Significance
Accumulation of a lipid-rich substance (myelin sheath) around the axon of neurons enables the nerve cells to transmit information faster. However, the molecular mechanism that controls this important process is not well understood. Here, we report that the GTPase phosphoinositide 3-kinase enhancer is a regulator of myelin sheath formation during development and myelin repair.
Abstract
Oligodendrocyte (OL) differentiation and myelin development are complex events regulated by numerous signal transduction factors. Here, we report that phosphoinositide-3 kinase enhancer L (PIKE-L) is required for OL development and myelination. PIKE-L expression is up-regulated when oligodendrocyte progenitor cells commit to differentiation. Conversely, depleting phosphoinositide-3 kinase enhancer (PIKE) expression by shRNA prevents oligodendrocyte progenitor cell differentiation. In both conventional PIKE knockout (PIKE−/−) and OL-specific PIKE knockout mice, the number of OLs is reduced in the corpus callosum. PIKE−/− OLs also display defects when forming myelin sheath on neuronal axons during neonatal development, which is partially rescued when PTEN is ablated. In addition, Akt/mTOR signaling is impaired in OL-enriched tissues of the PIKE−/− mutant, leading to reduced expression of critical proteins for myelin development and hypomyelination. Moreover, myelin repair of lysolecithin-induced lesions is delayed in PIKE−/− brain. Thus, PIKE plays pivotal roles to advance OL development and myelinogenesis through Akt/mTOR activation.
Efficient propagation of action potentials depends on the presence of myelin sheath that spirals around the axon. As a membrane extension from oligodendrocytes (OLs), the myelin sheath has a unique lipid-rich composition that allows electrical insulation for high-speed conduction and fidelity of signal transfer (1). Generation of OLs is a developmentally regulated process, which involves the proliferation of oligodendrocyte progenitor cells (OPCs) at the germinal subventricular zones (SVZ), migration throughout the CNS, differentiation into mature OLs, and adhesion to the axon to form myelin (2). Although most OPCs first appear in early neonatal brain, maturation and myelination of OLs in rodents occur largely in postnatal life between P10 and P60 (1). The timing of s differentiation and myelin formation requires highly localized signaling mechanisms, which involves the coordinated activation/inactivation of Wnt/β-catenin, Hedgehog/Gli1, Jagged1/Notch, and PI3K/Akt/mTOR cascades (3). Disruption of these pathways via gene manipulation or modulation of their regulators results in defective OL development. For example, PI3K depletion causes reduced myelin expression in the cerebral cortex and striatum (4). On the other hand, mutation of PTEN, the negative regulator of PI3K/Akt cascade, causes thickening and unraveling of the myelin sheath surrounding hypertrophic axons in the corpus callosum (CC) (5).
Phosphoinositide 3-kinase enhancer L (PIKE-L) is a CNS-specific GTPase that belongs to the centaurin family (6, 7). It participates in numerous cellular events to regulate neuronal activity and survival. Our previous studies show that PIKE-L interacts with both netrin receptor (UNC5H) and metabotropic glutamate receptor I (mGlu1) to prevent apoptotic cell death (8, 9). In addition, PIKE-L controls cell-surface trafficking of 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid receptor and the formation of long-term potentiation in the postsynaptic neurons (10). Moreover, PIKE controls the neuronal dendritogenesis and survival through maintaining the integrity of the PI3K/Akt pathway (11). Indeed, PIKE is an important molecular switch to control the cellular PI3K/Akt activation as it links extracellular stimuli including netrin, glutamate, and neurotrophins to the intrinsic PI3K/Akt activities (12–14). Nevertheless, the functions of PIKE-L in nonneuronal cells such as OLs and astrocytes still remain unexplored. In this paper, we report that PIKE-L signals through the PI3K/Akt pathway to advance CNS myelinogenesis.
Results
Expression of PIKE-L Is Markedly Up-Regulated During OL Development.
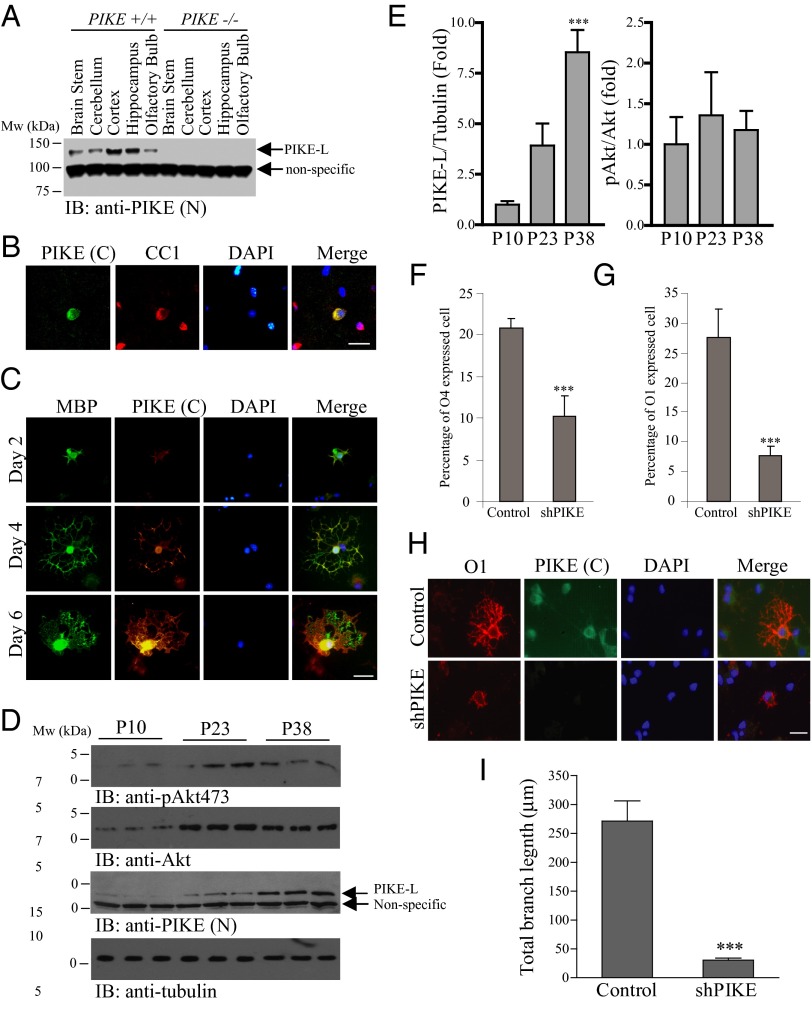
