutants. Also, the cochlea has a basal part that is converted into a more vestibular like system and an apex that is more cochlear like indicating different effects of Lmx1a along the length of the cochlea. There is a great enlargement of the posterior canal crista as well as the two patches of neglected papilla (NP) extending into the wide cochlear duct. The middle column shows the transformation of the ear in the Lmx1a null mutant. The saccular macula fuses with the organ of Corti, and there is no approximation of the scala tympani with the base of the organ of Corti but only with the apex (D). There is a dramatic increase in size of the posterior canal crista and neglected papilla. AC, anterior canal crista; HC, horizontal canal crista; DR, ductus reuniens; RW, round window; SM, scala media; UCSF; utriculo-saccular foramen. Modified after (Nichols et al. 2008)
A somewhat similar fusion of the utricular and saccular macula with the basal tip of the organ of Corti is also found in another mutant (Kopecky et al. 2011) (Fig. 5). However, while epithelia can be contiguous, there is nevertheless a distinction between basal turn organ of Corti and saccular macula HCs. Interestingly, in these mutants there is a complete loss of the basal hook region (Kopecky et al. 2012), the high frequency end of the organ of Corti that is a unique mammalian addition in frequency range. Importantly, both mutants show a different pattern of changes in the apex and the base which we will explore further below in their possible evolutionary significance.
Figure 5.
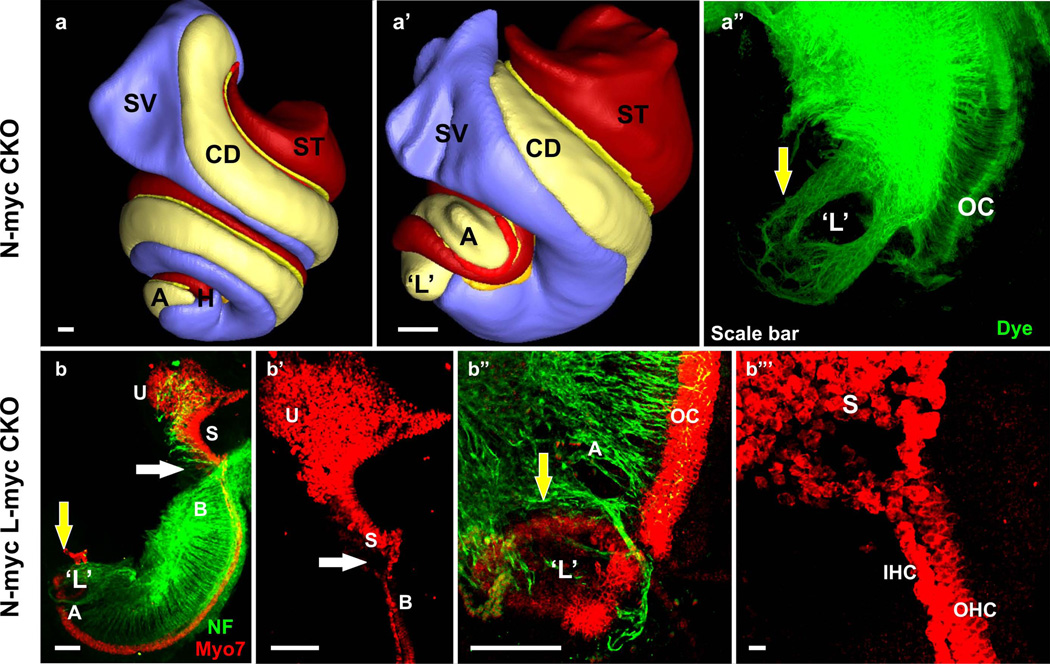
Three dimensional reconstruction of the inner ear shows the different regions of the cochlea in wildtype (a). Deletion of N-Myc results in severe truncation of the cochlear duct and aberration in the appropriate segregation of the different regions of the cochlea (b). In particular, the apex of the N-Myc CKO cochlea forms a circular sac-like structure resembling a ‘lagena’ (yellow arrow in b’). Dye tracing from the cochlear nucleus shows afferent innervation to the abnormally formed circular sac or ‘transformed lagena’. Double knockout (dCKO) of N-Myc and L-Myc adds to the severity in the N-Myc CKO phenotype (c-c”‘). Myo7a immunohistochemistry shows abnormal fusion of the hair cells from the basal tip with the saccular hair cells (white arrows in c-c”). The dCKO also shows formation of the segregated circular sac from the apical tip of the cochlea (yellow arrows in c, c”‘). The shortened cochlea forms multiple rows of hair cells in the apex (c”). Neurofilament immunohistochemistry shows the overshooting of the fibers to this circular sac (c”‘). A, apex; B, base; CD, cochlear duct; L, lagena; OC, organ of Corti; S, saccule; SV, scala vestibuli; ST, scala tympani; U, utricle. Bar indicates 100 µm. Modified after (Kopecky et al. 2011; Pan et al. 2011; Kopecky et al. 2012).
Finally, yet another null mutant can turn the therian organ of Corti into a monotreme-like ‘basilar papilla’ with up to 16 rows of HCs and a shortened, tear-drop like epithelium (Pauley et al. 2006). Moreover, like in monotremes, the outermost rows of outer hair cells show a continuous change in their polarity (Ladhams and Pickles 1996; Pauley et al. 2006). Obviously, the next step should be to combine mutations in two or three of these genes to investigate how the shortening of the organ of Corti can combine with the incomplete separation of the utricle/saccule/organ of Corti.
2.c. Evidence that the lagenar macula is not lost but integrated into the apex of the cochlea
As will be apparent below, numerous data on otherwise unexplainable and highly idiosyncratic aspects of organ of Corti development can make sense in light of the above-outlined evolution of the ear. Barring heterochronic shifts in development, most development of the ear proceeds along evolutionary progression. For example, the lagenar macula evolved already in the common sarcopterygian/actinopterygian ancestor in the common saccular recess and may represent a part of the common macula of jawless vertebrates (Lewis et al. 1985). This ancient sensory organ was transposed in sarcopterygians into the apex of the newly evolved lagenar recess, presumably around the same time the basilar papilla evolved near the orifice of the lagenar recess. Thus, the lagenar macula is evolutionarily speaking older than the basilar papilla and both show various degrees of loss among tetrapods (lagenar macula is lost in some caecilians and all therians, basilar papilla is lost in derived salamanders and caecilians). However, a lagenar macula is recognized in three different positions among craniates (as part of a common macula in the undivided ‘common macula’ of jawless vertebrates; as a separate lagenar macula next to the saccular macula in the saccular recess; and as an epithelium at the tip of the lagenar recess (Lewis et al. 1985; Fritzsch 1992). It needs to be stressed that the use of the term ‘lagena’ for this topologically variable epithelium has been introduced over 100 years ago and has never been questioned despite that fact that this epithelium is more variable than any other epithelium of the vertebrate ear.
