Abstract
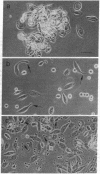
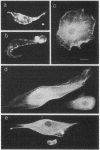
Foreign objects that enter the hemocoel of Drosophila melanogaster larvae are encapsulated by one type of blood cell, the lamellocyte, yet eggs of the parasitoid wasp Leptopilina heterotoma remain unencapsulated in D. melanogaster larval hosts that have many lamellocytes. Here we demonstrate that shortly after a female wasp oviposits in the hemocoel the lamellocytes undergo morphological changes and lose their adhesiveness. These affected blood cells are eventually destroyed as the parasitoid egg continues its development. The factor responsible for lamellocyte destruction, lamellolysin, is contained in an accessory gland of the female reproductive system and is injected along with the egg into the host hemocoel. Lamellolysin does not alter the morphology or the defense functions of the other types of blood cells in the host.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson K. M., Vinson S. B., Stoltz D. B., Summers M. D. Virus in a parasitoid wasp: suppression of the cellular immune response in the parasitoid's host. Science. 1981 Feb 6;211(4482):582–583. doi: 10.1126/science.7455695. [DOI] [PubMed] [Google Scholar]
- Nappi A. J. Inhibition by parasites of melanotic tumour formation in Drosophila melanogaster. Nature. 1975 May 29;255(5507):402–404. doi: 10.1038/255402a0. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Immunofluorescence and immunocytochemical procedures with affinity purified antibodies: tubulin-containing structures. Methods Cell Biol. 1982;24:97–132. doi: 10.1016/s0091-679x(08)60650-0. [DOI] [PubMed] [Google Scholar]
- RIZKI M. T. The influence of glucosamine-hydrochloride on cellular adhesiveness in Drosophila melanogaster. Exp Cell Res. 1961 Jun;24:111–119. doi: 10.1016/0014-4827(61)90252-x. [DOI] [PubMed] [Google Scholar]
- Rizki R. M., Rizki T. M. Cell interactions in the differentiation of a melanotic tumor in Drosophila. Differentiation. 1979;12(3):167–178. doi: 10.1111/j.1432-0436.1979.tb01002.x. [DOI] [PubMed] [Google Scholar]
- Rizki T. M., Rizki R. M. Blood cell surface changes in Drosophila mutants with melanotic tumors. Science. 1983 Apr 1;220(4592):73–75. doi: 10.1126/science.6402819. [DOI] [PubMed] [Google Scholar]
- Stoltz D. B., Vinson S. B. Penetration into caterpillar cells of virus-like particles injected during oviposition by parasitoid ichneumonid wasps. Can J Microbiol. 1979 Feb;25(2):207–216. doi: 10.1139/m79-032. [DOI] [PubMed] [Google Scholar]
- Weber K., Pollack R., Bibring T. Antibody against tuberlin: the specific visualization of cytoplasmic microtubules in tissue culture cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):459–463. doi: 10.1073/pnas.72.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]