PIKE-L is a brain-specific GTPase (8). Immunoblot analysis revealed that PIKE-L is abundantly expressed in the cortex and hippocampus with a sustained level found in the cerebellum, olfactory bulb, and brainstem (Fig. 1A). The broad expression of PIKE-L in different brain regions suggests that PIKE-L may function in various cell types in the CNS in addition to its well-known roles in neuronal survival and synaptic plasticity. Using a homemade antibody that recognizes the C terminus of PIKE-L or a commercial polyclonal antibody against recombinant PIKE, we found that PIKE-L resided in the cell body and the processes of primary cultured OLs (Fig. S1A). This antibody was highly specific as no signal was detected in PIKE knockout (PIKE−/−) neurons (Fig. S1B). We also found that PIKE-L could be detected in mature oligodendrocytes (CC1+) in the brain of young adult [postnatal day 30 (P30)] mice (Fig. 1B). It is noteworthy that some OLs in the mouse CC did not display positive PIKE signaling, suggesting that PIKE-L may exhibit a selective expression pattern among the OL subpopulations.
Fig. 1.
Expression of PIKE-L in OLs is a developmentally regulated process. (A) Expression analysis of PIKE-L in various brain regions. Brains from wild-type (P7) and PIKE−/− mice were collected and dissected into different parts. The amount of total PIKE-L protein was examined using immunoblotting. (B) Expression of PIKE-L in OLs of CC of P30 mouse. (Scale bar, 20 μm.) (C) Increased expression of PIKE-L in differentiating OPC. Cultured rat OPCs at different differentiation days were stained with antibodies that recognize the C terminus of PIKE-L or MBP. (Scale bar, 20 μm.) (D) Expression of PIKE-L is elevated in developing optic nerve. Optic nerves collected from C57BL/6 mice of different ages were homogenized, and the amount of PIKE-L, Akt, and tubulin was examined using immunoblotting. Akt phosphorylation on S473 was also determined. (E) Quantification of Akt phosphorylation and PIKE-L expression shown in D (***P < 0.001 vs. P10, one-way ANOVA; n = 3). (F) OPCs isolated from rat were infected with control adenovirus or shPIKE-expressing adenovirus during OL differentiation. The number of O4+ cells was visualized by IF staining and quantified (***P < 0.001, Student t test; n = 6). (G) OPCs isolated from rat were infected with control adenovirus or shPIKE-expressing adenovirus during OL differentiation. The number of O1+ cells was visualized by IF staining and quantified (***P < 0.001, Student t test; n = 6). (H) PIKE-L depletion causes aberrant morphology in mature OLs. (Scale bar, 20 μm.) (I) Quantitation of dendritic length of cultured OLs after adenovirus infection (***P < 0.001, Student t test; n = 4).
Expression of PIKE-L could be detected in the soma, processes, and myelin sheath formed by mature OLs expressing myelin basic protein (MBP) (Fig. 1C). Importantly, the amount of PIKE protein increased when the OPCs were approaching maturation. Up-regulation of PIKE mRNA was also detected upon induced differentiation of the CG4 OPC cell line (Fig. S2), further supporting differentiation-enhanced, cell-autonomous up-regulation of PIKE expression in OPCs. In addition, the amount of PIKE-L was progressively elevated in the mouse optic nerve, which is highly enriched of OLs (15). During myelinogenesis, the amount of PIKE-L increased at least eightfold between postnatal day 10 and 38 (Fig. 1 D and E). An increasing Akt S473 phosphorylation was also observed (Fig. 1D), which was probably a result of enhanced protein accumulation as the amount of total Akt also increased during the OL development (Fig. 1 D and E). Presumably, PIKE-L expression is up-regulated during OL differentiation to cope with the increased amount of total Akt for maintaining a constant Akt activity.
PIKE Is Essential for OL Development in Culture and in Vivo.
To ask if PIKE-L plays essential roles in advancing OPC differentiation, we infected rat OPC cultures with control adenovirus or adenovirus expressing shRNA against PIKE-L (16) upon induced differentiation. PIKE-L depletion decreased the presence of cells expressing the well-established differentiation markers O4+ and O1+ in the OL lineage (Fig. 1 F and G). In addition, the process outgrowth was curtailed in PIKE-L–depleted cells (Fig. 1 H and I), suggesting that PIKE-L is a pivotal intrinsic factor important not only for the timing of differentiation but also for the morphological development of OLs.