During development of mammals, apical HCs exit the cell cycle first but initiate differentiation only days later when the expansion of a transcription factor that initiates differentiation, Atoh1, has reached them (Ruben 1967; Chen et al. 2002; Matei et al. 2005; Pan et al. 2011). Cell cycle exit, but not delayed expression of Atoh1, indicates a pattern closely related to vestibular HC proliferation. It is notable that premature expression of Atoh1 in certain mutant mice transforms only the apical part of the organ of Corti into an area with a mixed distribution of IHCs and OHCs (Jahan, Pan et al. 2010). Equally consistent with this idea that the apex of the organ of Corti may be a transformed lagenar macula is the expression of the neurotrophin Bdnf in the apex first (even if no HCs form). This expression sequence is similar to vestibular organs but is unlike the basal turn of the organ of Corti (Fritzsch et al. 2005), which depends on neurotrophic support from the unique presence of Ntf3, a neurotrophin with little functional role in the vestibular organs (Fritzsch et al. 2004). Indeed, misexpression of Bdnf under Ntf3 promoter control lures vestibular fibers to innervate aberrantly the basal, high frequency end of the mammalian organ of Corti (Tessarollo et al. 2004) confirming that Bdnf is the neurotrophin for the embryonic apical spiral ganglion cell survival whereas Ntf3 is playing this role in the basal turn (Farinas et al. 2001; Yang et al. 2011).
One of the most profound pieces of evidence for the vestibular nature of the apex is the transformation of the apex into an expanded space with a large sensory patch of HCs that bears little resemblance to the organ of Corti HCs (Kopecky et al. 2011). In fact, mice without these transcription factors have a clear gap between a recognizable organ of Corti and a discrete apical patch of HCs (Fig. 5) that has a different pattern of innervation (Kopecky et al. 2012). Moreover, these apical HCs are not sitting on a basilar membrane but rather on limbic tissue, indicating that organ of Corti interaction with limbic tissue is essential for normal cochlear and organ of Corti development. This is further supported by experimental data showing that loss of periotic tissue either in tissue culture (Van de Water 1983) or by extruding the cochlea into the brain cavity (Bouchard et al. 2010) results in complete loss of coiling of the cochlear duct, loss of formation of an organ of Corti but formation of a large ‘lagenar recess’ devoid of any sensory HCs comparable to certain amphibians (Lewis et al. 1985; Fritzsch and Wake 1988). The molecular basis for these otic-periotic tissue interactions are becoming clear and seem to be diffusible factors (Pirvola et al. 2004) and transcription factors (Braunstein et al. 2008; Ahn et al. 2009). These data suggest that coiling of the cochlea can be undone by altered epithelial/periotic mesenchyme interactions, transforming the cochlear duct into a large recess comparable to the lagenar recess.
Additional evidence that the base and the apex of the organ of Corti respond differentially to various mutations is provided another mouse lacking a transcription factor. These mice have a normal apex but a transformed base displaying a mix of vestibular and organ of Corti-like HCs (Nichols et al. 2008). While details of the molecular basis of this transformation are unclear, it is obvious that some of the molecular markers defining an organ of Corti must have changed their expression and that there is a different interaction with the periotic mesenchyme that may cause more vestibular type of HC formation. Indeed, it is noteworthy that Atoh1 transfection mediated differentiation of HCs in the greater epithelial ridge results in vestibular HC-like transformation with a limited viability (Yang et al. 2012). Combined, these data minimally suggest that the apex and the base of the organ of Corti are radically different in the use of the genes outlined above, but it may also indicate that the apical part of the organ of Corti is a transformed lagenar macula, still responding to low frequency sound much like the ancestral lagenar macula. It is important to note here that all mammals, no matter what the frequency range is, always have the apex of the organ of Corti responding to low and the base to high frequencies (Lewis et al. 1985). In contrast, in certain reptiles the basilar papilla next to a functional lagenar macula can actually have the frequency distribution with either the low range or the high range pointing toward the lagenar macula (Manley et al. 1999; Manley 2011). The constancy of mammals with respect to the frequency distribution may be related to the proposed transformation of the lagenar macula into the cochlear apex that locks in the low frequency reception to the apex.
In summary, the vertebrate middle ear is well known for the transformation of the hyomandibular bone into the columella/stapes of tetrapods and the transformation of the primary jaw joints into the hammer (malleus) and anvil (incus) of the middle ear (Reichert 1837; Hopson and Crompton 1969; Fritzsch 1999; Clack and Allin 2004). We propose a comparable transformation of the inner ear of mammals: the ancestral lagenar macula of sarcopterygians has become integrated into the apex of the therian organ of Corti. This implies that the sensory patch of the ancestral lagenar macula, which evolved into the basilar papilla at the orifice of the lagenar recess when the lagenar macula was displaced from the saccular recess into the lagenar recess, was fused together again in ancestral therians to form a unified organ of Corti. In contrast to progressive evolution of the middle ear, evolution of the organ of Corti may revert the development of two discrete epithelia found in Latimeria and most tetrapods, the basilar papilla and lagena, back into the formation of a single epithelium. However, this newly fused sensory epithelium is very distinct from the ancestral macula lagena found in the saccular recess of ancestral gnathostomes. Future molecular data will likely show similarities between the apex and the vestibular organs, in particular the saccule and utricle, beyond those already listed above.
2.d. Making inner and outer hair cells and turning them into the stereotyped organ of Corti
Tetrapod basilar papillae show a rich variation in HC patterning and distribution, except for mammals (Lewis et al. 1985; Manley 2011). In contrast to all other tetrapods, all mammals show two types of distinct HCs, the IHCs medial to the tunnel of Corti and the OHCs lateral to the tunnel of Corti. While the function of IHCs and OHCs in hearing has been clarified (Lewis et al. 1985; Manley 2010), neither the evolution of the two types of hair cells nor their development is molecularly understood. Some mutational studies indicate that specification of two different types of HCs may be affected by certain genes as null mutants of these genes lose IHCs or OHCs (Deol 1981; Brooker et al. 2006; Holley et al. 2010; Ahmed et al. 2012; Huh et al. 2012; Pan et al. 2012). In addition, HCs provide feed-forward and possibly feedback loops of diffusible factors (Huh et al. 2012) and transcription factors (Jahan et al. 2012; Pan et al. 2012) which secure specific cellular differentiation of supporting cells (Jacques et al. 2007; Doetzlhofer et al. 2009; Basch et al. 2011; Fritzsch et al. 2011). Best known examples include the differential distribution of Fgf8 in IHCs (Pirvola et al. 2000; Jacques et al. 2007) and Fgfr3 in pillar and Deiters’ cells (Puligilla et al. 2007; Huh et al. 2012). Absence of Fgfr3 or Fgf8 leads to altered pillar cell development (Jacques et al. 2007; Puligilla et al. 2007). Recently genes associated with the development of subsets of OHCs and supporting cells (Huh et al. 2012) or only all OHCs (Ahmed et al. 2012) have been identified. However, how IHCs form (Pirvola et al. 2000; Jahan, Kersigo et al. 2010) and how certain genes manage to regulate formation of only some HCs remains unclear.