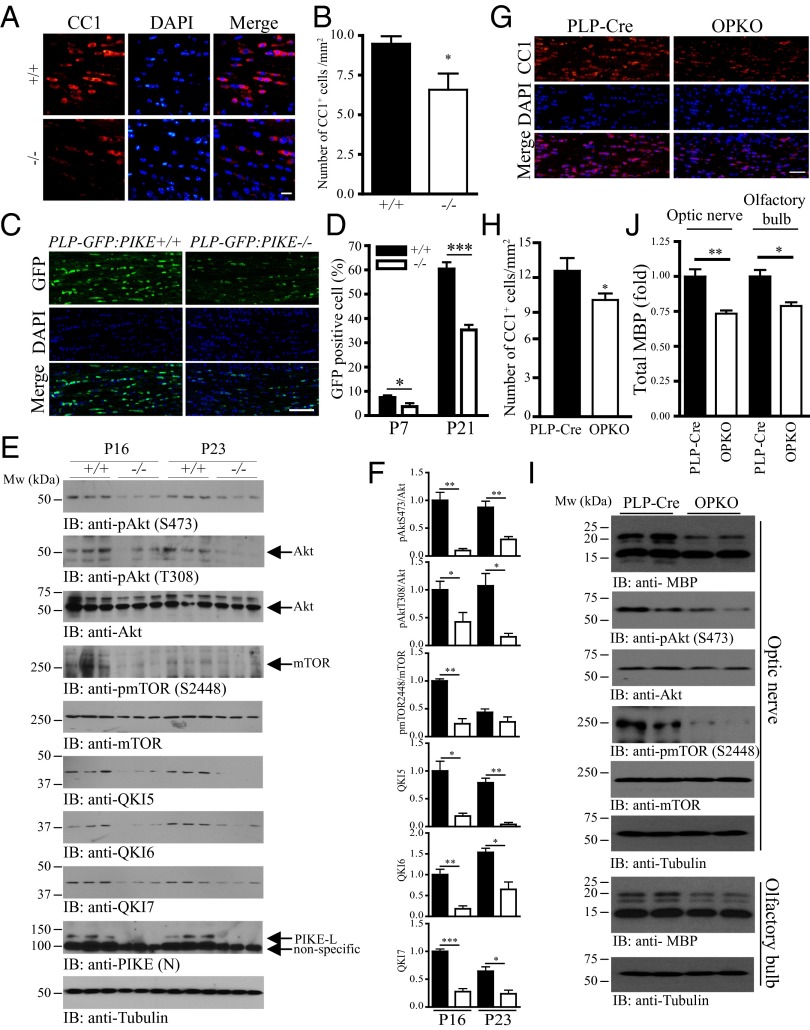
To further assess the role of PIKE-L in OL differentiation in vivo, we examined the amount of OLs in the whole-body PIKE knockout (PIKE−/−) mice (17). We found that the number of cells expressing the OL maturation marker CC1 in CC was significantly lower in the P16 PIKE-null mice (Fig. 2 A and B), suggesting that loss of PIKE impairs OL development in vivo. Furthermore, we crossed the PIKE−/− mice with the myelin proteolipid (PLP) promoter-driven GFP transgenic mice (18) to generate a double-engineered mutant line with all of the cells in the OL lineage that are marked by GFP expression (PLP-GFP:PIKE−/−). The number of GFP-positive cells in the CC increased drastically between postnatal day 7 and 21 in both PLP-GFP:PIKE+/+ controls and PLP-GFP:PIKE−/− mice. However, the density of GFP+ cells in the CC was significantly reduced in PLP-GFP:PIKE−/− mice at both ages (Fig. 2 C and D). We observed a reduction of OPC in PIKE−/− mice as the knockout animals displayed less NG2, the marker of OPC, staining in the SVZ (Fig. S3), suggesting that the lower amount of mature OLs due to PIKE deficiency is a result of defective OL lineage development, possibly involving both proliferation and differentiation.
Fig. 2.
The number of mature OLs and Akt/mTOR signaling are reduced in PIKE-ablated brain. (A) PIKE−/− brain contains fewer mature OLs. Brains from wild-type and PIKE−/− mice (P16) were collected, and the mature OLs in CC were visualized using IF staining against CC1. (Scale bar, 20 μm.) (B) Quantification of CC1+ cell in PIKE−/− CC (*P < 0.05, Student t test; n = 3). (C) PLP-GFP:PIKE−/− brain contains fewer mature OLs in CC. Brains of PLP-GFP:PIKE+/+ and PLP-GFP:PIKE−/− mice (P21) were collected, and the OLs in the CC were visualized and counted using confocal microscopy. (Scale bar, 50 μm.) (D) Quantification of GFP+ cells in the CC of PLP-GFP:PIKE+/+ and PLP-GFP:PIKE−/− mice (*P < 0.05, ***P < 0.001, Student t test; n = 3). (E) Optic nerves from wild-type (+/+) and PIKE−/− (−/−) mice of different ages (P16 and P23) were collected and homogenized. Expression of Akt, mTOR, QK5, QK6, QK7, PIKE-L, and tubulin was examined by immunoblotting. Phosphorylations of Akt and mTOR were also determined. (F) Quantification of the band intensity shown in E (solid bars, PIKE+/+; open bars, PIKE−/−; *P < 0.05, **P < 0.01, ***P < 0.001, Student t test; n = 3). (G) OPKO mice contain fewer OLs in the CC. Mature OLs in CC were visualized using IF staining against CC1. (Scale bar, 50 μm.) (H) Quantification of CC1+ cells in the CC of control and OPKO mice (*P < 0.05, Student t test; n = 4). (I) Reduced MBP expression and Akt/mTOR phosphorylations in OPKO mice. Optic nerves and the olfactory bulbs from the control and OPKO mice after tamoxifen administration were collected. Expression of MBP, Akt, mTOR, and tubulin was then examined using immunoblotting. Phosphorylations of Akt and mTOR were also determined. (J) Quantification of the MBP protein amount shown in I (*P < 0.05, Student t test; n = 3).
PIKE-L Is Required for Maintaining Akt/mTOR Signaling During Myelinogenesis.
PIKE−/− neurons display reduced PI3K/Akt activities (11). Because Akt and mTOR are important factors that trigger myelination (19), we tested whether Akt signaling in PIKE−/− OLs was impaired. Akt phosphorylation on both T308 and S473 was significantly reduced in the optic nerve of P16 and P23 PIKE−/− mice (Fig. 2 E and F), verifying that PIKE-L is indeed an essential factor in maintaining Akt activity in OLs. Phosphorylation on mTOR S2448 was also diminished in the optic nerve of P16 PIKE−/− mice (Fig. 2 E and F). Furthermore, the selective RNA-binding protein QKI that performs critical functions in promoting OL differentiation and myelination (20) was also significantly reduced in PIKE−/− mice (Fig. 2 E and F), consistent with the previous report that QKI is a target of mTOR during OPC differentiation (21). Hence, PIKE-L plays a critical role in maintaining the intact Akt/mTOR-signaling cascades in OLs during differentiation and myelin development.
PIKE−/− mice have reduced number of neurons in the cortex (11). Because neuronal impairment could also affect OL development through axon–glia interaction, we next set out to address whether the loss of PIKE in OLs alone can impair OL development in a cell-autonomous manner. We generated OL-specific PIKE knockout (OPKO) mice by crossing the transgenic PIKE flox/flox (22) animals with mice carrying a tamoxifen-inducible, Cre-mediated recombination system driven by the proteolipid protein 1 (PLP-1) promoter (23) (Fig. S4A). After tamoxifen administration, PIKE-L expression was significantly reduced in the OL-enriched optic nerve but not in the cortex of OPKO mice (Fig. S4B). Similar to the observation in PIKE−/− brain, the number of mature OLs was also reduced in the OPKO CC (Fig. 2 G and H). Expression of MBP was also impaired in the same tissue (Fig. 2 I and J), suggesting the absence of PIKE in the OL is sufficient to impair myelin development. Moreover, Akt and mTOR phosphorylations were reduced in the optic nerve of OPKO mice (Fig. 2I). Thus, these observations clearly demonstrate the essential role of PIKE in OLs for advancing OL development.
Loss of PIKE Causes Reduction of Proteins Critical for OL and Myelin Development.
MBP is an essential structural myelin protein that is regulated by Akt, mTOR, and QKI (24). As expected, the total amount of MBP protein was significantly reduced in P16 and P23 PIKE−/− optic nerves (Fig. 3 A and B). Interestingly, expression of 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP), a membrane-associated protein abundantly expressed in myelin-forming OLs, was not affected in PIKE−/− optic nerves, indicating a target-specific control of PIKE-L on myelin protein expression (Fig. 3 A and B). This is consistent with our previous observation that the mRNAs encoding MBP and CNP are differentially regulated by QKI (25).
Fig. 3.
PIKE−/− axons are hypomyelinated. (A) Defective expression of MBP in the optic nerve of PIKE−/− mice. Optic nerves from wild-type (+/+) and PIKE−/− (−/−) mice of different ages (P16 and P23) were collected. Expression of MBP, CNP, PIKE-L, and tubulin were then examined using immunoblotting. (B) Quantification of the protein amount shown in A (solid bars, PIKE+/+; open bars, PIKE−/−; *P < 0.05, Student t test; n = 3). (C) IF staining of MBP in the CC of wild-type, PIKE−/−, and PIKE−/−PTEN−/− double-knockout mice (P7). (Scale bar, 50 μm.) (D) Defective MBP expression and Akt phosphorylation in PIKE−/− mice could be rescued by PTEN ablation. Optic nerves from wild-type, PIKE−/−, and PIKE−/−PTEN−/− double-knockout mice (P23) were collected. Expression of MBP, PTEN, Akt, PIKE-L, and tubulin were then examined using immunoblotting. Phosphorylation of Akt was also tested. (E) Quantification of the protein amount shown in D (*P < 0.05, one-way ANOVA; n = 3). (F) Mature OLs in the CC of wild-type, PIKE−/−, and PIKE−/−PTEN−/− mice (P23) were visualized using IF staining against CC1. (Scale bar, 20 μm.) (G) Quantification of CC1+ cells in CC of wild-type, PIKE−/−, and PIKE−/−PTEN−/− mice (*P < 0.05, one-way ANOVA). (H) Representative electron micrograph of CC in cross-section from wild-type and PIKE−/− mice at P16. (Scale bar, 20 μm.) (I) Axon diameter of wild-type (+/+) and PIKE−/− (−/−) neurons in the CC (n = 6). (J) Histogram representing the G-ratio of wild-type (+/+) and PIKE−/− (−/−) axons (***P < 0.001, Student t test; n = 6).
To test whether such impairment in initial myelination is due to defective PI3K/Akt activities, we performed immunofluorescent (IF) staining in the brain of PIKE−/−PTEN−/− double-knockout mice, in which removal of PI3K/Akt suppressor PTEN phosphatase was demonstrated to rescue the PI3K/Akt signaling caused by the loss of PIKE (11). Unlike MBP+ OLs in the PIKE−/− neonates that harbor curtailed processes in the CC, MBP+ myelinating OLs in the PIKE−/−PTEN−/− neonates successfully extend myelin sheath along axon tracts, comparable to what was observed in wild-type controls (Fig. 3C), indicating that deficiency in PI3K/Akt activity underlies the defects in myelinating OLs. In addition, depleting PTEN in PIKE−/− mice also leads to rescued expression of MBP and Akt phosphorylation in the optic nerve (Fig. 3D) as well as a significantly increased number of mature OLs in CC (Fig. 3 F and G), indicating that deficiency in PI3K/Akt activity underlies the defects in myelinating OLs in PIKE−/− brain.
To further assess if the loss of PIKE may result in hypomyelination, we performed electron microscopic analysis on axons in CC of PIKE−/− and wild-type littermate controls. PIKE−/− mice displayed moderate hypomyelination, with visible thinning of myelin on many axons (Fig. 3H). In contrast, axon diameters in the CC of wild-type and PIKE−/− mice were comparable (Fig. 3I). Quantitative analysis further revealed a significant increase of G-ratio (axon diameter/axon diameter plus myelin thickness) for the PIKE −/− axons (Fig. 3J).
Remyelination Is Impaired in PIKE−/− Mutant.