The growing expression set of transcription factors and diffusible morphogens (Basch et al. 2011; Fritzsch et al. 2011; Ohyama et al. 2011; Groves and Fekete 2012; Huh et al. 2012) culminate in the expression of the three closely related bHLH transcription factors of the atonal family members: Atoh1, Neurog1 and Neurod1 (Bermingham et al. 1999; Ma et al. 2000; Kim et al. 2001). Loss-of-function studies show that the restricted expression of bHLH transcription factors provides a crucial step in neurosensory development that cannot be replaced by other transcription factors (Fritzsch et al. 2010). These three bHLH transcription factors regulate neurosensory development through extensive intra- and intercellular interactions. For example, Neurog1 drives the expression of Neurod1 in sensory neurons (Ma et al. 1998) and deletion of either gene leads to neuronal loss. Deletion of Neurog1 also results in HC loss and premature expression of Atoh1 (Ma et al. 2000; Matei et al. 2005), which is essential for HC development (Bermingham et al. 1999). This suggests that Neurog1 inhibits Atoh1, which in turn cross-regulates expression of Neurog1 (Matei et al. 2005; Raft et al. 2007; Jahan et al. 2012). In addition, absence of Neurog1 increases expression of Neurod1 in HCs (Matei et al. 2005), possibly through disinhibition of Atoh1 expression. Neurod1 in turn suppresses Neurog1 (Jahan, Kersigo et al. 2010). Atoh1 also drives Neurod1 expression in HCs (Jahan et al. 2012; Pan et al. 2012), whereas Neurod1 inhibits Atoh1 expression in HCs and neurons (Jahan, Kersigo et al. 2010). Neurod1 loss results in degeneration of most sensory neurons but some neurons survive and turn into ‘intraganglionic’ HCs apparently through de-repression of Atoh1 (Jahan, Kersigo et al. 2010). In contrast to the apparent plasticity of neurons revealed with loss of Neurod1, targeted misexpression of Neurog1 under the Atoh1 promoter control does not convert HCs into neurons (Jahan et al. 2012), suggesting a high degree of HC commitment prior to Atoh1 expression.
Given that the bHLH transcription factors are ancient and long-term associated with inner ear neurosensory development (Fritzsch et al. 2010), it is logical to reason that the interactions thus far elucidated in the mouse ear for the formation of IHCs and OHCs should also be ancestral. Other factors must therefore tweak these interactions to develop a graded change of HCs as in the long and short HCs of the avian basilar papilla (Lewis et al. 1985). It would be important for the conclusions drawn here to show that levels of bHLH gene co-expression cannot only change HC type in the mouse organ of Corti but are equally effective in tetrapods that have no morphologically distinct HC types in the basilar papilla such as frogs. Ultimately, the intricate network of gene interactions, now revealed in the organ of Corti development, need to be understood in other tetrapods to specify where developmental steps differ to establish causality of the above-outlined developmental aspect for evolution. Transforming the basilar papilla of a frog into an organ of Corti-like structure using molecular manipulations with genes associated with the therian organ of Corti would not only establish homology between these dissimilar looking organs. Such an approach would also establish how networks of genes evolved to regulate developmental steps resulting ultimately in dissimilar looking organs (Wagner 2011).
2.e. Turning vestibular sensory neurons into spiral ganglion neurons
Evolution of a functional auditory system critically depends on evolving connections between an auditory epithelium and the auditory processing center in the brain, the cochlear (auditory) nuclei (Fritzsch et al. 2006; Duncan and Fritzsch 2012). Only with the establishment of such a unique information highway is it ensured that auditory information is processed in the brain independently from vestibular information and vice versa. Failure to completely segregate vestibular from auditory information flow would result in cross-information processing that is not conducive for either hearing or vestibular function. Given the close proximity of the saccular macula, basilar papilla and lagenar macula, it would be important to know the connections of these organs with the hindbrain. Unfortunately, detailed data exist only in therian mammals (Maklad and Fritzsch 2003), several amphibians (Will and Fritzsch 1988) and avians (Fischer et al. 1994), with even more limited data on other amniotes. To make sense of the evolution of the therian auditory pathways, it is necessary to collect data on the central projection of the lagenar macula, basilar papilla and saccule in a monotreme.
In fact, detailed information about the development of auditory and vestibular ganglion cell and their organ specific connections to the brain is only known for mammals and some data in birds. A number of genes have been identified that guide the sensory neuron development and allow them to be connected (Rubel and Fritzsch 2002; Yang et al. 2011). Only few molecular candidate genes have been shown to be uniquely associated with spiral ganglion neuron, but not vestibular ganglion neuron development. Loss of those genes results in absence only of spiral ganglion neurons (Karis et al. 2001; Bouchard et al. 2010; Duncan et al. 2011; Lu et al. 2011). For our evolutionary investigations we are missing critical sets of data on the molecular basis of the comparative development of the amphibian ear, the only other tetrapod ear in which the central projection development has been mapped in great detail (Will and Fritzsch 1988). Modern techniques (Dagle et al. 2000; Abu-Daya et al. 2012) could help in understanding molecular aspects of frog ear neurosensory development. Superior manipulation ability of developing frogs and well-established tracing techniques for nerve fibers (Fritzsch 1993) could further our understanding of the molecular basis of auditory projection development in amphibians, providing a broader basis for the evolutionary comparison of molecular developmental modules attempted here.