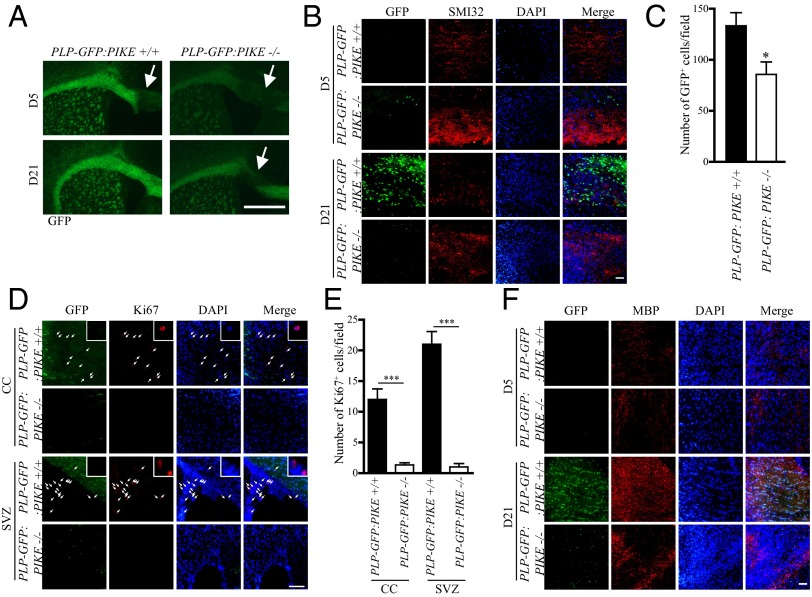
We further extended our study to investigate if PIKE is critical for CNS myelin repair after lysolecithin (LL)-induced CC demyelination. Five days (D5) and 21 days (D21) after LL injection, brains of PLP-GFP:PIKE+/+ and PLP-GFP:PIKE−/− mice were sectioned to examine the demyelination and remyelination. LL injection caused comparable levels of lesion in both genotypes at D5. However, PLP-GFP:PIKE−/− mice displayed significant defects in remyelination, with obvious demyelination still detected at D21, whereas PLP-GFP:PIKE+/+ mice largely repaired the lesion (Fig. 4A). In addition, PLP-GFP:PIKE−/− mice exhibited more intense staining of SMI32, a surrogate marker for axonal damage in pathological conditions, at both D5 and D21 (Fig. 4B). This result provides further support to our previous findings that PIKE is essential for neuronal integrity against various insults (11, 12, 16). Regarding the OL lineage, the numbers of regenerated GFP+ OLs in PLP-GFP:PIKE−/− were significantly reduced (Fig. 4C). Consistent with the previous report that demyelination triggers proliferation of neural precursor cells in the SVZ, which gives rise to proliferating OPCs that migrate to CC and further maturate on site for remyelination (26), we detected a large number of proliferating (Ki67+) cells in the SVZ as well as the demyelinated CC of PLP-GFP:PIKE+/+ mice at D5 (Fig. 4 D and E). However, Ki67+ cells were scarcely detectable in the SVZ and in the lesion area of the PLP-GFP:PIKE−/− mice at D5. Fewer number of Ki67+ cells were also detected in the SVZ of PLP-GFP:PIKE−/− mice compared with the PLP-GFP:PIKE+/+ control at D21 (Fig. S5). Finally, the loss of PIKE resulted in reduced MBP expression in the demyelinating lesion at both D5 and D21 in the PLP-GFP:PIKE−/− mice (Fig. 4F), further suggesting the essential role of PIKE in remyelination .
Fig. 4.
Delayed remyelination in PIKE−/− brain. (A) Representative coronal brain slices of PLP-GFP:PIKE+/+ and PLP-GFP:PIKE−/− mice (2-mo-old) collected at 5 (D5) and 21 (D21) days after LL injection. Damaged area is marked by the arrow. (Scale bar, 500 μm.) (B) SMI 32 staining in LL-injected CC after 5 (D5) and 21 (D21) days. (Scale bar, 50 μm.) (C) Number of GFP+ OLs in the damaged CC after LL injection (*P < 0.05, Student t test; n = 3). (D) Ki67 staining of the demyelinated CC in PLP-GFP:PIKE+/+ and PLP-GFP:PIKE−/− brains 5 d after LL injection. Cells that show positive Ki67 signals are indicated with arrows and are magnified in the Insets. (Scale bar, 50 μm.) (E) Number of Ki67+ cells in the CC and SVZ after LL injection (***P < 0.05, Student t test; n = 3). (F) IF staining of MBP in the LL-damaged CC. (Scale bar, 50 μm.)
Discussion
The essential functions of PIKE in neuronal survival, development, synaptic plasticity, and memory formation have been clearly demonstrated in our previous reports (8–12, 16). Accumulating evidence suggests that oligodendroglia also plays critical roles in governing neuronal functions, for example, via myelination-dependent saltatory conductance and proper signal transmission in the brain, which is essential for cognitive and motor behaviors (27). In the current study, we psrovide evidence that PIKE-L is also expressed in oligodendroglia, and the developmentally programmed PIKE up-regulation in OPCs governs OL differentiation and myelination via enhancing PI3K/Akt/mTOR signaling. We also demonstrate that depletion of PIKE affects the key genes that drive OL-dependent myelination and reduces the number of myelinating OLs during de novo development. Finally, the loss of PIKE impairs myelin lesion repair in the adult brain.
Although we found that PIKE depletion in cultured OPCs results in a prominent impairment of OPC differentiation, only mild myelination defect was observed in PIKE−/− CC. OLs are heterogeneous cells that express different receptors and transcription factors in a region-specific manner (28, 29). Because PIKE is expressed in a portion of OLs, PIKE ablation in mouse brain presumably affects the development or function of a particular type of OLs, which causes mild myelination defect. Therefore, PIKE −/− mice display only poor performance in the spatial memory tasks (Morris water maze and Y-maze) (11) but not any spontaneous epilepsy or aberrant coordinated movement as observed in other mouse models of myelinopathy (30, 31).