For example, manipulations of neurotrophins can result in rerouting of vestibular nerve fibers to the basal turn of the organ of Corti (Tessarollo et al. 2004). This changes the pattern of innervation of the basal turn of the organ of Corti into a pattern of innervation of basilar papilla in derived frogs (Fig. 6), indicating that arguments to use this altered pattern of innervation to suggest that the basilar papilla of derived frogs is not homologous to that of other amphibians or other tetrapods or Latimeria are not well justified. Clearly, one would not change the assumption of homology of the basal part of the organ of Corti simply because vestibular fibers can be rerouted to it. It is also noteworthy that the pattern of innervation of all lagenar maculae found in Chondrichthyes or actinopterygians in the lagenar recess is identical to that found in monotremes, other tetrapods and Latimeria (Fig. 6 B,B’, A’). In contrast, the organization of polarity of hair cells of the lagena is different from tetrapods (Fig. 6 B,B’, C) indicating that the innervation allows homology across species, no matter whether the lagenar macula is in its own recess or what the pattern of hair cell polarity is. This conclusion for the lagena macula is in line with variable hair cell polarity in the basilar papilla of various lizards (Manley 2011). Overall, it appears that the molecular basis of nerve fiber organization, once understood, could allow to establish homology of nerve trajectories not only on traditional criteria such as relative position but also on molecular cues used to guide specific nerve fibers to specific sensory epithelia. That such molecules must exist to establish how the sensory epithelia are connected to distinct aspects of the vestibular nuclei has been demonstrated in a detailed projection analysis that showed little reorganization of developing projections over time from distinctly polarized hair cells to discrete areas of the brain (Maklad et al. 2010).
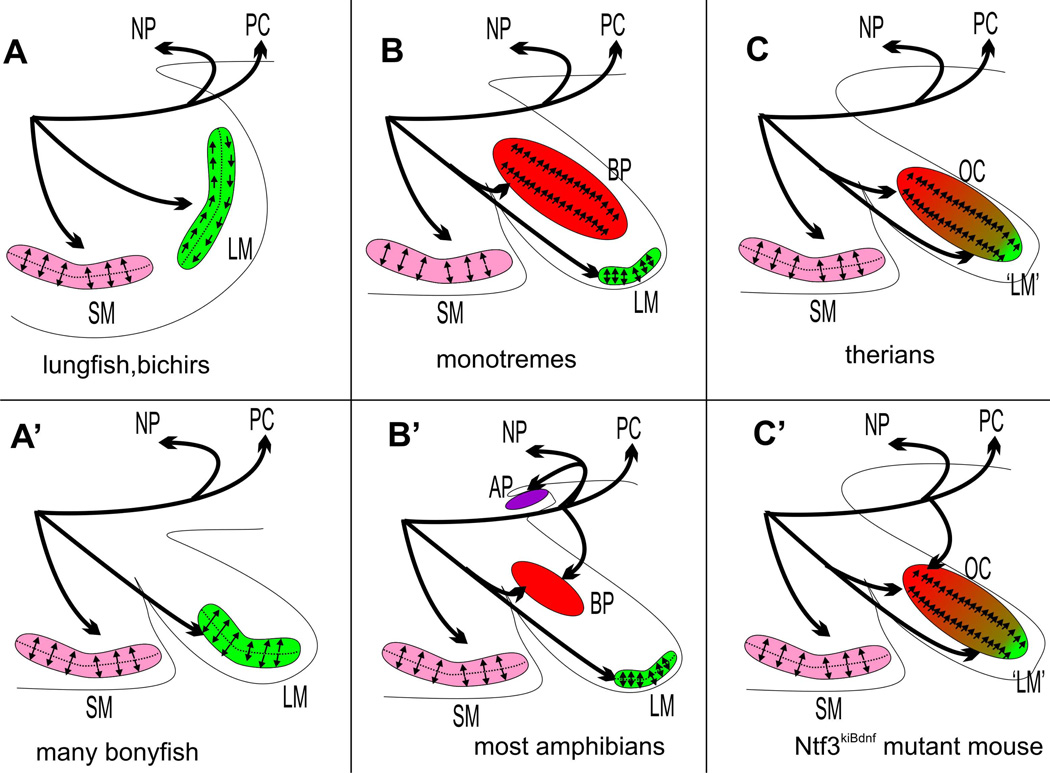
Figure 6.
This scheme shows the evolution of the innervation of the postero-ventral aspect of the gnathostome ear and the polarity pattern in three sensory epithelia, the saccular macula (SM), the lagenar macula (LM) and the basilar papilla (BP)/organ of Corti (OC). Note that basal sarcopterygians (lungfish) and actinopterygians (bichir) show a detailed identity in the lagenar hair cell polarity organization (A) that is distinct from that of tetrapods (B, B’) and also from the different pattern in many derived bonyfish that have a lagenar macula in its own recess (A’). Therian mammals (C) differ from monotremes (B) by the possible fusion of the lagenar macula with the basilar papilla (green and red) forming the organ of Corti. Note that innervation is highly stereotyped and similar across vertebrates except for amphibians with the innervation of the amphibian papilla of a neglected papilla (NP) twig (if the later exist) and formation of a new posterior twig to the basilar papilla coming off the posterior canal nerve in derived frogs (B’). A similar pattern of innervation can be generated in mice using a knockin of the neurotrophin Bdnf into the Ntf3 locus, turning the basal turn of the organ of Corti innervation into an innervation resembling the derived frog basilar papilla.
Summary and conclusion
We provide here an overview of ear changes in an evolutionary context of the currently best fitting taxonomic relationships. Based on changes in ear morphology and taxonomic relationships, we propose a simple scheme of progressive and regressive evolutionary events associated with the formation of a basilar papilla in sarcopterygian vertebrates and its subsequent diversification, culminating in the therian organ of Corti. We also present the even more puzzling alterations in position of the lagenar macula and its possible blending into the evolving organ of Corti as its apex. We also summarize data obtained from mutant mice that suggest the molecular basis for some of the critical steps identified for the organ of Corti evolution. Wherever possible we identified critical experiments that could support or falsify our evolutionary correlation with developmental events. Despite the existing gaps in our knowledge of molecular events specifying major aspects of developmental reorganizations underlying these evolutionary changes, the progress made over the last 15 years suggests that with proper expansion of the mammalian molecular insights onto other crucial tetrapods such as frogs and birds, we will be able to close many of these gaps in the near future. Remaining issues require working with non-model organisms such as monotremes, Latimeria, or basal caecilians, all of which are difficult to raise in captivity and for which no genome has yet been published. Given the genomic progress in some of these organisms, molecular data will soon be available to allow comparative genomic analysis of sarcopterygian ear development. This would allow comparing exomes of mice, chicken, frogs and other sarcopterygians for a full complement of all the genetic alterations underlying the evolution of the developmental modules of the ancestral sarcopterygian ear without a basilar papilla into a mammalian ear with its organ of Corti, the sound pressure receiver enabling our daily verbal communications. The ultimate proof for completion of our prepositions expanded here would be to modify development of one species to approximate the pattern found in another, distantly related species. Proving the present suggestions beyond reasonable doubt requires the transformation of a frog basilar papilla into a therian organ of Corti by proper molecular means. This could parallel the clear evidence for signal similarity across phyla generated by rescuing mammalian hair cell development with the fly atonal gene (Wang et al. 2002). This experiment demonstrated that the atonal fly gene, with limited sequence similarity to the mammalian Atoh1 gene, is nevertheless functionally equivalent, establishing the deep homology of mechanosensory cells now widely accepted based on additional evidence (Fritzsch et al. 2010).