In addition to the presence of essential growth factors, OL survival and myelination depends on a contact-mediated signal between axon and OL (32). In particular, the interaction between OL-derived integrin and axon-derived lamin that activates Akt in OLs is critical for myelination of the axon (33). Because the number of neurons in the PIKE−/− brain is lower than the wild-type control (11), it is reasonable to suspect that the insufficient number of axons or contact from PIKE−/− neurons is the major cause of the impaired OL differentiation. However, primary cultures of PIKE-depleted OPC display aberrant OL differentiation, which excludes the possibility of neuronal influence. Furthermore, OL development is impaired in OPKO mice, which provides strong evidence to support the importance of PIKE-L in OL differentiation. Actually, the up-regulation of PIKE-L during OL differentiation and myelinogenesis and the defective Akt/mTOR signaling in PIKE−/− OLs indicate that PIKE-L is a pivotal mediator that links the developmental signal to the Akt/mTOR cascade. As such, ablation or inactivation of PIKE-L might block the extracellular factors from using this pathway to initiate OL maturation and myelination. We have shown previously that PIKE-L is a downstream effecter of netrin 1 to protect neurons from apoptosis through substantiating the activity of PI3K/Akt (9). Given that netrin 1 also regulates OPC migration and differentiation (34), PIKE-L may be an executor of netrin 1 to promote OL differentiation. In addition, netrin 1 is required to induce OL branching and formation of myelin-like sheet in vitro (35), which fits with our observation that PIKE-L is necessary for OL complexity development.
We also found that the amount of QKI proteins is reduced in PIKE−/− OLs. QKI proteins are members of signal transduction and activation of the RNA (STAR) family that play an essential role in OL development (36). These proteins, such as MBP, myelin-associated glycoprotein, and actin-interacting protein 1, control the splicing and stability of mRNA that are crucial to OL differentiation (37). The RNA-binding activity of QKI is governed by Fyn kinase (38, 39), which is linked with PIKE function (9, 40). A recent report suggests that inhibition of mTOR reduces translation of QKI isoforms (21). Consistent with this view, expressions of QKI6 and QKI7 are decreased in PIKE−/− OLs, which is a logical consequence of the aberrant activities of the Akt/mTOR observed. Interestingly, PIKE ablation also causes a reduction of QKI5, whose expression is not regulated by mTOR (21). Thus, PIKE-L might control QKI-5 expression to balance the opposing functions of QKI isoforms in OPC proliferation and differentiation via undefined molecular mechanisms. The reduction of QKI, as a result of deficient PIKE-Akt-mTOR signaling aberrantly, affects the down-stream targets of QKI, as represented by the preferential reduction of the direct QKI target MBP (41).
PIKE−/− mice also demonstrate a delayed remyelination after LL lesion, which indicates that PIKE-L is a crucial signaling molecule for both de novo myelinogenesis during normal development and myelin repair after lesion. During remyelination, new OLs are differentiated from OPCs localized in the damaged CC or from SVZ-originated progenitor cells (26, 42). Because activation of the PI3K/Akt signaling is required for OPC differentiation and survival, a variety of growth factors such as insulin-like growth factor 1 (IGF1), brain-derived neurotrophic factor (BDNF), and epidermal growth factor (EGF) that use PI3K/Akt/mTOR pathways to execute their physiological activities thus possess promising remyelination-promoting function (43–45). Collectively, our data support that the defective PI3K/Akt/mTOR cascades in PIKE−/− hinder regeneration of OPCs and differentiation of OLs in the damaged CC and SVZ of PIKE−/− brain, leading to a delayed recovery. Because the differentiation failure of OLs represents a major cause of defective remyelination in myelinopathies (46), the fact that PIKE-L controls OL differentiation and subsequent myelination makes it a crucial factor with important clinical implication. Presumably, augmentation of PIKE-L activity might represent a potential therapeutic strategy against various demyelination diseases.
Materials and Methods
Animals.
Doubled-engineered mice with GFP expression in the PIKE−/− OLs were generated by mating the whole-body PIKE−/− mice (10) with transgenic mice that carry a GFP controlled by PLP promoter (18). OPKO mice were generated by crossing the PIKE flox/flox mice with transgenic mice that carry the tamoxifen-inducible PLP1-promoter–driven Cre (Plp1-Cre/ERT) (The Jackson Laboratory). The double-engineered animals were bred to homozygosity. Genotyping of offspring was performed by PCR using genomic DNA from tail biopsies, and PCR genotyping was performed as described (22). One milligram of tamoxifen per mouse was administrated intraperitoneally into P22 OPKO mice once a day for 5 consecutive days. Four weeks later, the animals were killed to collect various CNS tissues. All animal experiments were approved and performed according to the care of experimental animal guidelines from Emory University.
OPC Culture and Differentiation.
Mixed glial cultures were prepared from the brains of neonatal SD rats (P1) and allowed to reach confluence. OPCs were removed by shaking. They were plated onto polylysine-coated coverslips and maintained in a defined serum-free medium with platelet-derived growth factor and fibroblast growth factor (10 and 20 ng/mL, respectively) to prevent differentiation. Mature OLs were obtained by culturing the OPCs in a defined medium lacking growth factors. Complexity of the cultured OLs was calculated by measuring the total branch length of the cell using the computer program ImageJ (National Institutes of Health).
Immunoprecipitation and Western Blotting.
Immunoprecipitation and Western blot analysis were performed as reported (10). Antibodies used in the Western blot analysis were obtained from Cell Signaling (pAkt, Akt, pmTOR, mTOR), Millipore (MBP), Sigma-Aldrich (tubulin), and Bethyl (QKI-5). Monoclonal antibodies against QKI-6 and QKI-7 were developed in collaboration with NeuroMab. Rabbit antibodies against the N-terminal of PIKE-L [PIKE(N)] were raised in our laboratory.