Acknowledgements
This work was supported by NIH grants R01 DC 005590 (BF), P30 DC 010362 (BF) and CTSA UL1RR024979 (BK). We express our thanks to the Roy. J. Carver foundation for the purchase of the Leica TCS SP5 confocal microscope and the Office of the Vice President for Research for support.
Citations
- Abu-Daya A, Khokha MK, Zimmerman LB. The hitchhiker's guide to Xenopus genetics. Genesis. 2012;50(3):164–175. doi: 10.1002/dvg.22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M, Wong Elaine ÂYM, Sun J, Xu J, Wang F, Xu P-X. Eya1-Six1 Interaction Is Sufficient to Induce Hair Cell Fate in the Cochlea by Activating Atoh1 Expression in Cooperation with Sox2. Developmental Cell. 2012;22(2):377–390. doi: 10.1016/j.devcel.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn KJ, Passero F, Jr, Crenshaw EB., 3rd Otic mesenchyme expression of Cre recombinase directed by the inner ear enhancer of the Brn4/Pou3f4 gene. Genesis. 2009;47(3):137–141. doi: 10.1002/dvg.20454. [DOI] [PubMed] [Google Scholar]
- Amemiya CT, Powers TP, Prohaska SJ, Grimwood J, Schmutz J, Dickson M, Miyake T, Schoenborn MA, Myers RM, Ruddle FH, Stadler PF. Complete HOX cluster characterization of the coelacanth provides further evidence for slow evolution of its genome. Proc Natl Acad Sci U S A. 2010;107(8):3622–3627. doi: 10.1073/pnas.0914312107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch ML, Ohyama T, Segil N, Groves AK. Canonical Notch signaling is not necessary for prosensory induction in the mouse cochlea: insights from a conditional mutant of RBPjkappa. J Neurosci. 2011;31(22):8046–8058. doi: 10.1523/JNEUROSCI.6671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund AM, Brown MC. Central trajectories of type II spiral ganglion cells from various cochlear regions in mice. Hear Res. 1994;75(1–2):121–130. doi: 10.1016/0378-5955(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284(5421):1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bernstein P. The ear region of Latimeria chalumnae: functional and evolutionary implications. Zoology (Jena) 2003;106(3):233–242. doi: 10.1078/0944-2006-00119. [DOI] [PubMed] [Google Scholar]
- Bok J, Chang W, Wu DK. Patterning and morphogenesis of the vertebrate inner ear. Int J Dev Biol. 2007;51(6–7):521–533. doi: 10.1387/ijdb.072381jb. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Busslinger M, Xu P, De Caprona D, Fritzsch B. PAX2 and PAX8 cooperate in mouse inner ear morphogenesis and innervation. BMC Dev. Biol. 2010;10:89. doi: 10.1186/1471-213X-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein EM, Crenshaw EB, 3rd, Morrow BE, Adams JC. Cooperative function of Tbx1 and Brn4 in the periotic mesenchyme is necessary for cochlea formation. J Assoc Res Otolaryngol. 2008;9(1):33–43. doi: 10.1007/s10162-008-0110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133(7):1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Carroll RL. The Palaeozoic Ancestry of Salamanders, Frogs and Caecilians. Zoological Journal of the Linnean Society. 2007;150:1–140. [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129(10):2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Christensen-Dalsgaard J, Brandt C, Wilson M, Wahlberg M, Madsen PT. Hearing in the African lungfish (Protopterus annectens): pre-adaptation to pressure hearing in tetrapods? Biol Lett. 2011;7(1):139–141. doi: 10.1098/rsbl.2010.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack JA. Homologies in the fossil record: the middle ear as a test case. Acta Biotheor. 1993;41(4):391–409. doi: 10.1007/BF00709373. [DOI] [PubMed] [Google Scholar]
- Clack JA. The evolution of tetrapod ears and the fossil record. Brain Behav Evol. 1997;50(4):198–212. doi: 10.1159/000113334. [DOI] [PubMed] [Google Scholar]
- Clack JA. Patterns and processes in the early evolution of the tetrapod ear. J Neurobiol. 2002;53(2):251–264. doi: 10.1002/neu.10129. [DOI] [PubMed] [Google Scholar]
- Clack JA, Allin EF. The evolution of single and multiple-ossicle ears in fishes and tetrapods. In: Manley GA, Popper AN, Fay RR, editors. Evolution of the vertebrate auditory system. New York: Springer Verlag; 2004. [Google Scholar]
- Dagle JM, Littig JL, Sutherland LB, Weeks DL. Targeted elimination of zygotic messages in Xenopus laevis embryos by modified oligonucleotides possessing terminal cationic linkages. Nucleic Acids Res. 2000;28(10):2153–2157. doi: 10.1093/nar/28.10.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deol MS. The inner ear in Bronx waltzer mice. Acta Otolaryngol. 1981;92(3–4):331–336. doi: 10.3109/00016488109133269. [DOI] [PubMed] [Google Scholar]
- Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell. 2009;16(1):58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JS, Fritzsch B. Transforming the vestibular system one molecule at a time: the molecular and developmental basis of vertebrate auditory evolution. Adv Exp Med Biol. 2012;739:173–186. doi: 10.1007/978-1-4614-1704-0_11. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Lim KC, Engel JD, Fritzsch B. Limited inner ear morphogenesis and neurosensory development are possible in the absence of GATA3. Int J Dev Biol. 2011;55(3):297–303. doi: 10.1387/ijdb.103178jd. [DOI] [PubMed] [Google Scholar]
- Ehret G, Keilwerth E, Kamada T. The lung-eardrum pathway in three treefrog and four dendrobatid frog species: some properties of sound transmission. J Exp Biol. 1994;195:329–343. doi: 10.1242/jeb.195.1.329. [DOI] [PubMed] [Google Scholar]
- Elliott KL, Fritzsch B. Transplantation of Xenopus laevis ears reveals the ability to form afferent and efferent connections with the spinal cord. Int J Dev Biol. 2010;54(10):1443–1451. doi: 10.1387/ijdb.103061ke. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21(16):6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay RR. Comparative psychoacoustics. Hear Res. 1988;34(3):295–305. doi: 10.1016/0378-5955(88)90009-3. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Sienknecht UJ. The Inner ear. In: Moody SA, editor. Principles of Developmental Genetics. Philadelphia: Academic Press; 2007. [Google Scholar]
- Fischer FP, Eisensamer B, Manley GA. Cochlear and lagenar ganglia of the chicken. J Morphol. 1994;220(1):71–83. doi: 10.1002/jmor.1052200107. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. The inner ear of the coelacanth fish Latimeria has tetrapod affinities. Nature. 1987;327:153–154. doi: 10.1038/327153a0. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. Phylogenetic and ontogenetic origin of the dorsolateral auditory nucleus of anurans. In: Fritzsch B, Ryan M, Wilczynski W, Hetherington T, Walkowiak W, editors. The Evolution of the Amphibian Auditory System. New York: Wiley & Sons; 1988. [Google Scholar]
- Fritzsch B. The water-to-land transition: Evolution of the tetrapod basilar papilla, middle ear and auditory nuclei. In: Webster DB, Fay RR, Popper AN, editors. The Evolutionary Biology of Hearing. New York: Springer Verlag; 1992. [Google Scholar]
- Fritzsch B. Fast axonal diffusion of 3000 molecular weight dextran amines. J Neurosci Methods. 1993;50(1):95–103. doi: 10.1016/0165-0270(93)90060-5. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. Hearing in two worlds: Theoretical and realistic adaptive changes of the aquatic and terrestrial ear for sound reception. In: Fay RR, Popper AN, editors. Comparative Hearing: Fish and Amphibians. New York: Springer-Verlag; 1999. [Google Scholar]
- Fritzsch B. The ear of Latimeria chalumnae revisited. Zoology. 2003;106:243–248. doi: 10.1078/0944-2006-00120. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Pauley S, Soukup G. Molecular evolution of the vertebrate mechanosensory cell and ear. Int J Dev Biol. 2007;51(6–7):663–678. doi: 10.1387/ijdb.072367bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Eberl DF, Beisel KW. The role of bHLH genes in ear development and evolution: revisiting a 10-year-old hypothesis. Cell Mol Life Sci. 2010;67(18):3089–3099. doi: 10.1007/s00018-010-0403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Eberl DF, Beisel KW. The role of bHLH genes in ear development and evolution: revisiting a 10-year-old hypothesis. Cell Mol Life Sci. 2010 doi: 10.1007/s00018-010-0403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Glover JC. Evolution of the deuterostome central nervous system: an intercalation of developmental patterning processes with cellular specification processes. In: Kaas JH, editor. Evolution of Nervous Systems. Oxford: Academic Press; 2007. [Google Scholar]
- Fritzsch B, Jahan I, Pan N, Kersigo J, Duncan J, Kopecky B. Dissecting the molecular basis of organ of Corti development: Where are we now? Hear Res. 2011 doi: 10.1016/j.heares.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Matei VA, Nichols DH, Bermingham N, Jones K, Beisel KW, Wang VY. Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Dev Dyn. 2005;233(2):570–583. doi: 10.1002/dvdy.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Neary T. The octavolateralis system of mechanosensory and electrosensory organs. In: Heatwole H, editor. Amphibian Biology. Chipping Norton: Surrey Beatty & Sons; 1998. [Google Scholar]
- Fritzsch B, Pauley S, Feng F, Matei V, Nichols DH. The evolution of the vertebrate auditory system: transformations of vestibular mechanosensory cells for sound processing is combined with newly generated central processing neurons. International Journal of Comparative Psychology. 2006;19:1–24. [Google Scholar]
- Fritzsch B, Ryan M, Wilczynski W, Hetherington T, Walkowiak W. In: The Evolution of the Amphibian Auditory System. Northcutt RG, editor. New York: Wiley & Sons; 1988. [Google Scholar]
- Fritzsch B, Signore M, Simeone A. Otx1 null mutant mice show partial segregation of sensory epithelia comparable to lamprey ears. Dev Genes Evol. 2001;211(8–9):388–396. doi: 10.1007/s004270100166. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Wake MH. The inner ear of gymnophione amphibians and its nerve supply: a comparative study of regressive events in a complex sensory system. Zoomorphol. 1988;108:210–217. [Google Scholar]
- Gehring WJ. Chance and necessity in eye evolution. Genome Biol Evol. 2011;3:1053–1066. doi: 10.1093/gbe/evr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe B, Carr EC, Casseday JH, Fritzsch B, Köppl C. The Evolution of Central Pathways and Their Neural Processing Patterns. In: Manley GA, Popper AN, Fay RR, editors. Springer Handbook of Auditory Research: Evolution of the Vertebrate Auditory System. New York: Spinger-Verlag; 2004. [Google Scholar]
- Groves AK, Fekete DM. Shaping sound in space: the regulation of inner ear patterning. Development. 2012;139(2):245–257. doi: 10.1242/dev.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond KL, Whitfield TT. The developing lamprey ear closely resembles the zebrafish otic vesicle: otx1 expression can account for all major patterning differences. Development. 2006;133(7):1347–1357. doi: 10.1242/dev.02306. [DOI] [PubMed] [Google Scholar]
- Holley M, Rhodes C, Kneebone A, Herde MK, Fleming M, Steel KP. Emx2 and early hair cell development in the mouse inner ear. Dev Biol. 2010;340(2):547–556. doi: 10.1016/j.ydbio.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopson JA, Crompton AW. Origin of Mammals. In: Dobzhansky T, Hecht MK, Steere WC, editors. Evolutionary Biology. New York: Appleton-Century-Crofts; 1969. [Google Scholar]
- Huh SH, Jones J, Warchol ME, Ornitz DM. Differentiation of the Lateral Compartment of the Cochlea Requires a Temporally Restricted FGF20 Signal. PLoS Biol. 2012;10(1):e1001231. doi: 10.1371/journal.pbio.1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques BE, Montcouquiol ME, Layman EM, Lewandoski M, Kelley MW. Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development. 2007;134(16):3021–3029. doi: 10.1242/dev.02874. [DOI] [PubMed] [Google Scholar]