Immunofluorescent Staining.
Immunofluorescent staining was performed using either a fluorescence or confocal microscope as previously reported (10). Antibodies used in the IFs were obtained from Rockland Immunochemicals (CENTG1), Calbochem (CC1), Millipore (MBP, O1 and O4), Covance (SMI32), and BD Biosciences (Ki67).
Electron Microscopy Analysis.
After anesthetization, animals were perfused with 2.5% glutaraldehyde. The brains were then collected and cut into 0.3-μm vibratome sections. Slices were then postfixed with 1% osmium. After dehydration, ultrathin sections of the area of interest were cut using ultramicrotome, mounted on grids, counterstained with uranyl acetate and lead citrate, and examined with a Hitachi H-7500 Electron Microscope at Emory University’s Robert P. Apkarian Integrated Electron Microscopy Core.
Lysolecithin-Induced Focal Demyelination.
Adult (2-mo-old) mice were deeply anesthetized with chloral hydrate and positioned in a stereotaxic frame (Kopf). Focal demyelination was induced by injection of 2 µL of 1% lysolecithin (Sigma) in 0.9% NaCl into CC using coordinates of 1.1 mm anterior to the bregma, 1 mm lateral, and 2.5 mm deep from the skull surface. Because the PIKE−/− brain was smaller (11), the injection coordinates were adjusted accordingly (1.05 mm anterior to the bregma, 0.95 mm lateral, and 2.38 mm deep from the skull surface). At 5 or 21 d after LL injection, animals were subjected to transcardial perfusion and brains were harvested for IF analysis of myelin lesion and repair.
Statistical Analysis.
Results were expressed as mean ± SEM and were considered significant when P < 0.05. Statistical analysis of the data was performed by the computer program GraphPad Prism (GraphPad Software).
Supplementary Material
Acknowledgments
The authors thank Reed Otten for reading the manuscript. This work is supported by National Institutes of Health Grant R01NS045627 (to K.Y.) and Grant R01NS056097 (to Y.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318185111/-/DCSupplemental.
References
- 1.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81(2):871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 2.Bradl M, Lassmann H. Oligodendrocytes: Biology and pathology. Acta Neuropathol. 2010;119(1):37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taveggia C, Feltri ML, Wrabetz L. Signals to promote myelin formation and repair. Nat Rev Neurol. 2010;6(5):276–287. doi: 10.1038/nrneurol.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tohda C, Nakanishi R, Kadowaki M. Learning deficits and agenesis of synapses and myelinated axons in phosphoinositide-3 kinase-deficient mice. Neurosignals. 2006-2007;15(6):293–306. doi: 10.1159/000108936. [DOI] [PubMed] [Google Scholar]
- 5.Harrington EP, et al. Oligodendrocyte PTEN is required for myelin and axonal integrity, not remyelination. Ann Neurol. 2010;68(5):703–716. doi: 10.1002/ana.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye K. PIKE/nuclear PI 3-kinase signaling in preventing programmed cell death. J Cell Biochem. 2005;96(3):463–472. doi: 10.1002/jcb.20549. [DOI] [PubMed] [Google Scholar]
- 7.Ye K, Snyder SH. PIKE GTPase: A novel mediator of phosphoinositide signaling. J Cell Sci. 2004;117(Pt 2):155–161. doi: 10.1242/jcs.00924. [DOI] [PubMed] [Google Scholar]
- 8.Rong R, et al. PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci. 2003;6(11):1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- 9.Tang X, et al. Netrin-1 mediates neuronal survival through PIKE-L interaction with the dependence receptor UNC5B. Nat Cell Biol. 2008;10(6):698–706. doi: 10.1038/ncb1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan CB, et al. PIKE-mediated PI3-kinase activity is required for AMPA receptor surface expression. EMBO J. 2011;30(20):4274–4286. doi: 10.1038/emboj.2011.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan CB, et al. Phosphoinositide 3-kinase enhancer regulates neuronal dendritogenesis and survival in neocortex. J Neurosci. 2011;31(22):8083–8092. doi: 10.1523/JNEUROSCI.1129-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan CB, et al. Essential role of PIKE GTPases in neuronal protection against excitotoxic insults. Adv Biol Regul. 2012;52(1):66–76. doi: 10.1016/j.advenzreg.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Y, Liu Z, Ye K. Phosphoinositol lipids bind to phosphatidylinositol 3 (PI3)-kinase enhancer GTPase and mediate its stimulatory effect on PI3-kinase and Akt signalings. Proc Natl Acad Sci USA. 2005;102(46):16853–16858. doi: 10.1073/pnas.0507365102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu R, et al. Cdk5-mediated regulation of the PIKE-A-Akt pathway and glioblastoma cell invasion. Proc Natl Acad Sci USA. 2008;105(21):7570–7575. doi: 10.1073/pnas.0712306105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colello RJ, Devey LR, Imperato E, Pott U. The chronology of oligodendrocyte differentiation in the rat optic nerve: Evidence for a signaling step initiating myelination in the CNS. J Neurosci. 1995;15(11):7665–7672. doi: 10.1523/JNEUROSCI.15-11-07665.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z, et al. Neuroprotective actions of PIKE-L by inhibition of SET proteolytic degradation by asparagine endopeptidase. Mol Cell. 2008;29(6):665–678. doi: 10.1016/j.molcel.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan CB, et al. Deficiency of phosphoinositide 3-kinase enhancer protects mice from diet-induced obesity and insulin resistance. Diabetes. 2010;59(4):883–893. doi: 10.2337/db09-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci. 2002;22(3):876–885. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narayanan SP, Flores AI, Wang F, Macklin WB. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J Neurosci. 2009;29(21):6860–6870. doi: 10.1523/JNEUROSCI.0232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Tian D, Ku L, Osterhout DJ, Feng Y. The selective RNA-binding protein quaking I (QKI) is necessary and sufficient for promoting oligodendroglia differentiation. J Biol Chem. 2007;282(32):23553–23560. doi: 10.1074/jbc.M702045200. [DOI] [PubMed] [Google Scholar]
- 21.Tyler WA, et al. Proteomic identification of novel targets regulated by the mammalian target of rapamycin pathway during oligodendrocyte differentiation. Glia. 2011;59(11):1754–1769. doi: 10.1002/glia.21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan CB, et al. The association of phosphoinositide 3-kinase enhancer A with hepatic insulin receptor enhances its kinase activity. EMBO Rep. 2011;12(8):847–854. doi: 10.1038/embor.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doerflinger NH, Macklin WB, Popko B. Inducible site-specific recombination in myelinating cells. Genesis. 2003;35(1):63–72. doi: 10.1002/gene.10154. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Zhang Y, Li D, Feng Y. Destabilization and mislocalization of myelin basic protein mRNAs in quaking dysmyelination lacking the QKI RNA-binding proteins. J Neurosci. 2000;20(13):4944–4953. doi: 10.1523/JNEUROSCI.20-13-04944.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Feng Y. Distinct molecular mechanisms lead to diminished myelin basic protein and 2′,3′-cyclic nucleotide 3′-phosphodiesterase in qk(v) dysmyelination. J Neurochem. 2001;77(1):165–172. doi: 10.1046/j.1471-4159.2001.t01-1-00224.x. [DOI] [PubMed] [Google Scholar]
- 26.Nait-Oumesmar B, et al. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11(12):4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- 27.Inagawa K, Watanabe S, Tsukada Y, Mikoshiba K. The role of myelination in learning performance observed in two strains of myelin-deficient mutant mice (shiverer and mld) Behav Neural Biol. 1988;50(2):184–192. doi: 10.1016/s0163-1047(88)90871-0. [DOI] [PubMed] [Google Scholar]
- 28.Du Y, Fischer TZ, Lee LN, Lercher LD, Dreyfus CF. Regionally specific effects of BDNF on oligodendrocytes. Dev Neurosci. 2003;25(2–4):116–126. doi: 10.1159/000072261. [DOI] [PubMed] [Google Scholar]
- 29.Kitada M, Rowitch DH. Transcription factor co-expression patterns indicate heterogeneity of oligodendroglial subpopulations in adult spinal cord. Glia. 2006;54(1):35–46. doi: 10.1002/glia.20354. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths I, et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280(5369):1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- 31.Lappe-Siefke C, et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33(3):366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- 32.Piaton G, Gould RM, Lubetzki C. Axon-oligodendrocyte interactions during developmental myelination, demyelination and repair. J Neurochem. 2010;114(5):1243–1260. doi: 10.1111/j.1471-4159.2010.06831.x. [DOI] [PubMed] [Google Scholar]
- 33.Barros CS, et al. Beta1 integrins are required for normal CNS myelination and promote AKT-dependent myelin outgrowth. Development. 2009;136(16):2717–2724. doi: 10.1242/dev.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spassky N, et al. Directional guidance of oligodendroglial migration by class 3 semaphorins and netrin-1. J Neurosci. 2002;22(14):5992–6004. doi: 10.1523/JNEUROSCI.22-14-05992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajasekharan S, et al. Netrin 1 and Dcc regulate oligodendrocyte process branching and membrane extension via Fyn and RhoA. Development. 2009;136(3):415–426. doi: 10.1242/dev.018234. [DOI] [PubMed] [Google Scholar]
- 36.Feng Y, Bankston A. The star family member QKI and cell signaling. Adv Exp Med Biol. 2010;693:25–36. [PubMed] [Google Scholar]
- 37.Zhao L, Mandler MD, Yi H, Feng Y. Quaking I controls a unique cytoplasmic pathway that regulates alternative splicing of myelin-associated glycoprotein. Proc Natl Acad Sci USA. 2010;107(44):19061–19066. doi: 10.1073/pnas.1007487107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, et al. Tyrosine phosphorylation of QKI mediates developmental signals to regulate mRNA metabolism. EMBO J. 2003;22(8):1801–1810. doi: 10.1093/emboj/cdg171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Z, Ku L, Chen Y, Feng Y. Developmental abnormalities of myelin basic protein expression in fyn knock-out brain reveal a role of Fyn in posttranscriptional regulation. J Biol Chem. 2005;280(1):389–395. doi: 10.1074/jbc.M405973200. [DOI] [PubMed] [Google Scholar]
- 40.Tang X, Feng Y, Ye K. Src-family tyrosine kinase fyn phosphorylates phosphatidylinositol 3-kinase enhancer-activating Akt, preventing its apoptotic cleavage and promoting cell survival. Cell Death Differ. 2007;14(2):368–377. doi: 10.1038/sj.cdd.4402011. [DOI] [PubMed] [Google Scholar]
- 41.Zhao L, et al. QKI binds MAP1B mRNA and enhances MAP1B expression during oligodendrocyte development. Mol Biol Cell. 2006;17(10):4179–4186. doi: 10.1091/mbc.E06-04-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zawadzka M, et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6(6):578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.VonDran MW, Singh H, Honeywell JZ, Dreyfus CF. Levels of BDNF impact oligodendrocyte lineage cells following a cuprizone lesion. J Neurosci. 2011;31(40):14182–14190. doi: 10.1523/JNEUROSCI.6595-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10(8):990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- 45.Mason JL, Xuan S, Dragatsis I, Efstratiadis A, Goldman JE. Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. J Neurosci. 2003;23(20):7710–7718. doi: 10.1523/JNEUROSCI.23-20-07710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhlmann T, et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131(Pt 7):1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.