- Jahan I, Kersigo J, Pan N, Fritzsch B. Neurod1 regulates survival and formation of connections in mouse ear and brain. Cell Tissue Res. 2010;341(1):95–110. doi: 10.1007/s00441-010-0984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Calisto LE, Morris KA, Kopecky B, Duncan JS, Beiseld KW, Fritzsch B. Expression of Neurog1 instead of Atoh1 can partially rescue organ of Corti cell survival. PLoS One. 2012;7(1):e30853. doi: 10.1371/journal.pone.0030853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Fritzsch B. Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea. PLoS One. 2010;5(7):e11661. doi: 10.1371/journal.pone.0011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B. Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol. 2001;429(4):615–630. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kelly M, Chen P. Shaping the mammalian auditory sensory organ by the planar cell polarity pathway. Int J Dev Biol. 2007;51(6–7):535–547. doi: 10.1387/ijdb.072344mk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersigo J, D'Angelo A, Gray B, Soukup GA, Fritzsch B. The role of sensory organs and the forebrain for the development of the craniofacial shape as revealed by Foxg1-cre mediated microRNA loss. Genesis. 2011 doi: 10.1002/dvg.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434(7036):1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128(3):417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SK, Hill JK, Hwang CH, Lin ZS, Millen KJ, Wu DK. Lmx1a maintains proper neurogenic, sensory, and non-sensory domains in the mammalian inner ear. Dev Biol. 2009;333(1):14–25. doi: 10.1016/j.ydbio.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. Conditional deletion of N-Myc disrupts neurosensory and non-sensory development of the ear. Dev Dyn. 2011;240(6):1373–1390. doi: 10.1002/dvdy.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky BJ, Jahan I, Fritzsch B. Correct Timing Between Proliferation and Differentiation is Necessary for Normal Inner Ear Development and Auditory Hair Cell Viability. Dev Dyn. 2012 doi: 10.1002/dvdy.23910. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladhams A, Pickles JO. Morphology of the monotreme organ of Corti and macula lagena. J Comp Neurol. 1996;366(2):335–347. doi: 10.1002/(SICI)1096-9861(19960304)366:2<335::AID-CNE11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Ladich F, Popper AN. Parallel evolution of fish hearing organs. In: Manley GA, Popper AN, Fay RR, editors. Evolution of the vertebrate auditory system. New York: Springer Verlag; 2004. [Google Scholar]
- Leake PA, Hradek GT, Bonham BH, Snyder RL. Topography of auditory nerve projections to the cochlear nucleus in cats after neonatal deafness and electrical stimulation by a cochlear implant. J Assoc Res Otolaryngol. 2008;9(3):349–372. doi: 10.1007/s10162-008-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis ER, Leverenz EL, Bialek WS. The vertebrate inner ear. Boca Raton: CRC Press; 1985. [Google Scholar]
- Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419(6904):300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- Lu CC, Appler JM, Houseman EA, Goodrich LV. Developmental profiling of spiral ganglion neurons reveals insights into auditory circuit assembly. J Neurosci. 2011;31(30):10903–10918. doi: 10.1523/JNEUROSCI.2358-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZX, Ruf I, Schultz JA, Martin T. Fossil evidence on evolution of inner ear cochlea in Jurassic mammals. Proc Biol Sci. 2011;278(1702):28–34. doi: 10.1098/rspb.2010.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1(2):129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20(3):469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. The developmental segregation of posterior crista and saccular vestibular fibers in mice: A carbocyanine tracer study using confocal microscopy. Develop. Brain Research. 2002;135:1–17. doi: 10.1016/s0165-3806(01)00327-3. [DOI] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. Partial segregation of posterior crista and saccular fibers to the nodulus and uvula of the cerebellum in mice, and its development. Brain Res Dev Brain Res. 2003;140(2):223–236. doi: 10.1016/s0165-3806(02)00609-0. [DOI] [PubMed] [Google Scholar]
- Maklad A, Kamel S, Wong E, Fritzsch B. Development and organization of polarity-specific segregation of primary vestibular afferent fibers in mice. Cell Tissue Res. 2010;340(2):303–321. doi: 10.1007/s00441-010-0944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA. The origin and evolution of high-frequency hearing in (most) mammals. Hear Res. 2010;270(1–2):2–3. doi: 10.1016/j.heares.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Manley GA. Lizard auditory papillae: an evolutionary kaleidoscope. Hear Res. 2011;273(1–2):59–64. doi: 10.1016/j.heares.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Manley GA, Jones TA. The development and evolution of a tonotopic organization in the cochlea. Hear Res. 2011;277(1–2):1–3. doi: 10.1016/j.heares.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Manley GA, Koppl C. Phylogenetic development of the cochlea and its innervation. Curr Opin Neurobiol. 1998;8(4):468–474. doi: 10.1016/s0959-4388(98)80033-0. [DOI] [PubMed] [Google Scholar]
- Manley GA. What have lizard ears taught us about auditory physiology? Hear Res. 2008;238(1–2):3–11. doi: 10.1016/j.heares.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Manley GA, Koppl C, Sneary M. Reversed tonotopic map of the basilar papilla in Gekko gecko. Hear Res. 1999;131(1–2):107–116. doi: 10.1016/s0378-5955(99)00021-0. [DOI] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005;234(3):633–650. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millot J, Anthony J. Anatomie de Latimeria chalumnae. Centre National de la Recherche Scientifique; 1965. [Google Scholar]
- Morsli H, Tuorto F, Choo D, Postiglione MP, Simeone A, Wu DK. Otx1 and Otx2 activities are required for the normal development of the mouse inner ear. Development. 1999;126(11):2335–2343. doi: 10.1242/dev.126.11.2335. [DOI] [PubMed] [Google Scholar]
- Nayagam BA, Muniak MA, Ryugo DK. The spiral ganglion: connecting the peripheral and central auditory systems. Hear Res. 2011;278(1–2):2–20. doi: 10.1016/j.heares.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DH, Pauley S, Jahan I, Beisel KW, Millen KJ, Fritzsch B. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008;334(3):339–358. doi: 10.1007/s00441-008-0709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normile D. Evolution of language. Experiments probe language's origins and development. Science. 2012;336(6080):408–411. doi: 10.1126/science.336.6080.408. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Basch ML, Mishina Y, Lyons KM, Segil N, Groves AK. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J Neurosci. 2011;30(45):15044–15051. doi: 10.1523/JNEUROSCI.3547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoruwa OE, Weston MD, Sanjeevi DC, Millemon AR, Fritzsch B, Hallworth R, Beisel KW. Evolutionary insights into the unique electromotility motor of mammalian outer hair cells. Evol Dev. 2008;10(3):300–315. doi: 10.1111/j.1525-142X.2008.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Duncan J, Kopecky B, Fritzsch B. A novel Atoh1 ‘self-terminating’ mouse model reveals the necessity of proper Atoh1 expression level and duration for inner ear hair cell differentiation and viability. PLoS One. 2012;7(1):e30358. doi: 10.1371/journal.pone.0030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. Conditional deletion of Atoh1 using Pax2-Cre results in viable mice without differentiated cochlear hair cells that have lost most of the organ of Corti. Hear Res. 2011;275(1–2):66–80. doi: 10.1016/j.heares.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Lai E, Fritzsch B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev Dyn. 2006;235(9):2470–2482. doi: 10.1002/dvdy.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev Dyn. 2003;227(2):203–215. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Spencer-Dene B, Xing-Qun L, Kettunen P, Thesleff I, Fritzsch B, Dickson C, Ylikoski J. FGF/FGFR-2(IIIb) signaling is essential for inner ear morphogenesis. J Neurosci. 2000;20(16):6125–6134. doi: 10.1523/JNEUROSCI.20-16-06125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Zhang X, Mantela J, Ornitz DM, Ylikoski J. Fgf9 signaling regulates inner ear morphogenesis through epithelial-mesenchymal interactions. Dev Biol. 2004;273(2):350–360. doi: 10.1016/j.ydbio.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Platt C, Jorgensen JM, Popper AN. The inner ear of the lungfish Protopterus. J Comp Neurol. 2004;471(3):277–288. doi: 10.1002/cne.20038. [DOI] [PubMed] [Google Scholar]
- Puligilla C, Feng F, Ishikawa K, Bertuzzi S, Dabdoub A, Griffith AJ, Fritzsch B, Kelley MW. Disruption of fibroblast growth factor receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Dev Dyn. 2007;236(7):1905–1917. doi: 10.1002/dvdy.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft S, Koundakjian EJ, Quinones H, Jayasena CS, Goodrich LV, Johnson JE, Segil N, Groves AK. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134(24):4405–4415. doi: 10.1242/dev.009118. [DOI] [PubMed] [Google Scholar]
- Raincrow JD, Dewar K, Stocsits C, Prohaska SJ, Amemiya CT, Stadler PF, Chiu CH. Hox clusters of the bichir (Actinopterygii, Polypterus senegalus) highlight unique patterns of sequence evolution in gnathostome phylogeny. J Exp Zool B Mol Dev Evol. 2011;316(6):451–464. doi: 10.1002/jez.b.21420. [DOI] [PubMed] [Google Scholar]
- Reichert C. Ueber die Visceralbogen der Wierbelthiere im allgemeinen und deren Metamorphose bei den Saeugethieren und Voegeln. A. Anat. Phys. 1837:120–222. [Google Scholar]
- Retzius G. Das Gehörorgan der Wirbeltiere. 1881;Vol. I [Google Scholar]
- Retzius G. Das Gehörorgan der Wirbeltiere: II. Das Gehörorgan der Amnioten. Stockholm: Samson und Wallin; 1884. [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;220(Suppl):1–44. [PubMed] [Google Scholar]
- Ryugo DK, Parks TN. Primary innervation of the avian and mammalian cochlear nucleus. Brain Res Bull. 2003;60(5–6):435–456. doi: 10.1016/s0361-9230(03)00049-2. [DOI] [PubMed] [Google Scholar]
- Shan Y, Gras R. 43 genes support the lungfish-coelacanth grouping related to the closest living relative of tetrapods with the Bayesian method under the coalescence model. BMC Res Notes. 2011;4:49. doi: 10.1186/1756-0500-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubin N, Tabin C, Carroll S. Deep homology and the origins of evolutionary novelty. Nature. 2009;457(7231):818–823. doi: 10.1038/nature07891. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Sumiyama K, Amemiya CT. A living fossil in the genome of a living fossil: harbinger transposons in the coelacanth genome. Mol Biol Evol. 2012;29(3):985–993. doi: 10.1093/molbev/msr267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman M, Narins P. Evolution of the amphibian ear. In: Manley GA, Fay RR, Popper AN, editors. Evolution of the vertebrate auditory system. New York: Springer-Verlag; 2004. [Google Scholar]
- Takezaki N, Figueroa F, Zaleska-Rutczynska Z, Takahata N, Klein J. The phylogenetic relationship of tetrapod, coelacanth, and lungfish revealed by the sequences of forty-four nuclear genes. Mol Biol Evol. 2004;21(8):1512–1524. doi: 10.1093/molbev/msh150. [DOI] [PubMed] [Google Scholar]
- Tessarollo L, Coppola V, Fritzsch B. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J Neurosci. 2004;24(10):2575–2584. doi: 10.1523/JNEUROSCI.5514-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bergeijk WA. Evolution of the sense of hearing in vertebrates. Amer. Zoologist. 1966;6:371–377. doi: 10.1093/icb/6.3.371. [DOI] [PubMed] [Google Scholar]
- Van de Water TR. Embryogenesis of the inner ear: “In vitro studies”. In: Romand R, editor. Development of auditory and vestibular systems. New York: Academic Press; 1983. [Google Scholar]
- Vater M, Meng J, Fox RC. Hearing organ evolution and specializations: early and later mammals. In: Manley GA, Popper AN, Fay RR, editors. Evolution fo the vertebrate auditory system. New York: Springer Verlag; 2004. [Google Scholar]
- Voss SE, Rosowski JJ, Merchant SN, Peake WT. Acoustic responses of the human middle ear. Hear Res. 2000;150(1–2):43–69. doi: 10.1016/s0378-5955(00)00177-5. [DOI] [PubMed] [Google Scholar]
- Wagner A. The origins of evolutionary innovations. Oxford: Oxford Press; 2011. [Google Scholar]
- Wang VY, Hassan BA, Bellen HJ, Zoghbi HY. Drosophila atonal fully rescues the phenotype of Math1 null mice: new functions evolve in new cellular contexts. Curr Biol. 2002;12(18):1611–1616. doi: 10.1016/s0960-9822(02)01144-2. [DOI] [PubMed] [Google Scholar]
- Wever EG. The evolution of vertebrate hearing. In: Keidel WD, Neff WD, editors. Auditory System. Berlin: Springer Verlag; 1974. [Google Scholar]
- Will U, Fritzsch B. The octavus nerve of amphibians: Patterns of afferents and efferents. In: Fritzsch B, Ryan M, Wilczynski W, Hetherington T, Walkowiak W, editors. The Evolution of the Amphibian Auditory System. New York: Wiley and Sons; 1988. [Google Scholar]
- Yang J, Bouvron S, Lv P, Chi F, Yamoah EN. Functional features of trans-differentiated hair cells mediated by Atoh1 reveals a primordial mechanism. J Neurosci. 2012;32(11):3712–3725. doi: 10.1523/JNEUROSCI.6093-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Kersigo J, Jahan I, Pan N, Fritzsch B. The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of Corti. Hear Res. 2011;278(1–2):21–33. doi: 10.1016/j.heares.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]